Microplastic Particles’ Effects on Aquatic Organisms and Their Role as Transporters of Organic Pollutants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Particle Sizes and Preparation of Microplastics (MP)
2.2. Preparation of Pure Microplastics
2.3. MP Behavior Tests in an Aqueous Solution
2.4. Preparation of Contaminated Microplastics
2.5. Organism Obtaining
2.6. Bioassays
2.7. Acute Toxicity Bioassays
2.8. Chronic Toxicity Bioassay
2.9. Statistical Analysis
3. Results and Discussion
3.1. MP Particles’ Behavior in an Aqueous Solution
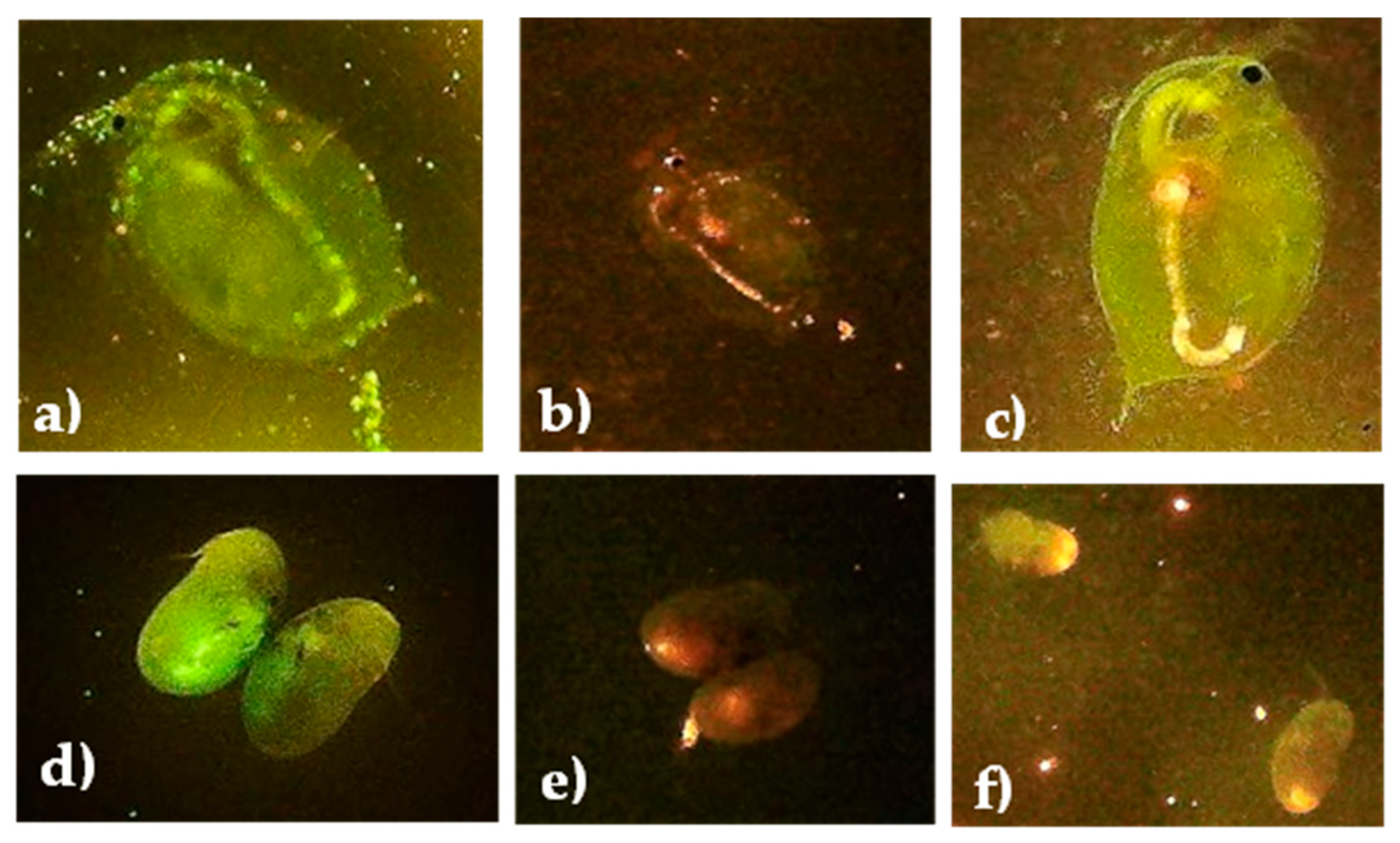
3.2. Plastic Particle Ingestion among Test Organisms
3.3. Acute Toxicity from Pure MP in D. magna and Podocopid Ostracods
3.4. Acute Toxicity from Polluted MP in D. magna and Podocopid Ostracods
3.5. Chronic Toxicity of Pure MP in D. magna
3.6. Chronic Toxicity of Contaminated MP in D. magna
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. ¿Qué es el Plástico? 2015. Available online: https://legacy.plasticseurope.org/es/about-plastics/what-are-plastics (accessed on 11 May 2023).
