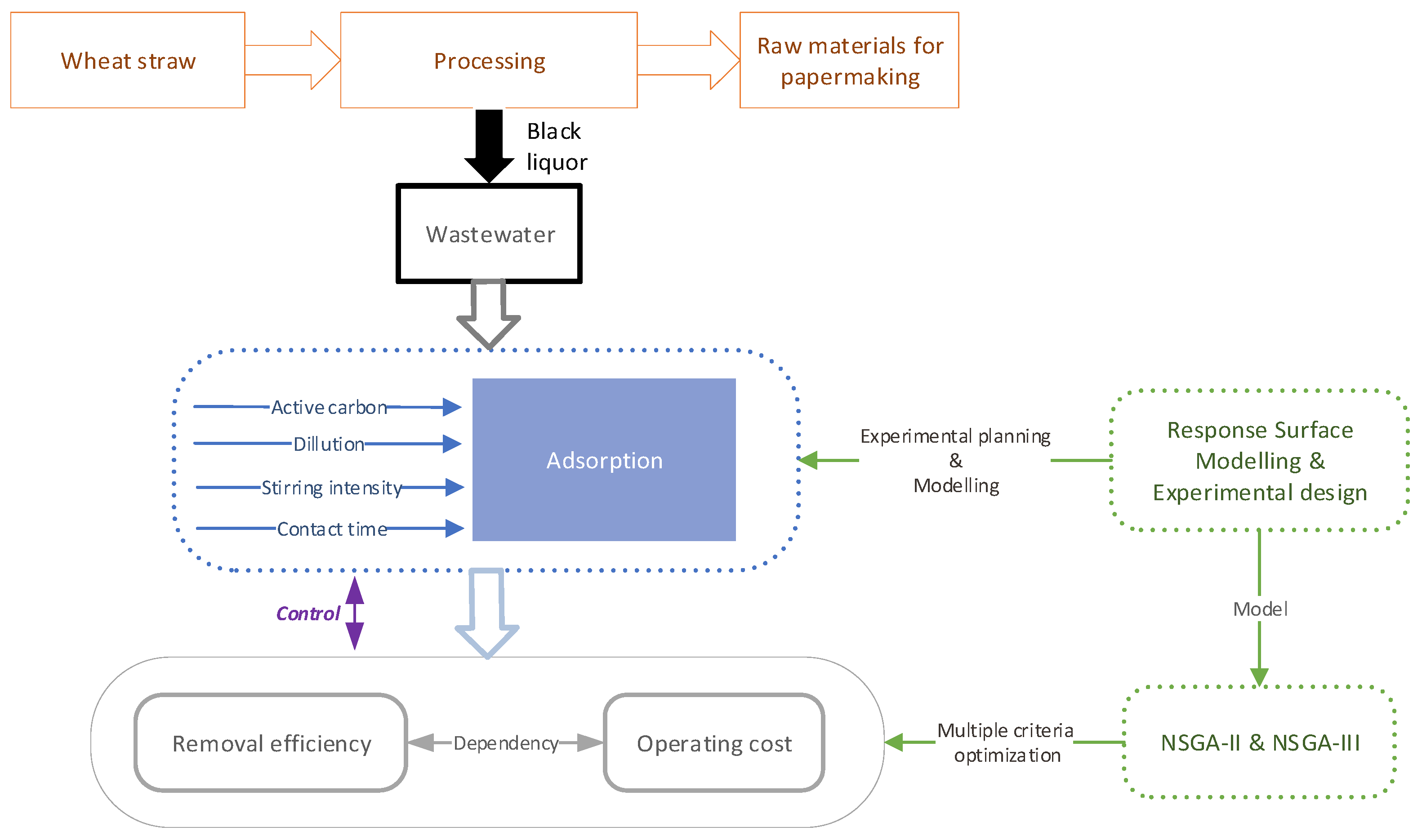
Technological and Economic Optimization of Wheat Straw Black Liquor Decolorization by Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Equipment
2.3. Batch Experiments
2.4. Experimental Design and Operating Cost
2.5. Multi-Objective Optimization
Subject to x ∈ Φ,
3. Results and Discussions
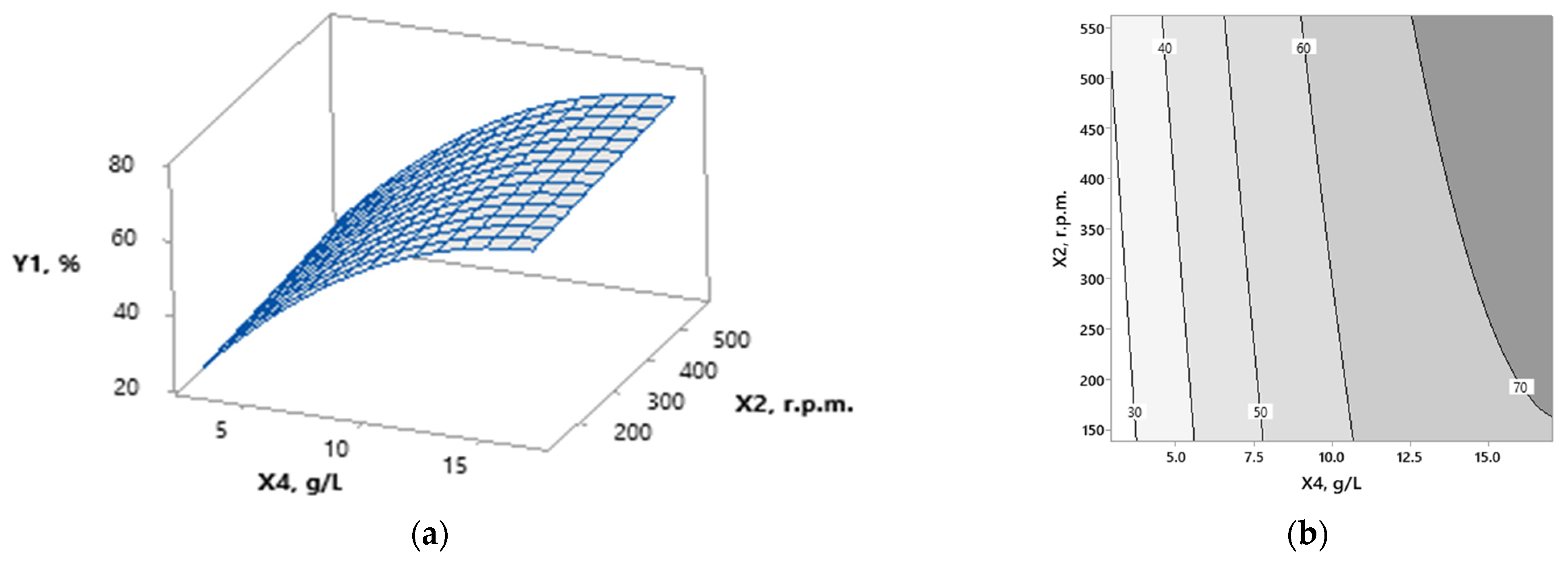
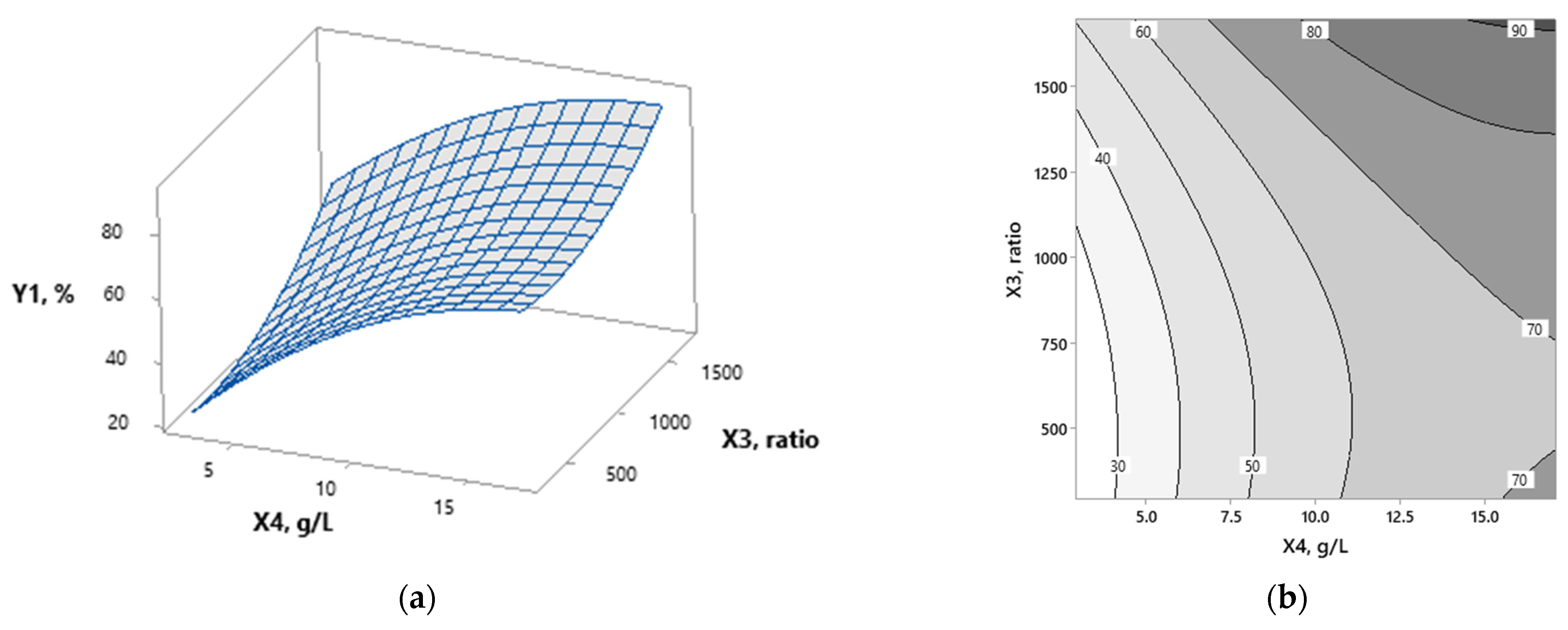
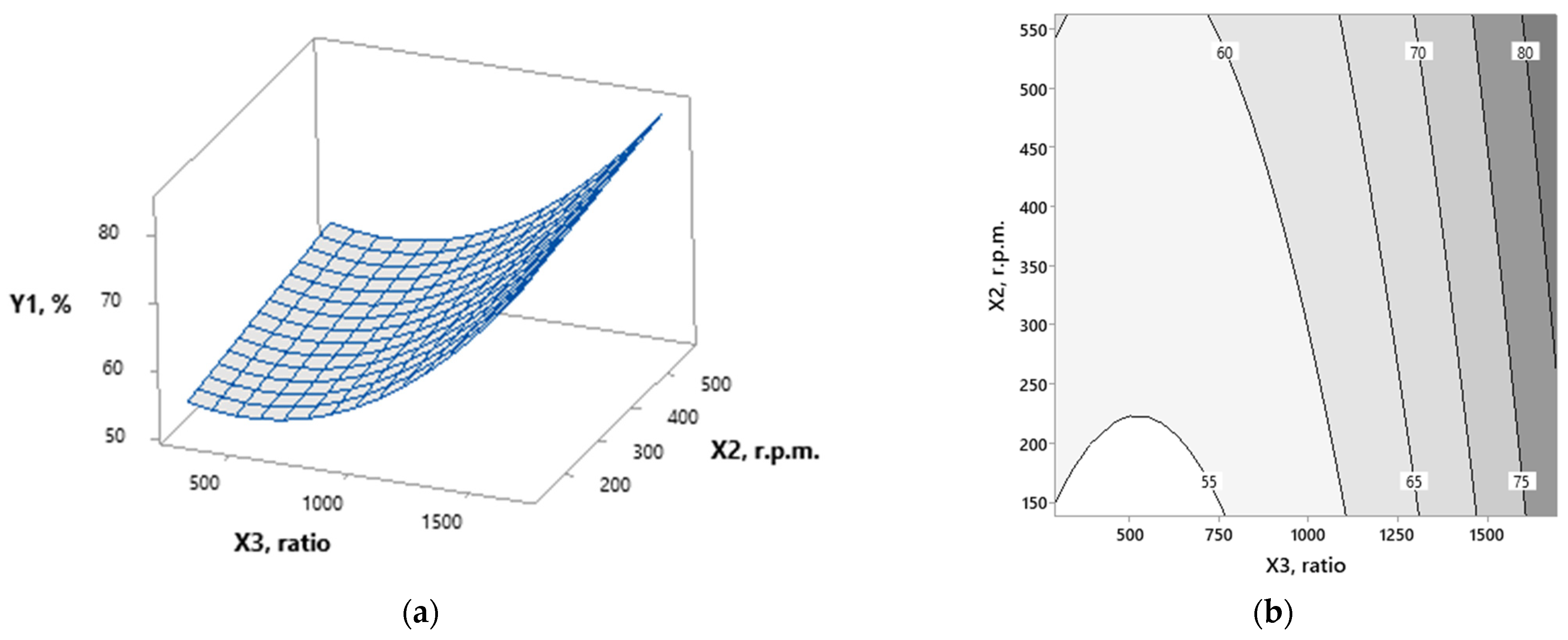
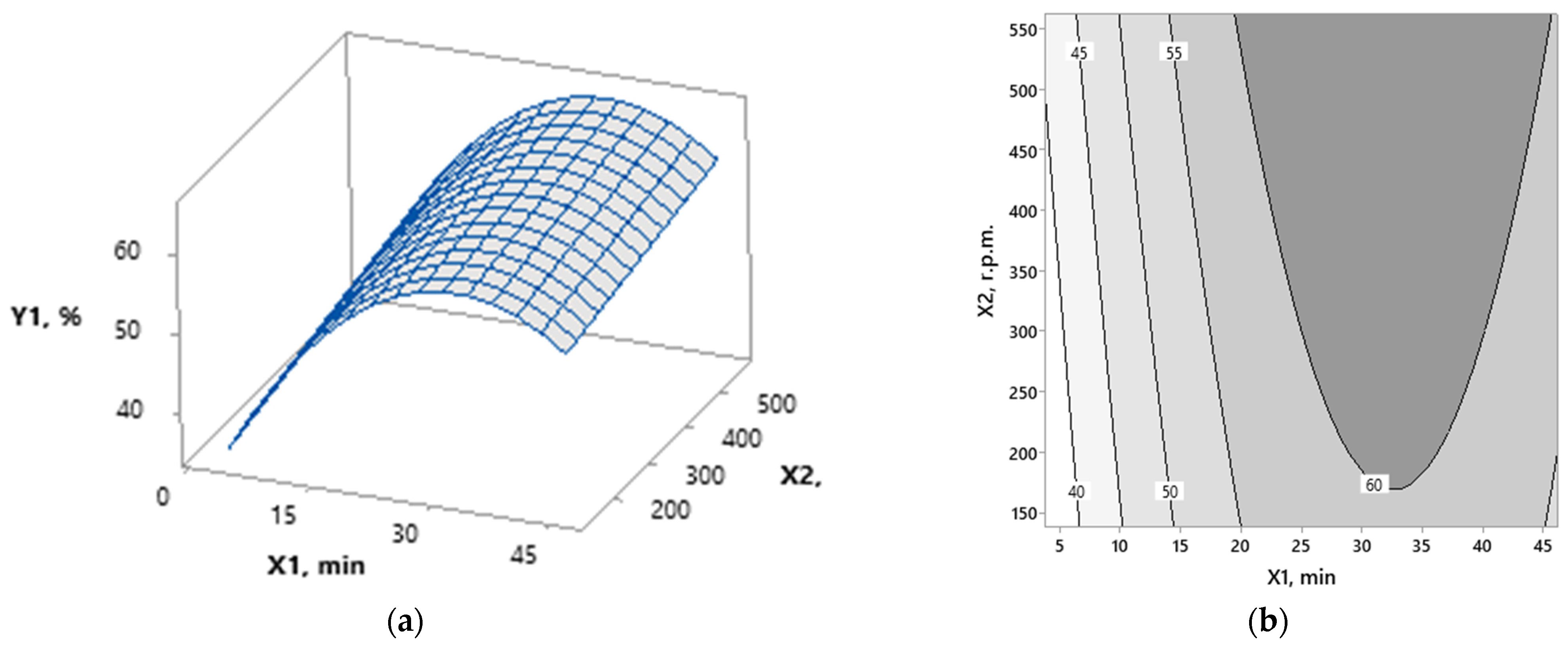
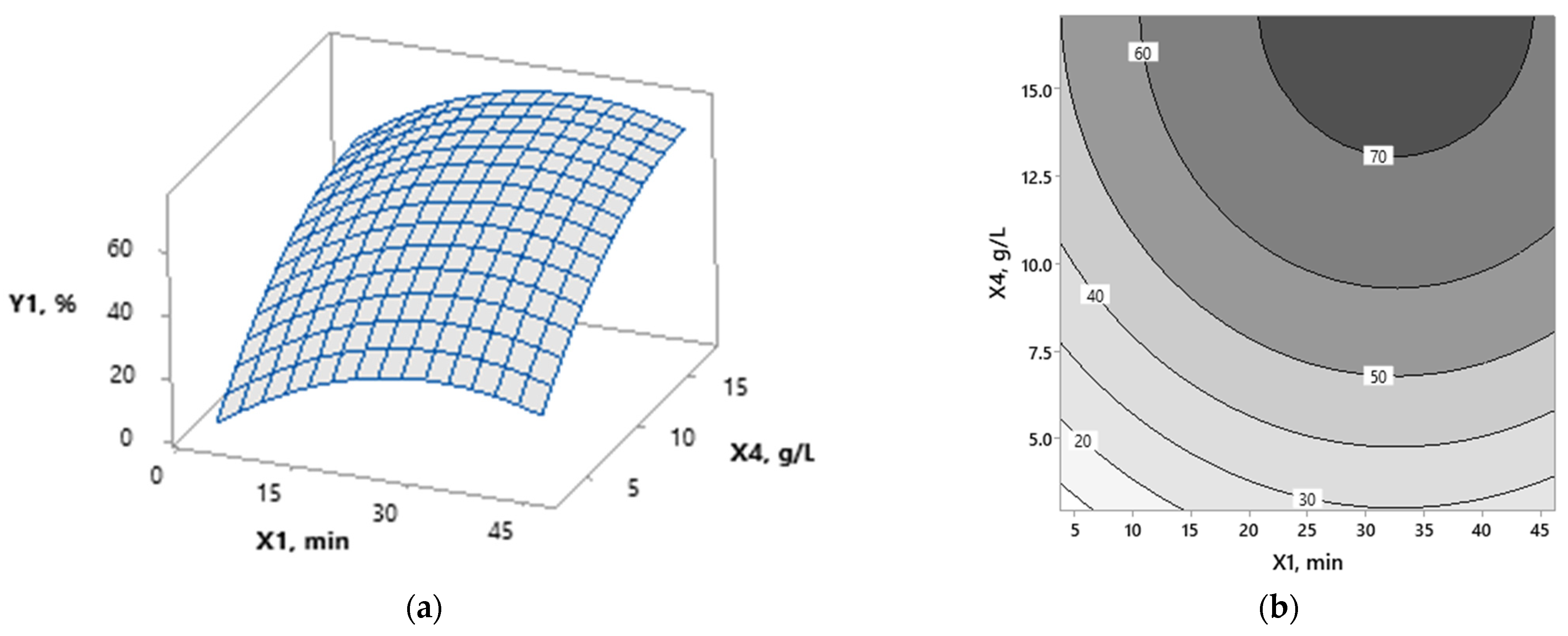
3.1. Modeling
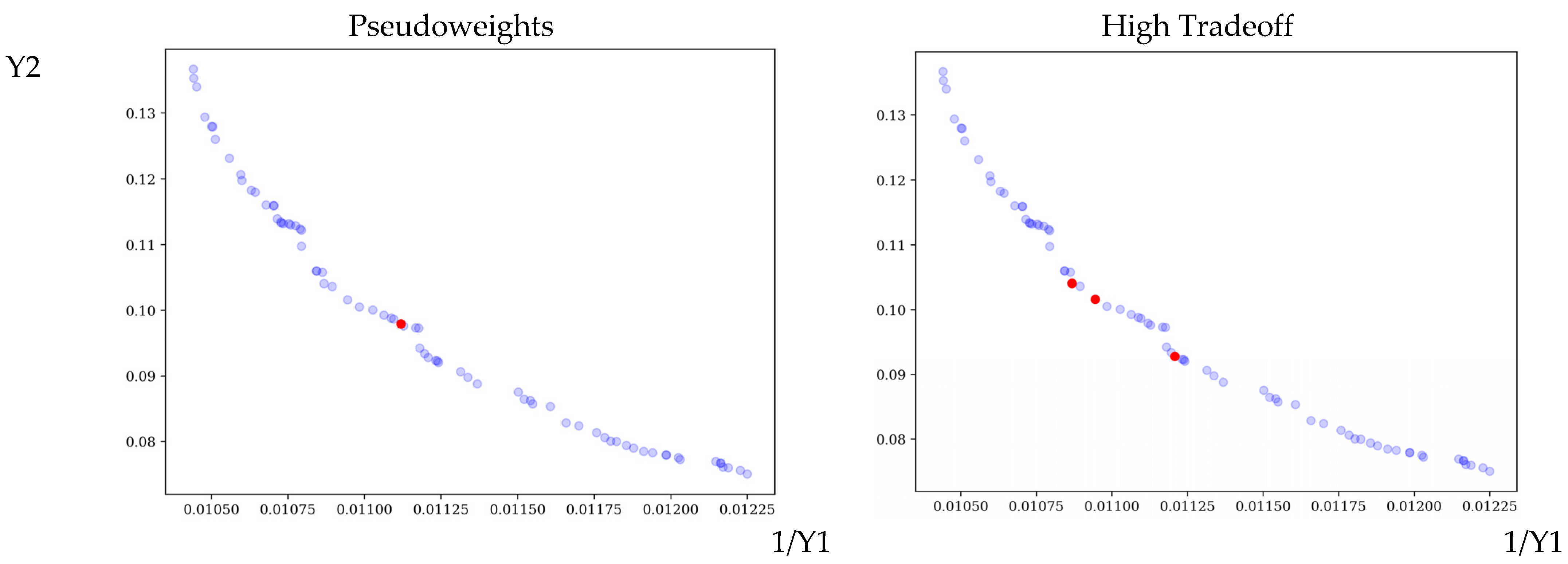
3.2. Optimization
4. Limitations and Future Work
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, G.; Swain, J.; Kay, M.; Forster, C.F. The treatment of pulp and paper mill effluent: A review. Bioresour. Technol. 2001, 77, 275–286. [Google Scholar] [CrossRef]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Li, N.; An, X.; Xiao, X.; An, W.; Zhang, Q. Recent advances in the treatment of lignin in papermaking wastewater. World J. Microbiol. Biotechnol. 2022, 38, 116. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.K.; Raj, P.V.R.P.; Kamble, S.; Pratap, S.; Gupta, S.; Belhadi, A. A deep learning-based approach for performance assessment and prediction: A case study of pulp and paper industries. Ann. Oper. Res. 2022. [Google Scholar] [CrossRef]
- Haq, I.; Kalamdhad, A.S.; Pandey, A. Genotoxicity evaluation of paper industry wastewater prior and post-treatment with laccase producing Pseudomonas putida MTCC 7525. J. Clean. Prod. 2022, 342, 130981. [Google Scholar] [CrossRef]
- Ali, M.; Sreekrishnan, T.R. Aquatic toxicity from pulp and paper mill effluents: A review. Adv. Environ. Res. 2001, 5, 175–196. [Google Scholar] [CrossRef]
- Baturina, M.A.; Kononova, O.N. Impact of Wastewater from the Pulp and Paper Industry on Aquatic Zoocenoses: A Review of the Literature. Contemp. Probl. Ecol. 2021, 14, 579–587. [Google Scholar] [CrossRef]
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manag. 2015, 158, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, A.K.; Bilal, M.; Prasad, S.; Rameshwari, K.R.T.; Chandra, R. Chapter 17—Paper and pulp mill wastewater: Characterization, microbial-mediated degradation, and challenges. In Nanotechnology in Paper and Wood Engineering; Bhat, R., Kumar, A., Nguyen, T.A., Sharma, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 371–387. [Google Scholar]
- Patel, K.; Patel, N.; Vaghamshi, N.; Shah, K.; Duggirala, S.M.; Dudhagara, P. Trends and strategies in the effluent treatment of pulp and paper industries: A review highlighting reactor options. Curr. Res. Microb. Sci. 2021, 2, 100077. [Google Scholar] [CrossRef]
- Owens, J.W. The hazard assessment of pulp and paper effluents in the aquatic environment: A review. Environ. Toxicol. Chem. 1991, 10, 1511–1540. [Google Scholar] [CrossRef]
- Bajpai, P.; Bajpai, P.K. Biological colour removal of pulp and paper mill wastewaters. J. Biotechnol. 1994, 33, 211–220. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M. Strategies for Decolorization and Detoxification of Pulp and Paper Mill Effluent. In Reviews of Environmental Contamination and Toxicology Volume 212; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; pp. 113–136. [Google Scholar]
