Multi-Dimensional Surface Water Quality Analyses in the Manawatu River Catchment, New Zealand
Abstract
:1. Introduction
2. Materials and Methods
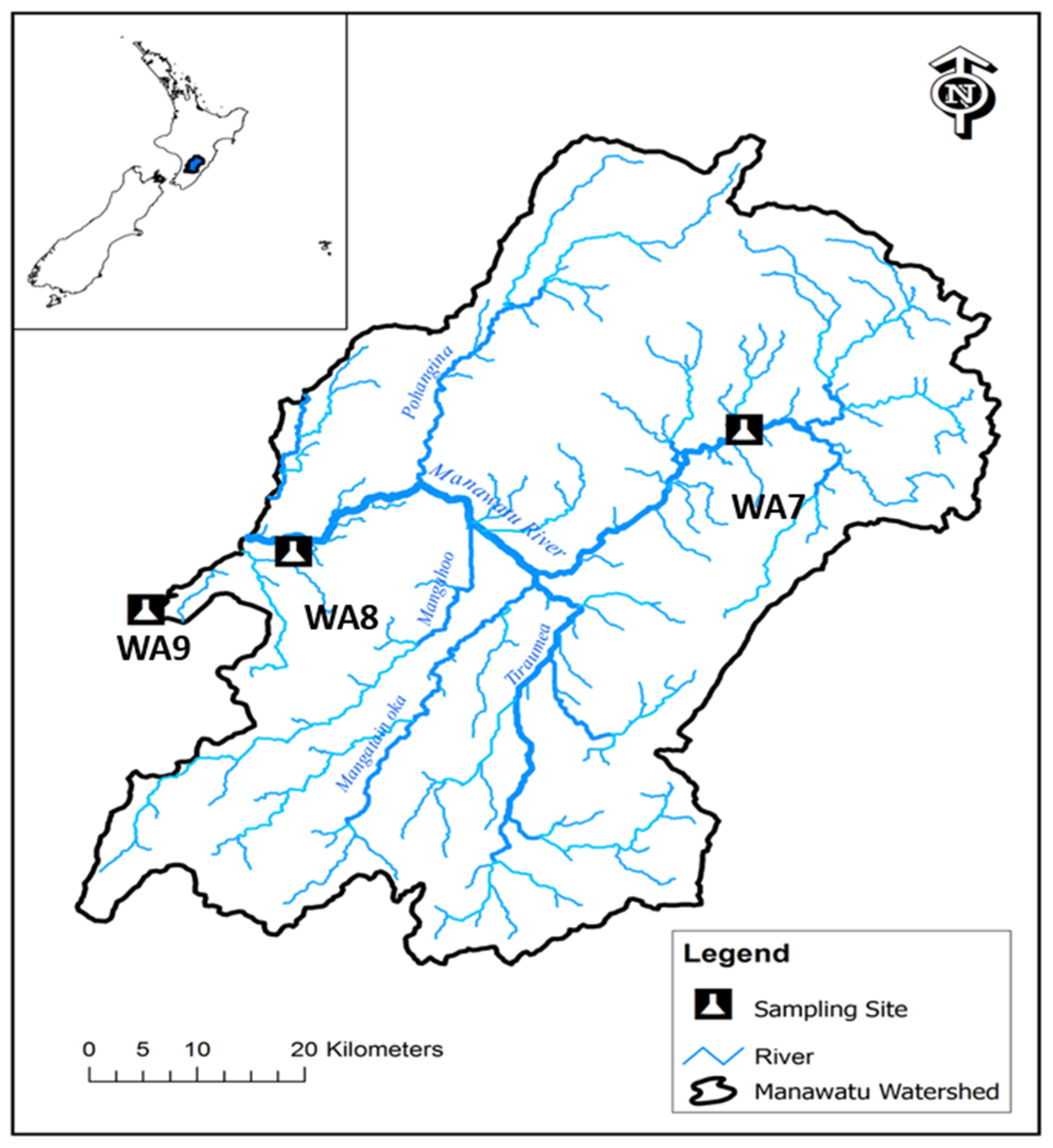
2.1. Study Area Physical Geography
2.2. Water Quality
2.3. Statistical Analysis
2.4. Land Use Land Cover (LULC) Mapping
3. Results
3.1. Land Use Map and Analysis
3.2. Landscape Connectivity
3.3. Seasonality and Trends in Water Quality in the Manawatu Catchment
3.4. Correlation Matrix for the Different Sites
3.5. Potential Pollution Sources Using the Positive Matrix Factorization Method
3.6. Model Performance of PMF for Manawatu Catchment
4. Discussion
4.1. Land Connectivity and Effects on Water Quality
4.2. Water Quality Assessment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jéquier, E.; Constant, F. Water as an Essential Nutrient: The Physiological Basis of Hydration. Eur. J. Clin. Nutr. 2010, 64, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Mandal, P.; Upadhyay, R.; Hasan, A. Seasonal and Spatial Variation of Yamuna River Water Quality in Delhi, India. Environ. Monit. Assess. 2010, 170, 661–670. [Google Scholar] [CrossRef]
- Almeida, C.A.; Quintar, S.; González, P.; Mallea, M.A. Influence of Urbanization and Tourist Activities on the Water Quality of the Potrero de Los Funes River (San Luis–Argentina). Environ. Monit. Assess. 2007, 133, 459–465. [Google Scholar] [CrossRef]
- Chelsea Nagy, R.; Graeme Lockaby, B.; Kalin, L.; Anderson, C. Effects of Urbanization on Stream Hydrology and Water Quality: The Florida Gulf Coast. Hydrol. Process. 2012, 26, 2019–2030. [Google Scholar] [CrossRef]
- Peters, N.E. Effects of Urbanization on Stream Water Quality in the City of Atlanta, Georgia, USA. Hydrol. Process. 2009, 23, 2860–2878. [Google Scholar] [CrossRef]
- Tenebe, I.T.; Emenike, C.P.; Daniel Chukwuka, C. Prevalence of Heavy Metals and Computation of Its Associated Risk in Surface Water Consumed in Ado-Odo Ota, South-West Nigeria. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 882–904. [Google Scholar] [CrossRef]
- ul Hassan, Z.; Shah, J.A.; Kanth, T.A.; Pandit, A.K. Influence of Land Use/Land Cover on the Water Chemistry of Wular Lake in Kashmir Himalaya (India). Ecol. Process. 2015, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Zhan, J.; Yan, H.; Wu, F.; Deng, X. Evaluation of the Impacts of Land Use on Water Quality: A Case Study in The Chaohu Lake Basin. Sci. World J. 2013, 2013, e329187. [Google Scholar] [CrossRef] [Green Version]
- Tenebe, I.T.; Ogbiye, A.S.; Omole, D.O.; Emenike, P.C. Estimation of Longitudinal Dispersion Co-Efficient: A Review. Cogent Eng. 2016, 3, 1216244. [Google Scholar] [CrossRef]