- Jambeck, J.; Geyer, R.; Wilcox, C.; Siegler, T.; Perryman, M.; Andrady, A.; Narayan, R.; Lavender Law, K. Plastic waste inputs from land into the ocean. Mar. Pollut. 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Cózara, A.; Echevarría, F.; González-Gordillo, J.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, A.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Environ. Sci. 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodall, L.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.; Narayanaswamy, B.; Thompson, R. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [Green Version]
- Moore, C. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shim, W.; Kwon, J. Sorption capacity of plastic debris for hydrophobic organic chemicals. Sci. Total Environ. 2014, 470–471, 1545–1552. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Ganesh Kumar, A.; Anjana, K.; Hinduja, M.; Sujitha, K.; Dharani, G. Review on plastic wastes in marine environment—Biodegradation and biotechnological solutions. Mar. Pollut. Bull. 2020, 150, 110733. [Google Scholar] [CrossRef]
- Andrady, A. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Andrady, A. The plastic in microplastics: A review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, H.; Hou, J. Progress on the Effects of Microplastics on Aquatic Crustaceans: A Review. Int. J. Mol. Sci. 2023, 24, 5523. [Google Scholar] [CrossRef]
- Eriksen, M.; Lebreton, L.; Carson, H.; Thiel, M.; Moore, C.; Borerro, J.; Galgani, F.; Ryan, P.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar]
- Tourinho, P.; Kočí, V.; Loureiro, S.; Gestel, C. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, S.; Pandey, R.; Yu, Z.; Kumar, M.; Khoo, K.S.; Kumar Thakur, T.; Loke Show, P. Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterizes, nutrient storage and its cycling. Trends Anal. Chem. 2023, 158, 116869. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Davison, P.; Asch, R.G. Plastic ingestion by mesopelagic fishes in the North Pacific Subtropical Gyre. Mar. Ecol. Prog. Ser. 2011, 432, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E. Microplastic pollution in lakes and lake shoreline sediments—A case study on Lake Bolsena and Lake Chiusi (central Italy). Environ. Pollut. 2016, 213, 648–657. [Google Scholar] [CrossRef]
- Forrest, S.; Holman, L.; Murphy, M.; Vermaire, M. Citizen science sampling programs as a technique for monitoring microplastic pollution: Results, lessons learned and recommendations for working with volunteers for monitoring plastic pollution in freshwater ecosystems. Environ. Monit. Assess. 2019, 191, 172. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Desforges, J.P.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.; Vethaak, A.D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Duis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016, 28, 2. [Google Scholar] [CrossRef] [Green Version]