- Kumar, A.; Srivastava, N.K.; Gera, P. Removal of color from pulp and paper mill wastewater- methods and techniques—A review. J. Environ. Manag. 2021, 298, 113527. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Pathan, S.; Bose, S. The Journey of Alternative and Sustainable Substitutes for “Single-Use” Plastics. Adv. Sustain. Syst. 2021, 5, 2100085. [Google Scholar] [CrossRef]
- Tan, J.; Tiwari, S.K.; Ramakrishna, S. Single-Use Plastics in the Food Services Industry: Can It Be Sustainable? Mater. Circ. Econ. 2021, 3, 7. [Google Scholar] [CrossRef]
- As’ad Mahpuz, A.S.; Muhamad Sanusi, N.A.S.; Jusoh, A.N.C.; Amin, N.J.M.; Musa, N.F.; Sarabo, Z.; Othman, N.Z. Manifesting sustainable food packaging from biodegradable materials: A review. Environ. Qual. Manag. 2022, 32, 379–396. [Google Scholar] [CrossRef]
- Degli Esposti, M.; Morselli, D.; Fava, F.; Bertin, L.; Cavani, F.; Viaggi, D.; Fabbri, P. The role of biotechnology in the transition from plastics to bioplastics: An opportunity to reconnect global growth with sustainability. FEBS Open Bio 2021, 11, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoez, W.; Dahab, I.; Ragab, E.M.; Abdelsalam, O.A.; Mustafa, A. Bio- and oxo-degradable plastics: Insights on facts and challenges. Polym. Adv. Technol. 2021, 32, 1981–1996. [Google Scholar] [CrossRef]
- Phelan, A.; Meissner, K.; Humphrey, J.; Ross, H. Plastic pollution and packaging: Corporate commitments and actions from the food and beverage sector. J. Clean. Prod. 2022, 331, 129827. [Google Scholar] [CrossRef]
- Johansen, M.R.; Christensen, T.B.; Ramos, T.M.; Syberg, K. A review of the plastic value chain from a circular economy perspective. J. Environ. Manag. 2022, 302, 113975. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, V.L. Comparative study of polystyrene/chemically modified wheat straw composite for green packaging application. Polym. Bull. 2020, 77, 1307–1326. [Google Scholar] [CrossRef]
- Kim, T.; Bamford, J.; Gracida-Alvarez, U.R.; Benavides, P.T. Life Cycle Greenhouse Gas Emissions and Water and Fossil-Fuel Consumptions for Polyethylene Furanoate and Its Coproducts from Wheat Straw. ACS Sustain. Chem. Eng. 2022, 10, 2830–2843. [Google Scholar] [CrossRef]
- Mohammad Rahmani, A.; Gahlot, P.; Moustakas, K.; Kazmi, A.A.; Shekhar Prasad Ojha, C.; Tyagi, V.K. Pretreatment methods to enhance solubilization and anaerobic biodegradability of lignocellulosic biomass (wheat straw): Progress and challenges. Fuel 2022, 319, 123726. [Google Scholar] [CrossRef]
- Puițel, A.C.; Suditu, G.D.; Danu, M.; Ailiesei, G.-L.; Nechita, M.T. An Experimental Study on the Hot Alkali Extraction of Xylan-Based Hemicelluloses from Wheat Straw and Corn Stalks and Optimization Methods. Polymers 2022, 14, 1662. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef] [PubMed]
- Janaswamy, S.; Yadav, M.P.; Hoque, M.; Bhattarai, S.; Ahmed, S. Cellulosic fraction from agricultural biomass as a viable alternative for plastics and plastic products. Ind. Crops Prod. 2022, 179, 114692. [Google Scholar] [CrossRef]