- Tenebe, I.T.; Ogbiye, A.S.; Omole, D.O.; Emenike, P.C. Parametric Evaluation of the Euler-Lagrangian Approach for Tracer Studies. Desalin. Water Treat. 2018, 109, 344–349. [Google Scholar] [CrossRef]
- Larned, S.T.; Moores, J.; Gadd, J.; Baillie, B.; Schallenberg, M. Evidence for the Effects of Land Use on Freshwater Ecosystems in New Zealand. N. Z. J. Mar. Freshw. Res. 2020, 54, 551–591. [Google Scholar] [CrossRef]
- Woldeab, B.; Ambelu, A.; Mereta, S.T.; Beyene, A. Effect of Watershed Land Use on Tributaries’ Water Quality in the East African Highland. Environ. Monit. Assess. 2018, 191, 36. [Google Scholar] [CrossRef] [PubMed]
- Julian, J.P.; Gardner, R.H. Land Cover Effects on Runoff Patterns in Eastern Piedmont (USA) Watersheds. Hydrol. Process. 2014, 28, 1525–1538. [Google Scholar] [CrossRef]
- Dymond, J.R.; Serezat, D.; Ausseil, A.-G.E.; Muirhead, R.W. Mapping of Escherichia Coli Sources Connected to Waterways in the Ruamahanga Catchment, New Zealand. Environ. Sci. Technol. 2016, 50, 1897–1905. [Google Scholar] [CrossRef]
- Taiwo, A.M. Source Identification and Apportionment of Pollution Sources to Groundwater Quality in Major Cities in Southwest, Nigeria/Identifikacija Izvora Oneciscenja Podzemnih Voda u Vecim Gradovima Jugozapadne Nigerije. Geofizika 2012, 29, 157–175. [Google Scholar]
- Emenike, C.P.; Tenebe, I.T.; Omole, D.O.; Ngene, B.U.; Oniemayin, B.I.; Maxwell, O.; Onoka, B.I. Accessing Safe Drinking Water in Sub-Saharan Africa: Issues and Challenges in South–West Nigeria. Sustain. Cities Soc. 2017, 30, 263–272. [Google Scholar] [CrossRef]
- Emenike, C.P.; Tenebe, I.T.; Jarvis, P. Fluoride Contamination in Groundwater Sources in Southwestern Nigeria: Assessment Using Multivariate Statistical Approach and Human Health Risk. Ecotoxicol. Environ. Saf. 2018, 156, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Gulgundi, M.S.; Shetty, A. Identification and Apportionment of Pollution Sources to Groundwater Quality. Environ. Process. 2016, 3, 451–461. [Google Scholar] [CrossRef]
- Chounlamany, V.; Tanchuling, M.A.; Inoue, T. Spatial and Temporal Variation of Water Quality of a Segment of Marikina River Using Multivariate Statistical Methods. Water Sci. Technol. 2017, 76, 1510–1522. [Google Scholar] [CrossRef]
- Bruesewitz, D.A.; Hamilton, D.P.; Schipper, L.A. Denitrification Potential in Lake Sediment Increases Across a Gradient of Catchment Agriculture. Ecosystems 2011, 14, 341–352. [Google Scholar] [CrossRef]
