The Chemical Compatibility of Sand–Attapulgite Cut-Off Walls for Landfills
Abstract
:1. Introduction
2. Materials and Methods
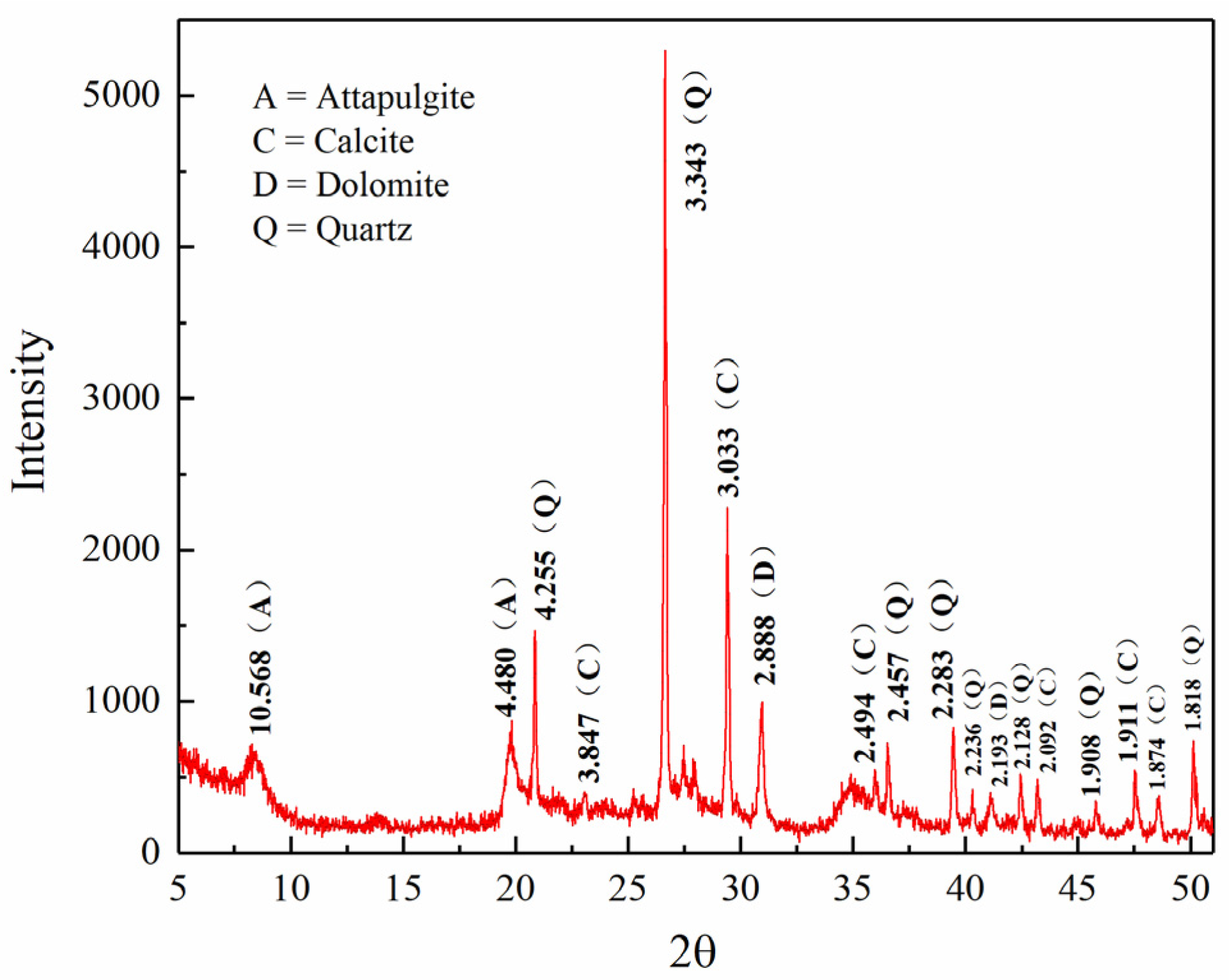
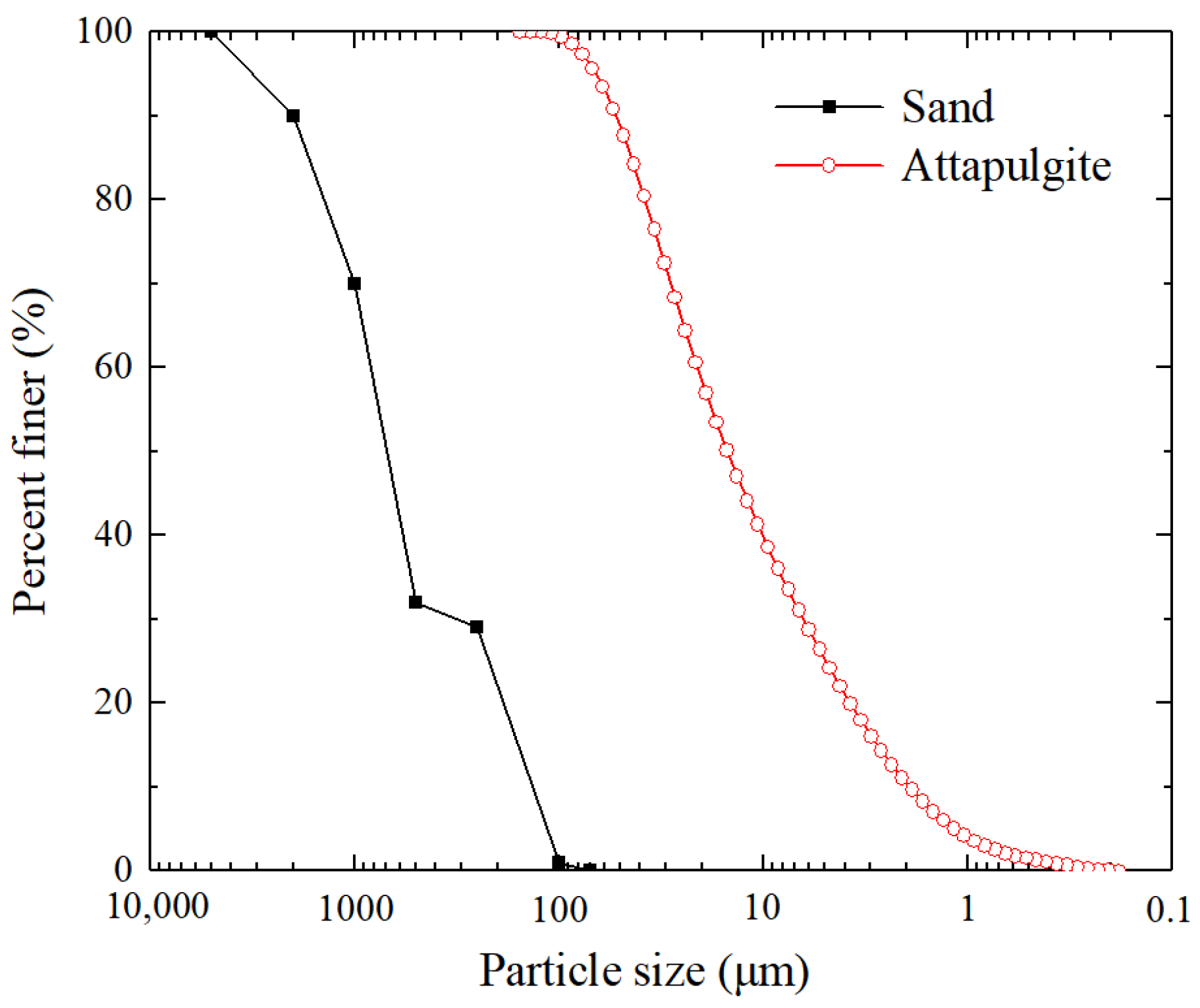
2.1. Materials and Permeate Solutions
2.2. Sample Preparation
2.3. Rigid-Wall Hydraulic Conductivity Tests
2.4. Determination of Bound Water Content
2.5. Mercury Intrusion Porosimetry
3. Results and Discussion
3.1. Effect of the Inorganic Contaminant on the Hydraulic Conductivity