- Data Bridge Market Research. Global Wheat Straw Market—Industry Trends and Forecast to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-wheat-straw-market (accessed on 5 August 2023).
- Foodand Agriculture Organization of United Nations. Crops and Livestock Products. Available online: https://www.fao.org/ (accessed on 5 August 2023).
- Holmatov, B.; Hoekstra, A.Y.; Krol, M.S. EU’s bioethanol potential from wheat straw and maize stover and the environmental footprint of residue-based bioethanol. Mitig. Adapt. Strateg. Glob. Chang. 2021, 27, 6. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a Wood-Inspired Binder Enabled Strong, Water Stable, and Biodegradable Paper for Plastic Replacement. Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar] [CrossRef]
- Su, Y.; Yang, B.; Liu, J.; Sun, B.; Cao, C.; Zou, X.; Lutes, R.; He, Z. Prospects for Replacement of Some Plastics in Packaging with Lignocellulose Materials: A Brief Review. BioResources 2018, 13, 27. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xia, Q.; Jing, S.; Li, C.; Chen, Q.; Chen, B.; Pang, Z.; Jiang, B.; Gan, W.; Chen, G.; et al. Strong, Hydrostable, and Degradable Straws Based on Cellulose-Lignin Reinforced Composites. Small 2021, 17, 2008011. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations; Springer: Cham, Switzerland, 2019; pp. 719–725. [Google Scholar]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Kim, S.-H. Lignocellulosic biomass as renewable feedstock for biodegradable and recyclable plastics production: A sustainable approach. Renew. Sustain. Energy Rev. 2022, 158, 112130. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of Wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Ho, B.-H. Kinetics and Performance of Biological Activated Carbon Reactor for Advanced Treatment of Textile Dye Wastewater. Processes 2022, 10, 129. [Google Scholar] [CrossRef]
- Issa, Y.S.; Hamad, K.I.; Algawi, R.J.; Humadi, J.I.; Al-Salihi, S.; Ahmed, M.A.; Hassan, A.A.; Jasim, A.-K.A. Removal efficiency and reaction kinetics of phenolic compounds in refinery wastewater by nano catalytic wet oxidation. Int. J. Renew. Energy Dev. 2023, 12, 508–519. [Google Scholar] [CrossRef]
- AlRubaiea, J.F.; Latteiff, F.A.; Mahdi, J.M.; Atiya, M.A.; Majdi, H.S. Desalination of Agricultural Wastewater by Solar Adsorption System: A Numerical Study. Int. J. Renew. Energy Dev. 2021, 10, 901–910. [Google Scholar] [CrossRef]
- Devi, M.M.; Saravanamurugan, S. Chapter 16—Biomass-derived carbonaceous materials and their applications. In Biomass, Biofuels, Biochemicals; Li, H., Saravanamurugan, S., Pandey, A., Elumalai, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 431–467. [Google Scholar]
- Sherugar, P.; Padaki, M.; Naik, N.S.; George, S.D.; Murthy, D.H.K. Biomass-derived versatile activated carbon removes both heavy metals and dye molecules from wastewater with near-unity efficiency: Mechanism and kinetics. Chemosphere 2022, 287, 132085. [Google Scholar] [CrossRef] [PubMed]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.a.H.; Harrison, J.; Rooney, D.W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020, 10, 2563. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, N.A.; Chai, Y.H.; Ismail, I.S.; Othman, M.F.H.; Yusup, S. Biomass as activated carbon precursor and potential in supercapacitor applications. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Surface properties of granular activated carbons from agricultural by-products and their effects on raw sugar decolorization. Bioresour. Technol. 2000, 71, 103–112. [Google Scholar] [CrossRef]