- Rehse, S.; Kloas, W.; Zarfl, C. Short-term exposure with high concentrations of pristine microplastic particles leads to immobilisation of Daphnia magna. Chemosphere 2016, 153, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rainieri, S.; Conlledo, N.; Larsen, B.; Granby, K.; Barranco, A. Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 2018, 162, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Sureda, A.; Tejada, S.; Faggio, C. Microplastic in marine organism: Environmental and toxicological effects. Environ. Toxicol. Pharmacol. 2018, 64, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Kim, S.; An, Y.J. Polystyrene nanoplastics inhibit reproduction and induce abnormal embryonic development in the freshwater crustacean Daphnia galeata. Sci. Rep. 2017, 7, 12095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mak, C.; Yeung, K.; Chan, K. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.; Pereira, B.; Fonseca, M.; Mestre, N.; Fonseca, T.; Ilharco, L.; Bebianno, M. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Zhang, L.; Wang, K.; Yu, X.; Zheng, Z.; Zheng, R. Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. Sci. Total Environ. 2018, 628–629, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.; Budarz, J.; Hernandez, L.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G. Microplastics as vectors of contaminants. Mar. Pollut. Bull. 2019, 146, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Tang, X.Y.; Zhu, Y.G.; Zheng, M.H.; Miao, Q.L. Polycyclic aromatic hydrocarbon (PAH) contamination in urban soils of Beijing. China Reign. Ent. 2005, 31, 822–828. [Google Scholar] [CrossRef]
- Hylland, K. Polycyclic aromatic hydrocarbon (PAH). Ecotoxicology in marine ecosystems. Toxicol. Environ. Health Part A 2006, 69, 109–123. [Google Scholar] [CrossRef]
- Henkler, F.; Stolpmann, K.; Luch, A. Exposure to Polycyclic Aromatic Hydrocarbons: Bulky DNA Adducts and Cellular Responses. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 107–131. [Google Scholar]
- Shimada, H.; Oginuma, M.; Hara, A.; Imamura, Y. 9,10-Phenanthrenequinone, a component of diesel exhaust particles, inhibits the reduction of 4-benzoylpyridine and all-trans-retinal and mediates superoxide formation through its redox cycling in pig heart. Chem. Res. Toxicol. 2004, 17, 1145–1150. [Google Scholar] [CrossRef]
- Otero-Paternina, A.; Cruz-Casallas, P.; Velasco-Santamaría, Y. Evaluación del efecto del hidrocarburo fenantreno sobre el crecimiento de Chlorella vulgaris (Chlorellaceae). Acta Biol. Colomb. 2013, 18, 87–98. [Google Scholar]
- Bartonitz, A.; Anyanwu, I.; Geist, J.; Imhof, H.; Reichel, J.; Graßmann, J.; Drewes, J.; Beggel, S. Modulation of PAH toxicity on the freshwater organism G. roeseli by microparticles. Environ. Pollut. 2020, 260, 113999. [Google Scholar] [CrossRef]
- Mai, Y.; Peng, S.; Li, H.; Lai, Z. Histological, biochemical and transcriptomic analyses reveal liver damage in zebrafish (Danio rerio) exposed to phenanthrene. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 225, 108582. [Google Scholar] [CrossRef]
- Karami, A.; Romano, N.; Galloway, T.; Hamzah, H. Virgin microplastics cause toxicity and modulate the impacts of phenanthrene on biomarker responses in African catfish (Clarias gariepinus). Environ. Res. 2016, 151, 58–70. [Google Scholar] [CrossRef]
- Reynaud, S.; Deschaux, P. The effects of polycyclic aromatic hydrocarbons on the immune system of fish: A review. Aquat. Toxicol. 2006, 77, 229–238. [Google Scholar] [CrossRef]
- Palma, P.; Palma, V.L.; Fernandes, R.M.; Bohn, A.; Soares, A.; Barbosa, I. Embryo-toxic effects of environmental concentrations of chlorpyrifos on the crustacean Daphnia magna. Ecotoxicol. Environ. Saf. 2009, 72, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, L.; Marino, D.; Monferrán, M.; Amé, M. Can a low concentration of an organophosphate insecticide cause negative effects on an aquatic macrophyte? Exposure of Potamogeton pusillus at environmentally relevant chlorpyrifos concentrations. Environ. Exp. Bot. 2017, 138, 139–147. [Google Scholar] [CrossRef]