- Julian, J.P.; de Beurs, K.M.; Owsley, B.; Davies-Colley, R.J.; Ausseil, A.-G.E. River Water Quality Changes in New Zealand over 26 Years: Response to Land Use Intensity. Hydrol. Earth Syst. Sci. 2017, 21, 1149–1171. [Google Scholar] [CrossRef] [Green Version]
- Schallenberg, M. Determining Reference Conditions for New Zealand Lakes. Sci. Conserv. 2019, 334, 1. [Google Scholar]
- OECD/FAO. Aglink-Cosimo Model Documentation—A Partial Equilibrium Model of World Agricultural Markets; FAO: Rome, Italy, 2022; ISBN 978-92-5-136969-2. [Google Scholar]
- Smith, D.G.; McBride, G.B. New Zealand’s National Water Quality Monitoring Network—Design and First Year’s Operation1. JAWRA J. Am. Water Resour. Assoc. 1990, 26, 767–775. [Google Scholar] [CrossRef]
- Davies-Colley, R.J.; Nagels, J.W.; Smith, R.A.; Young, R.G.; Phillips, C.J. Water Quality Impact of a Dairy Cow Herd Crossing a Stream. N. Z. J. Mar. Freshw. Res. 2004, 38, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Ballantine, D.J.; Davies-Colley, R.J. Water Quality Trends in New Zealand Rivers: 1989-2009. Environ. Monit. Assess. 2014, 186, 1939–1950. [Google Scholar] [CrossRef]
- Larned, S.; Snelder, T.; Unwin, M.; McBride, G. Water Quality in New Zealand Rivers: Current State and Trends. N. Z. J. Mar. Freshw. Res. 2016, 50, 389–417. [Google Scholar] [CrossRef]
- Mcdowell, R.W.; Larned, S.T.; Houlbrooke, D.J. Nitrogen and Phosphorus in New Zealand Streams and Rivers: Control and Impact of Eutrophication and the Influence of Land Management. N. Z. J. Mar. Freshw. Res. 2009, 43, 985–995. [Google Scholar] [CrossRef]
- Reff, A.; Eberly, S.I.; Bhave, P.V. Receptor Modeling of Ambient Particulate Matter Data Using Positive Matrix Factorization: Review of Existing Methods. J. Air Waste Manag. Assoc. 2007, 57, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.G.; McBride, G.B.; Bryers, G.G.; Wisse, J.; Mink, D.F.J. Trends in New Zealand’s National River Water Quality Network. N. Z. J. Mar. Freshw. Res. 1996, 30, 485–500. [Google Scholar] [CrossRef]
- Abbott, S.; Julian, J.P.; Kamarinas, I.; Meitzen, K.M.; Fuller, I.C.; McColl, S.T.; Dymond, J.R. State-Shifting at the Edge of Resilience: River Suspended Sediment Responses to Land Use Change and Extreme Storms. Geomorphology 2018, 305, 49–60. [Google Scholar] [CrossRef]
- Hicks, D.M.; Shankar, U.; McKerchar, A.I.; Basher, L.; Lynn, I.; Page, M.; Jessen, M. Suspended Sediment Yields from New Zealand Rivers. J. Hydrol. 2011, 50, 81–142. [Google Scholar]
- Land Air Water Aotearoa. Available online: https://www.lawa.org.nz/download-data/ (accessed on 14 June 2023).