- Mudoga, H.L.; Yucel, H.; Kincal, N.S. Decolorization of sugar syrups using commercial and sugar beet pulp based activated carbons. Bioresour. Technol. 2008, 99, 3528–3533. [Google Scholar] [CrossRef]
- Rashid, J.; Tehreem, F.; Rehman, A.; Kumar, R. Synthesis using natural functionalization of activated carbon from pumpkin peels for decolourization of aqueous methylene blue. Sci. Total Environ. 2019, 671, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, D.; Mousavi, S.A.; Nayeri, D. Low-cost activated carbon: Characterization, decolorization, modeling, optimization and kinetics. Int. J. Environ. Sci. Technol. 2020, 17, 3935–3946. [Google Scholar] [CrossRef]
- Osman, A.I.; Blewitt, J.; Abu-Dahrieh, J.K.; Farrell, C.; Al-Muhtaseb, A.a.H.; Harrison, J.; Rooney, D.W. Production and characterisation of activated carbon and carbon nanotubes from potato peel waste and their application in heavy metal removal. Environ. Sci. Pollut. Res. 2019, 26, 37228–37241. [Google Scholar] [CrossRef] [Green Version]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.M.; Abdel Rahim, E.A.; Fouzy, A.S.M. Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Kaghazchi, T. Agricultural Waste Conversion to Activated Carbon by Chemical Activation with Phosphoric Acid. Chem. Eng. Technol. 2007, 30, 649–654. [Google Scholar] [CrossRef]
- Naji, S.Z.; Tye, C.T. A review of the synthesis of activated carbon for biodiesel production: Precursor, preparation, and modification. Energy Convers. Manag. X 2022, 13, 100152. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of activated carbon from food waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Gayathiri, M.; Pulingam, T.; Lee, K.T.; Sudesh, K. Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism. Chemosphere 2022, 294, 133764. [Google Scholar] [CrossRef]
- Nechita, M.T.; Suditu, G.D.; Puițel, A.C.; Drăgoi, E.N. Differential evolution-based optimization of corn stalks black liquor decolorization using active carbon and TiO2/UV. Sci. Rep. 2021, 11, 18481. [Google Scholar] [CrossRef] [PubMed]
- Dashti, A.F.; Salman, N.A.S.; Adnan, R.; Zahed, M.A. Palm oil mill effluent treatment using combination of low cost chickpea coagulant and granular activated carbon: Optimization via response surface methodology. Groundw. Sustain. Dev. 2022, 16, 100709. [Google Scholar] [CrossRef]
- Rao, A.; Kumar, A.; Dhodapkar, R.; Pal, S. Adsorption of five emerging contaminants on activated carbon from aqueous medium: Kinetic characteristics and computational modeling for plausible mechanism. Environ. Sci. Pollut. Res. 2021, 28, 21347–21358. [Google Scholar] [CrossRef]