- Servicio Agrícola y Ganadero; División Protección Agrícola y Forestal; Subdepartamento de Plaguicidas y Fertilizantes. Informe de Venta de Plaguicidas de Uso Agrícola en Chile; SAG: Santiago, Chile, 2008. [Google Scholar]
- Nasser, F.; Lynch, I. Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. J. Proteom. 2016, 137, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secretaría de Medio Ambiente y Recursos Naturales; Báez, M.C.; Pica, Y.; Ronco, A. Ensayo de Toxicidad Aguda con el Cladócero Daphnia magna. Ensayos Toxicológicos para la Evaluación de Sustancias Químicas en Agua y Suelo. La Experiencia en México; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2008; pp. 17–32. [Google Scholar]
- Baltanás, A.; Mesquita-Joanes, F. Clase Ostrácoda. Orden Podocópida. Idea-Sea 2015, 74, 1–10. [Google Scholar]
- Palacios-Fest, M.R.; Park, L.E.; González-Porta, J.; Dix, G.R. Química de conchas de ostrácodos: Una alternativa para medir la contaminación por metales en sistemas acuáticos. Rev. Mex. Cienc. Geológicas 2003, 20, 139–153. [Google Scholar]
- Zocchi, M.; Sommaruga, R. Microplastics modify the toxicity of glyphosate on Daphnia magna. Sci. Total Environ. 2019, 697, 134194. [Google Scholar] [CrossRef] [PubMed]
- Ziajahromi, S.; Kumar, A.; Neale, P.; Leusch, F. Impact of Microplastic Beads and Fibers on Waterflea (Ceriodaphnia dubia) Survival, Growth, and Reproduction: Implications of Single and Mixture Exposures. Environ. Sci. Technol. 2017, 51, 13397–13406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; Deforce, E.; Kukulka, T. Distribution of surface plastic waste in the eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 2011, 48, 4732–4738. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Woodall, L.C.; Gwinnett, C.; Packer, M.; Thompson, R.C.; Robinson, L.F.; Paterson, G.L. Using a forensic science approach to minimize environmental contamination and to identify microfibres in marine sediments. Mar. Pollut. Bull. 2015, 95, 40–46. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Kang, J.H.; Kwon, O.Y.; Han, G.M. Large accumulation of micro-sized synthetic polymer particles in the sea surface microlayer. Environ. Sci. Technol. 2014, 48, 9014–9021. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [Green Version]
- de Alencastro, L.F. Évaluation de la Pollution par les Plastiques dans les Eaux de Surface en Suisse; Final Report EPFL—ENAC/IIE/GR-CEL; École Polytechnique Fédérale de Lausanne: Lausanne, Switzerland, 2014. [Google Scholar]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Lombardini, E.; Martellini, T.; Katsoyiannis, A.; Fossi, M.C.; Corsolini, S. Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 2017, 175, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Nel, H.A.; Froneman, P.W. A quantitative analysis of microplastic pollution along the south-eastern coastline of South Africa. Mar. Pollut. Bull. 2015, 101, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Roscher, L.; Meyer, M.S.; Hildebrandt, L.; Prume, J.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Spatial distribution of microplastics in sediments and surface waters of the southern North Sea. Environ. Pollut. 