- Davies-Colley, R.J.; Smith, D.G.; Ward, R.C.; Bryers, G.G.; McBride, G.B.; Quinn, J.M.; Scarsbrook, M.R. Twenty Years of New Zealand’s National Rivers Water Quality Network: Benefits of Careful Design and Consistent Operation1. JAWRA J. Am. Water Resour. Assoc. 2011, 47, 750–771. [Google Scholar] [CrossRef]
- ANZECC & ARMCANZ (2000) Guidelines. Available online: https://www.waterquality.gov.au/anz-guidelines/resources/previous-guidelines/anzecc-armcanz-2000 (accessed on 8 June 2023).
- US EPA. Positive Matrix Factorization 5.0 Fundamentals and User Guide. Available online: https://www.epa.gov/air-research/epa-positive-matrix-factorization-50-fundamentals-and-user-guide (accessed on 14 June 2023).
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]
- Aitchison, J. Measures of Location of Compositional Data Sets. Math. Geol. 1989, 21, 787–790. [Google Scholar] [CrossRef]
- Blake, S.; Henry, T.; Murray, J.; Flood, R.; Muller, M.R.; Jones, A.G.; Rath, V. Compositional Multivariate Statistical Analysis of Thermal Groundwater Provenance: A Hydrogeochemical Case Study from Ireland. Appl. Geochem. 2016, 75, 171–188. [Google Scholar] [CrossRef] [Green Version]
- Emenike, P.C.; Tenebe, I.; Ogarekpe, N.; Omole, D.; Nnaji, C. Probabilistic Risk Assessment and Spatial Distribution of Potentially Toxic Elements in Groundwater Sources in Southwestern Nigeria. Sci. Rep. 2019, 9, 15920. [Google Scholar] [CrossRef] [Green Version]
- Manousakas, M.; Papaefthymiou, H.; Diapouli, E.; Migliori, A.; Karydas, A.G.; Bogdanovic-Radovic, I.; Eleftheriadis, K. Assessment of PM2.5 Sources and Their Corresponding Level of Uncertainty in a Coastal Urban Area Using EPA PMF 5.0 Enhanced Diagnostics. Sci. Total. Environ. 2017, 574, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamarinas, I. Geospatial Analyses of Terrestrial-Aquatic Connections Across New Zealand and Their Influence on River Water Quality. Ph.D. Thesis, Texas State University, San Marcos, TX, USA, August 2018. [Google Scholar]
- Kamarinas, I.; Julian, J.P.; Hughes, A.O.; Owsley, B.C.; De Beurs, K.M. Nonlinear Changes in Land Cover and Sediment Runoff in a New Zealand Catchment Dominated by Plantation Forestry and Livestock Grazing. Water 2016, 8, 436. [Google Scholar] [CrossRef] [Green Version]
- Snelder, T.H.; McDowell, R.W.; Fraser, C.E. Estimation of Catchment Nutrient Loads in New Zealand Using Monthly Water Quality Monitoring Data. JAWRA J. Am. Water Resour. Assoc. 2017, 53, 158–178. [Google Scholar] [CrossRef]
- Kemker, C. Turbidity, Total Suspended Solids & Water Clarity. Available online: https://www.fondriest.com/environmental-measurements/parameters/water-quality/turbidity-total-suspended-solids-water-clarity/ (accessed on 14 June 2023).