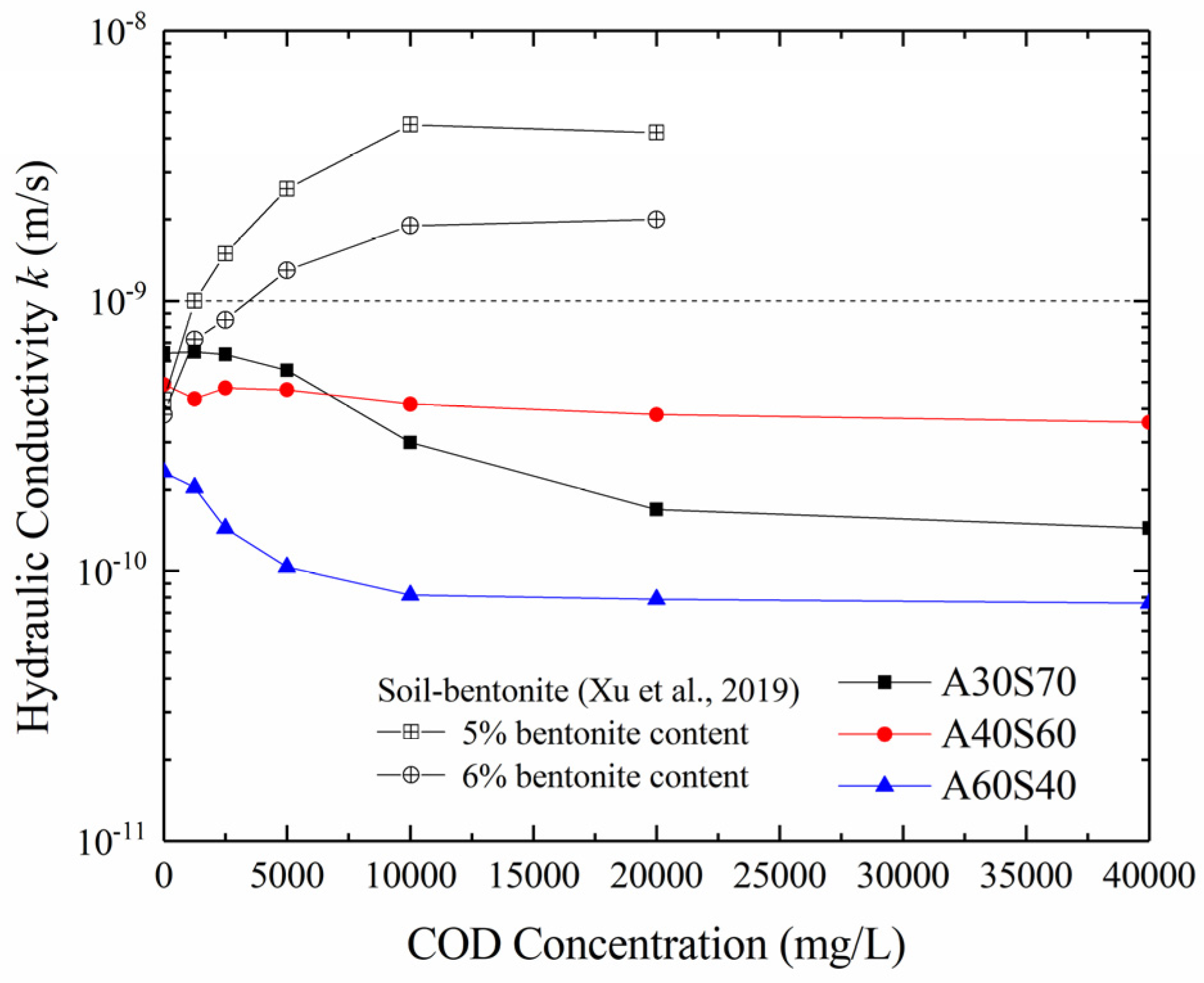
3.2. Effect of the Organic Contaminant on the Hydraulic Conductivity
3.3. Change in Pore Structure with the Contaminant Concentration
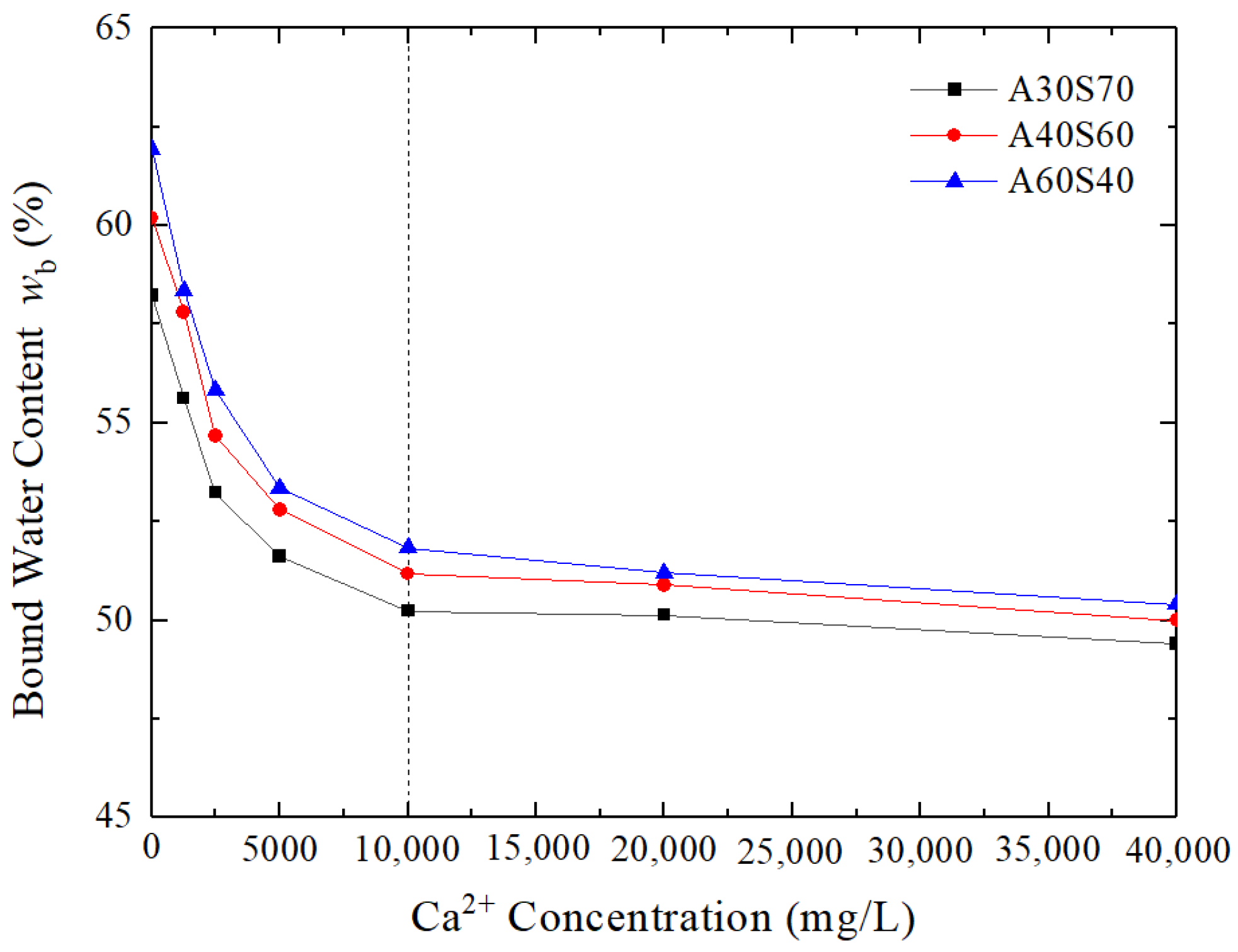
3.4. Change of Bound Water Content with Contaminant Concentration
4. Conclusions
- (1)
- As the Ca2+ concentration increased, the hydraulic conductivity of the sand–attapulgite cut-off wall first decreased, then increased, and then stabilized. Whether increasing or decreasing, it varied within a single order of magnitude. The results show that the sand–attapulgite cut-off wall has better chemical compatibility with inorganic contaminants than the soil–bentonite cut-off wall (the hydraulic conductivity increased by one to four orders of magnitude as the Ca2+ concentration increased). When the Ca2+ concentration exceeded 10,000 mg/L, the hydraulic conductivity of the sand–attapulgite cut-off wall was greater than 1.0 × 10−9 m/s.
- (2)
- The hydraulic conductivity of the sand–attapulgite cut-off wall with a COD concentration was divided into a decreasing stage (0 mg/L–10,000 mg/L) and a stable stage (10,000 mg/L–40,000 mg/L). The hydraulic conductivity of the sand–attapulgite cut-off wall was less than 1.0 × 10−9 m/s in both stages, and the range of variation as COD varied was within one order of magnitude, which indicates that the sand–attapulgite cut-off wall had good chemical compatibility with the COD solution.