2019, 252, 1719–1729. [Google Scholar] [CrossRef] [Green Version]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswam, Y.B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Faure, F.; Corbaz, M.; Baecher, H.; de Alencastro, L.F. Pollution due to plastics and microplastics in Lake Geneva and in the Mediterranean Sea. Arch Sci. 2012, 65, 157–164. [Google Scholar]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific Central Gyre Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Eriksen, M.; Mason, S.; Wilson, S.; Box, C.; Zellers, A.; Edwards, W. Microplastic pollution in the surface waters of the Laurentian Great Lakes. Mar. Pollut. Bull. 2013, 77, 177–182. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Thiel, M. Distribution and abundance of small plastic debris on beaches in the SE Pacific (Chile): A study supported by a citizen science project. Mar. Environ. Res. 2013, 87–88, 12–18. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, A.; Cao, S.; Sun, F.; Wang, L.; Guo, H.; Ji, R. Effects of nanoplastics and microplastics on toxicity, bioaccumulation, and environmental fate of phenanthrene in fresh water. Environ. Pollut. 2016, 219, 166–173. [Google Scholar] [CrossRef]
- Bonifacio, A.; Ballesteros, M.L.; Bonansea, R.; Filippi, I.; Amé, M.V.; Hued, A. Environmental relevant concentrations of a chlorpyrifos commercial formulation affect two neotropical fish species, Cheirodon interruptus and Cnesterodon decemmaculatus. Chemosphere 2017, 188, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Cormier, B.; Batel, A.; Cachot, J.; Bégout, M.L.; Braunbeck, T.; Cousin, X.; Keiter, S. Multi-laboratory hazard assessment of contaminated microplastic particles by means of enhanced fish embryo test with the zebrafish (Danio rerio). Front. Environ. Sci. 2019, 7, 135. [Google Scholar] [CrossRef] [Green Version]
- Bosker, T.; Olthof, G.; Vijver, M.; Baas, J.; Barmentlo, S. Significant decline of Daphnia magna population biomass due to microplastic exposure. Environ. Pollut. 2019, 250, 669–675. [Google Scholar] [CrossRef] [Green Version]
- EN ISO 6341; Water Quality—Determination of the Inhibition of the Mobility of Daphnia magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. International Organization for Standardization: Geneve, Switzerland, 2012.
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Publishing: Paris, France, 2004. [Google Scholar]
- OECD. OECD Guideline for Testing of Chemicals. No. 211, Daphnia magna Reproduction Test; OECD: Paris, France, 2008. [Google Scholar]
- Norberg-King, T.J. An Interpolation Estimate for Chronic Toxicity: The ICP Approach; NETAC Technical Report; United States Environmental Protection Agency: Duluth, MN, USA, 1988; pp. 5–88. [Google Scholar]
- Gulley, D.D.; Boelter, A.N.; Bergman, H.L. Toxstat/Datasys Statistical Software; Release 3.0; Department of Zoology and Physiology, University of Wyoming: Laramie, WY, USA, 1989. [Google Scholar]
- Eltemsah, Y.; Bøhn, T. Acute and chronic effects of polystyrene microplastics on juvenile and adult Daphnia magna. Environ. Pollut. 2019, 254 Pt A, 112919. [Google Scholar] [CrossRef]
- Ma, H.; Pu, S.; Liu, S.; Bai, Y.; Mandal, S.; Xing, B. Microplastics in aquatic environments: Toxicity to trigger ecological consequences. Environ. Pollut. 2020, 261, 114089. [Google Scholar] [CrossRef]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, M.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef]
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201–209. [Google Scholar] [CrossRef]