- Salim, I.; Sajjad, R.U.; Paule-Mercado, M.C.; Memon, S.A.; Lee, B.-Y.; Sukhbaatar, C.; Lee, C.-H. Comparison of Two Receptor Models PCA-MLR and PMF for Source Identification and Apportionment of Pollution Carried by Runoff from Catchment and Sub-Watershed Areas with Mixed Land Cover in South Korea. Sci. Total Environ. 2019, 663, 764–775. [Google Scholar] [CrossRef]
- Memon, S.; Paule, M.C.; Lee, B.-Y.; Umer, R.; Sukhbaatar, C.; Lee, C.-H. Investigation of Turbidity and Suspended Solids Behavior in Storm Water Run-off from Different Land-Use Sites in South Korea. Desalin. Water Treat. 2015, 53, 3088–3095. [Google Scholar] [CrossRef]
- Shrestha, S.; Kazama, F. Assessment of Surface Water Quality Using Multivariate Statistical Techniques: A Case Study of the Fuji River Basin, Japan. Environ. Model. Softw. 2007, 22, 464–475. [Google Scholar] [CrossRef]
- Ogwueleka, T.C. Use of Multivariate Statistical Techniques for the Evaluation of Temporal and Spatial Variations in Water Quality of the Kaduna River, Nigeria. Environ. Monit. Assess. 2015, 187, 137. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.A.S.; Gonçalves, A.d.A.; de Aragão, R.; de Amorim, J.R.A.; da Mota, P.V.M.; Srinivasan, V.S.; Garcia, C.A.B.; de Figueiredo, E.E. Spatial and Seasonal Variability of the Water Quality Characteristics of a River in Northeast Brazil. Environ. Earth Sci. 2019, 78, 68. [Google Scholar] [CrossRef]
- Reynolds, D.M. The Differentiation of Biodegradable and Non-Biodegradable Dissolved Organic Matter in Wastewaters Using Fluorescence Spectroscopy. J. Chem. Technol. Biotechnol. 2002, 77, 965–972. [Google Scholar] [CrossRef]
- Alves, D.D.; Riegel, R.P.; de Quevedo, D.M.; Osório, D.M.M.; da Costa, G.M.; do Nascimento, C.A.; Telöken, F. Seasonal Assessment and Apportionment of Surface Water Pollution Using Multivariate Statistical Methods: Sinos River, Southern Brazil. Environ. Monit. Assess. 2018, 190, 384. [Google Scholar] [CrossRef]
- Dils, R.M.; Heathwaite, A.L. The Controversial Role of Tile Drainage in Phosphorus Export from Agricultural Land. Water Sci. Technol. 1999, 39, 55–61. [Google Scholar] [CrossRef]
- Kazama, F.; Yoneyama, M. Nitrogen Generation in the Yamanashi Prefecture and Its Effects on the Groundwater Pollution. Environ. Sci. 2002, 15, 293–298. [Google Scholar] [CrossRef]
- Haji Gholizadeh, M.; Melesse, A.M.; Reddi, L. Water Quality Assessment and Apportionment of Pollution Sources Using APCS-MLR and PMF Receptor Modeling Techniques in Three Major Rivers of South Florida. Sci. Total Environ. 2016, 566–567, 1552–1567. [Google Scholar] [CrossRef]