- Alam, G.; Ihsanullah, I.; Naushad, M.; Sillanpää, M. Applications of artificial intelligence in water treatment for optimization and automation of adsorption processes: Recent advances and prospects. Chem. Eng. J. 2022, 427, 130011. [Google Scholar] [CrossRef]
- Altowayti, W.A.H.; Shahir, S.; Othman, N.; Eisa, T.A.E.; Yafooz, W.M.S.; Al-Dhaqm, A.; Soon, C.Y.; Yahya, I.B.; Che Rahim, N.A.N.b.; Abaker, M.; et al. The Role of Conventional Methods and Artificial Intelligence in the Wastewater Treatment: A Comprehensive Review. Processes 2022, 10, 1832. [Google Scholar] [CrossRef]
- Lowe, M.; Qin, R.; Mao, X. A Review on Machine Learning, Artificial Intelligence, and Smart Technology in Water Treatment and Monitoring. Water 2022, 14, 1384. [Google Scholar] [CrossRef]
- Mpongwana, N.; Rathilal, S. A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment. Water 2022, 14, 1550. [Google Scholar] [CrossRef]
- Singh, N.K.; Kazmi, A.A.; Starkl, M. A review on full-scale decentralized wastewater treatment systems: Techno-economical approach. Water Sci. Technol. 2014, 71, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, E.; Cinperi, N.C. Water efficiency and wastewater reduction in an integrated woolen textile mill. J. Clean. Prod. 2018, 201, 686–696. [Google Scholar] [CrossRef]
- Secula, M.S.; Cagnon, B.; Cretescu, I.; Diaconu, M.; Petrescu, S. Removal of an acid dye from aqueous solutions by adsorption on a commercial granular activated carbon: Equilibrium, kinetic and thermodynamic study. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2011, 12, 307. [Google Scholar]
- Suditu, G.D.; Drăgoi, E.N.; Apostică, A.G.; Mănăilă, A.M.; Radu, V.M.; Puițel, A.C.; Nechita, M.T. Artificial Intelligence-Based Tools for Process Optimization: Case Study—Bromocresol Green Decolorization with Active Carbon. Adsorpt. Sci. Technol. 2022, 2022, 8110436. [Google Scholar] [CrossRef]
- Iorio, A.; Li, X. Solving rotated multi-objective optimization problems using differential evolution. In AI 2004: Advances in Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Collette, Y.; Siarry, P. Multiobjective Optimization: Principles and Case Studies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Pavan, M.; Todeschini, R. Multicriteria decision-making methods. In Comprehensive Chemometrics [Recurso Electrónico]: Chemical and Biochemical Data Analysis; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1, pp. 585–615. [Google Scholar]
- Li, H.; Zhang, Q. Multiobjective optimization problems with complicated Pareto sets, MOEA/D and NSGA-II. IEEE Trans. Evol. Comput. 2009, 13, 284–302. [Google Scholar] [CrossRef]
- Deb, K.; Pratap, A.; Agarwal, S.; Meyarivan, T. A fast and elitist multiobjective genetic algorithm: NSGA-II. IEEE Trans. Evol. Comput. 2002, 6, 182–197. [Google Scholar] [CrossRef] [Green Version]
- Deb, K.; Jain, H. An evolutionary many-objective optimization algorithm using reference-point-based nondominated sorting approach, part I: Solving problems with box constraints. IEEE Trans. Evol. Comput. 2013, 18, 577–601. [Google Scholar] [CrossRef]