- Besseling, E.; Wang, B.; Lürling, M.; Koelmans, A.A. Nanoplastic affects growth of S. obliquus and reproduction of D. Magna. Environ. Sci. Technol. 2014, 48, 12336–12343. [Google Scholar] [CrossRef] [PubMed]
- Rist, S.; Baun, A.; Hartmann, N.B. Ingestion of micro- and nanoplastics in Daphnia magna—Quantification of body burdens and assessment of feeding rates and reproduction. Environ. Pollut. 2017, 228, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Beiras, R.; Bellas, J.; Cachot, J.; Cormier, B.; Cousin, X.; Engwall, M.; Gambardella, C.; Garaventa, F.; Keiter, S.; Le Bihanic, F.; et al. Ingestion and contact with polyethylene microplastics does not cause acute toxicity on marine zooplankton. J. Hazard. Mater. 2018, 360, 452–460. [Google Scholar] [CrossRef] [Green Version]
- Au, S.; Bruce, T.; Bridges, W.; Klaine, S. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ. Toxicol. Chem. 2015, 34, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Lee, W.T.; Chan, A.K.Y.; Lo, H.S.; Shin, P.K.S.; Cheung, S.G. Microplastic ingestion reduces energy intake in the clam Atactodea striata. Mar. Pollut. Bull. 2017, 124, 798–802. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Gambardella, C.; Morgana, S.; Ferrando, S.; Bramini, M.; Piazza, V.; Costa, E.; Garaventa, F.; Faimali, M. Effects of polystyrene microbeads in marine planktonic crustaceans. Ecotoxicol. Environ. Saf. 2017, 145, 250–257. [Google Scholar] [CrossRef]
- Sleight, V.A.; Bakir, A.; Thompson, R.C.; Henry, T.B. Assessment of microplastic-sorbed contaminant bioavailability through analysis of biomarker gene expression in larval zebrafish. Mar. Pollut. Bull. 2017, 116, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Frydkjær, C.K.; Iversen, N.; Roslev, P. Ingestion and Egestion of Microplastics by the Cladoceran Daphnia magna: Effects of Regular and Irregular Shaped Plastic and Sorbed Phenanthrene. Bull. Environ. Contam. Toxicol. 2017, 99, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Gil, I. Polyethylene microplastics increase the toxicity of chlorpyrifos to the marine copepod Acartia tonsa. Environ. Pollut. 2020, 260, 114059. [Google Scholar] [CrossRef] [PubMed]
- León, V.M.; García, I.; González, E.; Samper, R.; Fernández-González, V.; Muniategui-Lorenzo, S. Potential transfer of organic pollutants from littoral plastics debris to the marine environment. Environ. Pollut. 2018, 236, 442–453. [Google Scholar] [CrossRef]
- Solomon, K.R.; Williams, W.M.; Mackay, D.; Purdy, J.; Giddings, J.M.; Giesy, J.P. Properties and uses of chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States; Springer: New York, NY, USA, 2014; pp. 13–34. [Google Scholar]
- ATSDR. Toxicological Profile for Chlorpyrifos; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1997; 217p.
- Garrido, S.; Linares, M.; Campillo, J.A.; Albentosa, M. Effect of microplastics on the toxicity of chlorpyrifos to the microalgae Isochrysis galbana, clone t-ISO. Ecotoxicol. Environ. Saf. 2019, 173, 103–109. [Google Scholar] [CrossRef]
- Ogonowski, M.; Schür, C.; Jarsén, Å.; Gorokhova, E. The effects of natural and anthropogenic microparticles on individual fitness in Daphnia magna. PLoS ONE 2016, 11, e0155063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulden, C.E.; Place, A.R. Lipid accumulation and allocation in daphnid cladocera. Bull. Mar. Sci. 1993, 53, 106–114. [Google Scholar]