- Buck, O.; Niyogi, D.K.; Townsend, C.R. Scale-Dependence of Land Use Effects on Water Quality of Streams in Agricultural Catchments. Environ. Pollut. 2004, 130, 287–299. [Google Scholar] [CrossRef]
- Monaghan, R.M.; Hedley, M.J.; Di, H.J.; McDowell, R.W.; Cameron, K.C.; Ledgard, S.F. Nutrient Management in New Zealand Pastures—Recent Developments and Future Issues. N. Z. J. Agric. Res. 2007, 50, 181–201. [Google Scholar] [CrossRef] [Green Version]
- Ledgard, S.F. Nitrogen Cycling in Low Input Legume-Based Agriculture, with Emphasis on Legume/Grass Pastures. Plant Soil 2001, 228, 43–59. [Google Scholar] [CrossRef]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P. Nitrogen and Phosphorus Limitation of Phytoplankton Growth in New Zealand Lakes: Implications for Eutrophication Control. Ecosystems 2010, 13, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Özkundakci, D.; Hamilton, D.P.; Kelly, D.; Schallenberg, M.; de Winton, M.; Verburg, P.; Trolle, D. Ecological Integrity of Deep Lakes in New Zealand across Anthropogenic Pressure Gradients. Ecol. Indic. 2014, 37, 45–57. [Google Scholar] [CrossRef]











| Site Code | Catchment Area (km2) | Site Description |
|---|---|---|
| WA7 | 705.28 | Located upstream |
| WA8 | 3897.37 | Major river mid-stream located in the center of Palmerston North |
| WA9 | 4222.22 | Located downstream |
| Unit | Abbreviation | Missing Data (%) | Trigger Values (Lowland/Upland †) | |
|---|---|---|---|---|
| Flow rate | m3/s | Flow | 1.4 | |
| Water Temperature | °C | Tw | 0.3 | |
| Electrical conductivity | µScm−1 | EC | - | |
| Dissolved Oxygen | g/m3 | DO | 1.3 | 6 |
| Dissolved Oxygen (%) | % | DO% | 1.3 | 98/99 |
| pH | - | pH | 0.2 | 7.2/7.3 |
| Turbidity | NTU | Turb | - | 5.6/4.1 |
| Total Phosphorus | g/m3 | TP | 0.3 | 33/26 |
| Total Nitrogen | g/m3 | TN | 0.6 | 614/295 |
| Visual clarity | m−1 | Clar | - | 0.8/0.6 |
| Dissolved Reactive Phosphorus | g/m3 | DRP | - | 10/9 |
| Ammoniacal nitrogen | g/m3 | NH4-N | - | 21/10 |
| Nitrate | g/m3 | NO3-N | - | 444/167 |
| Land Use/ Land Cover Category | Connected Area in Floodplain (km2) | Connected Area Not in Floodplain (km2) | Area Not Connected (km2) | Total (km2) |
|---|---|---|---|---|
| (a) | ||||
| Shrub/grassland | 3.39 | 14.20 | 15.67 | 33.26 |
| Urban | 0.12 | 0.12 | 0.36 | 0.60 |
| Non-plantation forest | 2.69 | 10.92 | 10.99 | 24.60 |
| Plantation forest | 3.36 | 6.77 | 11.11 | 21.24 |
| Vegetated wetland | 0.00 | 0.01 | 0.03 | 0.04 |
| High-producing grassland | 73.47 | 163.76 | 386.83 | 624.06 |