- Nebro, A.J.; Durillo, J.J.; Machin, M.; Coello, C.A.C.; Dorronsoro, B. A Study of the Combination of Variation Operators in the NSGA-II Algorithm. In Advances in Artificial Intelligence; Bietza, C., Salmeron, A., Alonso-Betanzos, A., Hidalgo, J.I., Martinez, L., Troncoso, A., Corchado, E., Corchado, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8109, pp. 269–278. [Google Scholar]
- Zheng, F.; Zecchin, A.C.; Maier, H.R.; Simpson, A.R. Comparison of the searching behavior of NSGA-II, SAMODE, and borg MOEAs applied to water distribution system design problems. J. Water Resour. Plan. Manag. 2016, 142, 04016017. [Google Scholar] [CrossRef]
- Deb, K. Multi-Objective Optimisation Using Evolutionary Algorithms; Wiley: Chichester, UK, 2001. [Google Scholar]
- Rachmawati, L.; Srinivasan, D. Multiobjective Evolutionary Algorithm With Controllable Focus on the Knees of the Pareto Front. IEEE Trans. Evol. Comput. 2009, 13, 810–824. [Google Scholar] [CrossRef]
- Blank, J.; Deb, K. Pymoo: Multi-Objective Optimization in Python. IEEE Access 2020, 8, 89497–89509. [Google Scholar] [CrossRef]
- Heinze, G.; Wallisch, C.; Dunkler, D. Variable selection—A review and recommendations for the practicing statistician. Biom. J. 2018, 60, 431–449. [Google Scholar] [CrossRef] [Green Version]
- Shearer, L.; Pap, S.; Gibb, S.W. Removal of pharmaceuticals from wastewater: A review of adsorptive approaches, modelling and mechanisms for metformin and macrolides. J. Environ. Chem. Eng. 2022, 10, 108106. [Google Scholar] [CrossRef]
- Deb, K.; Sindhya, K.; Okabe, T. Self-adaptive simulated binary crossover for real-parameter optimization. In Proceedings of the 9th Annual Conference on Genetic and Evolutionary Computation, London, UK, 7–11 July 2007; pp. 1187–1194. [Google Scholar]
- Priddy, K.; Keller, P. Artificial Neural Networks:An Introduction; SPIE Press: Washington, DC, USA, 2005. [Google Scholar]
- Kumar, V.; Al-Gheethi, A.; Asharuddin, S.M.; Othman, N. Potential of cassava peels as a sustainable coagulant aid for institutional wastewater treatment: Characterisation, optimisation and techno-economic analysis. Chem. Eng. J. 2021, 420, 127642. [Google Scholar] [CrossRef]
- Yashni, G.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Dai-Viet, N.V.; Al-Kahtani, A.A.; Al-Sahari, M.; Nor Hazhar, N.J.; Noman, E.; Alkhadher, S. Bio-inspired ZnO NPs synthesized from Citrus sinensis peels extract for Congo red removal from textile wastewater via photocatalysis: Optimization, mechanisms, techno-economic analysis. Chemosphere 2021, 281, 130661. [Google Scholar] [CrossRef] [PubMed]
- Dadebo, D.; Nasr, M.; Fujii, M.; Ibrahim, M.G. Bio-coagulation using Cicer arietinum combined with pyrolyzed residual sludge-based adsorption for carwash wastewater treatment: A techno-economic and sustainable approach. J. Water Process Eng. 2022, 49, 103063. [Google Scholar] [CrossRef]
- Ano, J.; Henri Briton, B.G.; Kouassi, K.E.; Adouby, K. Nitrate removal by electrocoagulation process using experimental design methodology: A techno-economic optimization. J. Environ. Chem. Eng. 2020, 8, 104292. [Google Scholar] [CrossRef]
- Saad, M.S.; Kai, O.B.; Wirzal, M.D.H. Process modelling and techno economic analysis for optimal design of integrated electrocoagulation-membrane system for dye removal in wastewater. Chemosphere 2022, 306, 135623. [Google Scholar] [CrossRef]