- Van der Hoeven, N.; Gerritsen, A. Effects of chlorpyrifos on individuals and populations of Daphnia pulex in the laboratory and field. Environ. Toxicol. Chem. 2009, 16, 2438–2447. [Google Scholar] [CrossRef]





| MP Type | Size | Place | Location | Average MP Concentration | References |
|---|---|---|---|---|---|
| (N/I) | 80 μm | Swedish waters | Sweden | 150–2400 MP/m3 | [54] |
| Fibers | 2–3 mm and diameter < 0.1 mm | Water at 300 and 3500 m depth | NE North Atlantic and SW Indian Ocean in the Mediterranean Sea | 28–800 MP/L | [55] |
| Alkyd polymers and poli (acrilate/styrene) | 0.75 µm–5 mm ≈ | Goeje Island | South Korea | 195 MP/L | [56] |
| NI | NII | Ocean surface layer | Various sites | 0 to 8700 MP/m3 ≈ | [8] |
| NI | 63 µm–5 mm | NE Pacific, British Columbia coast | Canada | 8 and 9180 MP/m3 | [22] |
| 8975% fibers | NI | Ocean | Northeastern Pacific | 279 MP/m3 | [22] |
| NI | 0.5–5 mm ≈ | Yangtze Estuary | China | 4137 MP/m3 | [21] |
| NI | 0.5–20 mm | Danube River | Between Vienna (Austria) and Bratislava (Slovakia) | 0.055 MP/m3 | [57] |
| NI | 0.3–5 mm | Rhône River | Switzerland | 0.29 MP/m3 | [58] |
| 59NI | 0.33–2 mm | Northern shore cana L | Chicago, Illinois, USA | 1.94 MP/m3 upstream treatment plant. 17.93 MP/m3 downstream. | [59] |
| Mostly fibers | Mostly <300 μm | Amsterdam Canals | Netherlands | 100,000 MP/m3 | [23] |
| Fiber fragments and others, mostly PE and PP | NI | Ross Sea | Antarctica | 0.0032–1.18 MP/m3 | [60] |
| Fibers | NI | Southeastern coast of South Africa | South Africa | 257.9–1215 MP/m3 | [61] |
| PP, acrylates/polyurethane/varnish and polyamide | 86% <100 μm | Water surface | Southern area of the North Sea | 0.1–245.4 MP/m3 | [62] |
| PET and acrylic Fibers | 0.4–8.3 mm | Deep sea water | North Atlantic Ocean, Scotland | 70.8 MP/m3 | [63] |
| NI | 0.3–5 mm | Lake Geneva | Switzerland | 0.048 MP/m2 | [64] |
| Plastic film and fibers | <5 mm | North Pacific | North Pacific | 0.33 MP/m2 | [65] |
| NI | 0.3–5 mm | Lakes Geneva, Constance, Neuchâtel, Maggiore, Zurich, and Brienz | Switzerland | 0.091 MP/m2 | [58] |
| NI | 0.355 to 5 mm ≈ | Lakes Superior, Huron and Erie | Great Lakes of North America | 0.043 MP/m2 | [66] |
| Fragments, pellets/foams, facial cleanser micropearls | ≤1 mm | Lake Erie | Downstream from Detroit, Cleveland, and Erie, USA | 0.463 MP/m2 | [66] |
| NI | 0.355 to 5 mm ≈ | Lake Hovsgol | Mongolia | 0.001–0.044 MP/m2 | [67] |
| NI | NI | Rapa Nui shoreline | Chile | 805 MP/m2 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre-Martínez, G.; Carrizo, M.V.; Zenteno-Devaud, L. Microplastic Particles’ Effects on Aquatic Organisms and Their Role as Transporters of Organic Pollutants. Water 2023, 15, 2915. https://doi.org/10.3390/w15162915
Aguirre-Martínez G, Carrizo MV, Zenteno-Devaud L. Microplastic Particles’ Effects on Aquatic Organisms and Their Role as Transporters of Organic Pollutants. Water. 2023; 15(16):2915. https://doi.org/10.3390/w15162915
Chicago/Turabian StyleAguirre-Martínez, Gabriela, Maria Virginia Carrizo, and Lisette Zenteno-Devaud. 2023. "Microplastic Particles’ Effects on Aquatic Organisms and Their Role as Transporters of Organic Pollutants" Water 15, no. 16: 2915. https://doi.org/10.3390/w15162915
APA StyleAguirre-Martínez, G., Carrizo, M. V., & Zenteno-Devaud, L. (2023). Microplastic Particles’ Effects on Aquatic Organisms and Their Role as Transporters of Organic Pollutants. Water, 15(16), 2915. https://doi.org/10.3390/w15162915