| Open water | 0.49 | 0.28 | 0.49 | 1.26 |
| Barren/other | 0.05 | 0.08 | 0.09 | 0.22 |
| Perennial cropland | - | - | - | - |
| Annual cropland | - | - | - | - |
| Total (km2) | 83.57 | 196.14 | 425.57 | 705.28 |
| (b) | ||||
| Shrub/grassland | 47.51 | 294.89 | 207.40 | 549.80 |
| Urban | 1.93 | 2.45 | 17.53 | 21.91 |
| Non-plantation forest | 35.27 | 174.98 | 100.38 | 310.63 |
| Plantation forest | 11.78 | 28.27 | 50.55 | 90.60 |
| Vegetated wetland | 0.1 | 0.18 | 0.58 | 0.86 |
| High-producing grassland | 332.21 | 756.14 | 1799.32 | 2887.67 |
| Open water | 6.86 | 3.34 | 4.26 | 14.28 |
| Barren/other | 3.75 | 3.77 | 3.19 | 10.71 |
| Perennial cropland | 0.07 | 0.07 | 0.31 | 0.45 |
| Annual cropland | 1.43 | 1.19 | 7.66 | 10.28 |
| Total (km2) | 440.91 | 1265.28 | 2191.18 | 3897.37 |
| (c) | ||||
| Shrub/grassland | 52.08 | 319.23 | 225.73 | 597.04 |
| Urban | 5.30 | 5.73 | 39.29 | 50.32 |
| Non-plantation forest | 37.51 | 181.05 | 105.76 | 324.32 |
| Plantation forest | 14.09 | 35.27 | 60.82 | 110.18 |
| Vegetated wetland | 0.10 | 0.18 | 0.58 | 0.86 |
| High-producing grassland | 360.67 | 792.61 | 1944.12 | 3097.40 |
| Open water | 8.01 | 3.82 | 5.05 | 16.88 |
| Barren/other | 4.22 | 3.92 | 3.45 | 11.59 |
| Perennial cropland | 0.08 | 0.09 | 0.41 | 0.58 |
| Annual cropland | 1.85 | 1.70 | 10.08 | 13.63 |
| Total (km2) | 483.91 | 1343.60 | 2395.29 | 4222.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenebe, I.T.; Julian, J.P.; Emenike, P.C.; Dede-Bamfo, N.; Maxwell, O.; Sanni, S.E.; Babatunde, E.O.; Alves, D.D. Multi-Dimensional Surface Water Quality Analyses in the Manawatu River Catchment, New Zealand. Water 2023, 15, 2939. https://doi.org/10.3390/w15162939
Tenebe IT, Julian JP, Emenike PC, Dede-Bamfo N, Maxwell O, Sanni SE, Babatunde EO, Alves DD. Multi-Dimensional Surface Water Quality Analyses in the Manawatu River Catchment, New Zealand. Water. 2023; 15(16):2939. https://doi.org/10.3390/w15162939
Chicago/Turabian StyleTenebe, Imokhai T., Jason P. Julian, PraiseGod C. Emenike, Nathaniel Dede-Bamfo, Omeje Maxwell, Samuel E. Sanni, Eunice O. Babatunde, and Darlan D. Alves. 2023. "Multi-Dimensional Surface Water Quality Analyses in the Manawatu River Catchment, New Zealand" Water 15, no. 16: 2939. https://doi.org/10.3390/w15162939
APA StyleTenebe, I. T., Julian, J. P., Emenike, P. C., Dede-Bamfo, N., Maxwell, O., Sanni, S. E., Babatunde, E. O., & Alves, D. D. (2023). Multi-Dimensional Surface Water Quality Analyses in the Manawatu River Catchment, New Zealand. Water, 15(16), 2939. https://doi.org/10.3390/w15162939