| Independent Variables | Notation | Measure Units | Range | |||
|---|---|---|---|---|---|---|
| Coded | Uncoded | |||||
| From | To | From | To | |||
| Reaction time | X1 | min. | −1 | 1 | 10 | 40 |
| Stirrer rotation speed | X2 | r.p.m. | −1 | 1 | 200 | 500 |
| Black liquor dilution | X3 | ratio | −1 | 1 | 1:500 | 1:1500 |
| Activated carbon concentration | X4 | g/L | −1 | 1 | 5 | 15 |
| N | X1 | X2 | X3 | X4 | Y1, % | Y2 |
|---|---|---|---|---|---|---|
| 1 | 40 | 500 | 1:1500 | 15 | 83.42 | 0.99 |
| 2 | 10 | 500 | 1:1500 | 15 | 76.05 | 0.97 |
| 3 | 40 | 200 | 1:1500 | 15 | 82.23 | 0.98 |
| 4 | 10 | 200 | 1:1500 | 15 | 71.42 | 0.96 |
| 5 | 40 | 500 | 1:500 | 15 | 68.08 | 0.99 |
| 6 | 10 | 500 | 1:500 | 15 | 53.40 | 0.97 |
| 7 | 40 | 200 | 1:500 | 15 | 64.41 | 0.98 |
| 8 | 10 | 200 | 1:500 | 15 | 50.14 | 0.96 |
| 9 | 40 | 500 | 1:1500 | 5 | 55.20 | 0.35 |
| 10 | 10 | 500 | 1:1500 | 5 | 46.39 | 0.37 |
| 11 | 40 | 200 | 1:1500 | 5 | 53.03 | 0.38 |
| 12 | 10 | 200 | 1:1500 | 5 | 39.18 | 0.32 |
| 13 | 40 | 500 | 1:500 | 5 | 32.53 | 0.35 |
| 14 | 10 | 500 | 1:500 | 5 | 22.53 | 0.37 |
| 15 | 40 | 200 | 1:500 | 5 | 31.21 | 0.38 |
| 16 | 10 | 200 | 1:500 | 5 | 15.43 | 0.32 |
| 17 | 46.21 | 350 | 1:1000 | 10 | 62.29 | 0.66 |
| 18 | 3.79 | 350 | 1:1000 | 10 | 35.82 | 0.64 |
| 19 | 25 | 562.1 | 1:1000 | 10 | 62.96 | 0.66 |
| 20 | 25 | 137.9 | 1:1000 | 10 | 57.55 | 0.64 |
| 21 | 25 | 350 | 1:1707 | 10 | 70.55 | 0.65 |
| 22 | 25 | 350 | 1:293 | 10 | 71.24 | 0.65 |
| 23 | 25 | 350 | 1:1000 | 17.07 | 73.58 | 1.10 |
| 24 | 25 | 350 | 1:1000 | 2.93 | 29.77 | 0.197 |
| 25 | 25 | 350 | 1:1000 | 10 | 58.95 | 0.65 |
| 26 | 25 | 350 | 1:1000 | 10 | 60.27 | 0.65 |
| Source | DF | Seq SS | Contribution | Adj SS | Adj MS | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Regression | 8 | 8125.52 | 94.60% | 8125.52 | 1015.69 | 37.24 | 0 | |
| X1 | 1 | 884.5 | 10.30% | 605.21 | 605.21 | 22.19 | 0 | |
| X2 | 1 | 72.93 | 0.85% | 72.93 | 72.93 | 2.67 | 0.12 | |
| X3 | 1 | 1413.76 | 16.46% | 25.68 | 25.68 | 0.94 | 0.345 | |
| X4 | 1 | 4980.43 | 57.99% | 690.33 | 690.33 | 25.31 | 0 | |
| X1 × X1 | 1 | 380.33 | 4.43% | 370.26 | 370.26 | 13.58 | 0.002 | |
| X3 × X3 | 1 | 141.91 | 1.65% | 167.2 | 167.2 | 6.13 | 0.024 | |
| X4 × X4 | 1 | 237.56 | 2.77% | 237.56 | 237.56 | 8.71 | 0.009 | |
| X3 × X4 | 1 | 14.1 | 0.16% | 14.1 | 14.1 | 0.52 | 0.482 | |
| Error | 17 | 463.62 | 5.40% | 463.62 | 27.27 | |||
| Lack-of-Fit | 16 | 462.74 | 5.39% | 462.74 | 28.92 | 33.05 | 0.136 | |
| Pure error | 1 | 0.88 | 0.01% | 0.88 | 0.88 | |||
| Total | 25 | 8589.14 | 100.00% | |||||
| Sol No. | X1 | X2 | X3 | X4 | Yield (Y1, %) | Economic Cost (Y2, Euro) |
|---|---|---|---|---|---|---|
| 1 | 32.49 | 562.1 | 1:1694.92 | 16.78 | 95.54 | 0.1228 |
| 2 | 46.21 | 137.9 | 1:1694.92 | 17.07 | 84.68 | 0.1162 |
| 3 | 12.22 | 562.1 | 1:1694.92 | 17.07 | 83.41 | 0.1150 |
| Reaction Time, Min. | Stirrer Rotation Speed, r.p.m. | Dilution, Ratio | Activated Carbon Concentration, g/L | Y1, % | Y2, Euro | ||
|---|---|---|---|---|---|---|---|
| NSGA-II | Pseudoweights | 25.85 | 371.10 | 1:1705.25 | 13.48 | 89.93 | 0.0978 |
| High Tradeoff | 32.35 | 379.92 | 1:1703.91 | 13.51 | 91.36 | 0.1016 | |
| 31.90 | 392.12 | 1:1706.56 | 13.81 | 92.01 | 0.1041 | ||
| 30.33 | 377.36 | 1:1704.81 | 12.13 | 89.22 | 0.0928 | ||
| NSGA-III | Pseudoweights | 33.01 | 366.88 | 1:1694.85 | 11.28 | 86.61 | 0.0867 |
| High Tradeoff | 30.26 | 378.62 | 1:1703.44 | 10.12 | 83.95 | 0.0788 |
| Reaction Time, Min. | Stirrer Rotation Speed, r.p.m. | Dilution, Ratio | Activated Carbon Concentration, g/L | Y1, % | Y2, Euro | ||
|---|---|---|---|---|---|---|---|
| NSGA-II | Pseudoweights | 32.02 | 375.41 | 1:1697.17 | 7.38 | 74.42 | 0.0619 |
| High Tradeoff | 25.41 | 352.71 | 1:1703.38 | 6.52 | 69.28 | 0.0522 | |
| 30.09 | 549.34 | 1:1699.72 | 9.4 | 83.79 | 0.0874 | ||
| NSGA-III | Pseudoweights | 25.70 | 360.58 | 1:1690.29 | 8.89 | 78.14 | 0.0679 |
| High Tradeoff | 31.76 | 352.54 | 1:1705.42 | 8.79 | 79.69 | 0.0694 |
| Reaction Time, Min. | Stirrer Rotation Speed, r.p.m. | Dilution, Ratio | Activated Carbon Concentration, g/L | Y1, % | Y2, Euro | ||
|---|---|---|---|---|---|---|---|
| NSGA-II | Pseudoweights | 25.84 | 307.84 | 1:1706.45 | 12 | 87.85 | 0.0897 |
| High Tradeoff | 30.16 | 276.61 | 1:1706.78 | 16.61 | 92.33 | 0.1151 | |
| NSGA-III | Pseudoweights High Tradeoff | 28.41 | 305.62 | 1:1699.75 | 10.91 | 84.65 | 0.0794 |
| Wastewater Source | Method | Process Parameters | Optimization Methodology | Multi-Objective | Scale | Year, Ref. |
|---|---|---|---|---|---|---|
| synthetic | electrocoagulation | current intensity, electrode type, electrolyte type, pH, and time | Plackett-Burman design and Box-Behnken design | yes | cost per m3 | 2020, [87] |
| institutional | flocculation/ coagulation | pH, coagulant dosage, and settling time | RSM | no | annual operation cost | 2021, [84] |
| textile | photocatalysis | ZnO nanoparticle loading, pH, and Congo Red concentration | RSM | no | annual operation cost | 2021, [85] |
| textile | electrocoagulation | Voltage and time | Standard Least Squares Model | no | 15-yeartime frame | 2022, [88] |
| electrocoagulation + membrane | ||||||
| carwash | bio-coagulation | pH and coagulant dosage | RSM and Feed-forward ANN | no | 30 m3 of wastewater/day | 2022, [86] |
| adsorption | bed heights, flow rates | no | ||||
| black liquor | adsorption | Contact time, dilution, active carbon concentration, and stirring intensity | RSM, NSGA—II, and NSGA—III | yes | cost per each experiment | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suditu, G.D.; Drăgoi, E.N.; Puițel, A.C.; Nechita, M.T. Technological and Economic Optimization of Wheat Straw Black Liquor Decolorization by Activated Carbon. Water 2023, 15, 2911. https://doi.org/10.3390/w15162911
Suditu GD, Drăgoi EN, Puițel AC, Nechita MT. Technological and Economic Optimization of Wheat Straw Black Liquor Decolorization by Activated Carbon. Water. 2023; 15(16):2911. https://doi.org/10.3390/w15162911
Chicago/Turabian StyleSuditu, Gabriel Dan, Elena Niculina Drăgoi, Adrian Cătălin Puițel, and Mircea Teodor Nechita. 2023. "Technological and Economic Optimization of Wheat Straw Black Liquor Decolorization by Activated Carbon" Water 15, no. 16: 2911. https://doi.org/10.3390/w15162911