- (3)
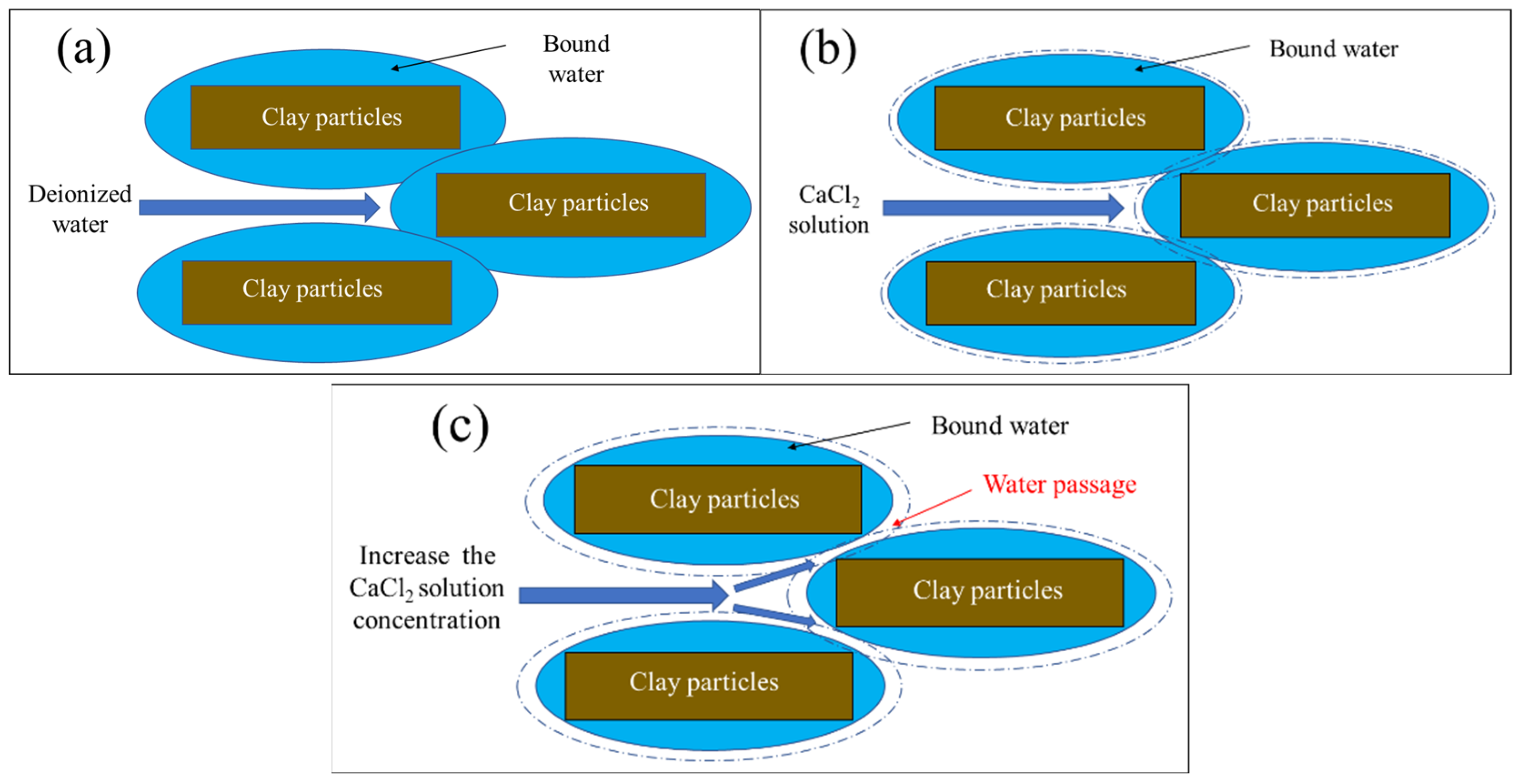
- The increase in the hydraulic conductivity of the sand–attapulgite cut-off wall due to the CaCl2 permeate can be explained in terms of bound water content and pore structure. The increase in Ca2+ concentration decreased the bound water content of the wall, and the CaCl2 solution increased the proportion of macropores and mesopores and decreased the proportion of small pores in the sand–attapulgite cut-off wall.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, L.J.; Gu, M.; Peng, Z. Study on the optimized mode of waste governance with sustainable urban development-case from China’s urban waste classified collection. Sustainability 2020, 12, 3706. [Google Scholar] [CrossRef]
- Solid Waste Treatment and Utilization Industry 2019 Development Report. China Environmental Protection Industry Association. China Environmental Protection Industry Development Report (2020); China Meteorological Press: Beijing, China, 2020; pp. 149–160.
- Xu, H.Q.; Zhu, W.; Qian, X.D.; Wang, S.W.; Fan, X.H. Studies on hydraulic conductivity and compressibility of backfills for soil-bentonite cutoff walls. Appl. Clay Sci. 2016, 132–133, 326–335. [Google Scholar] [CrossRef]
- Lee, T.; Benson, C.H. Flow past bench-scale vertical ground-water cutoff walls. J. Geotech. Geoenviron. 2000, 126, 511–520. [Google Scholar] [CrossRef]
- Xu, H.Q.; Shu, S.; Wang, S.W.; Zhou, A.Z.; Jiang, P.M.; Zhu, W.; Fan, X.H.; Chen, L. Studies on the chemical compatibility of soil-bentonite cut-off walls for landfills. J. Environ. Manag. 2019, 237, 155–162. [Google Scholar] [CrossRef] [PubMed]
- D’Appolonia, D.J. Soil-bentonite slurry trench cutoffs. J. Geotech. Geoenviron. 1980, 106, 399–417. [Google Scholar] [CrossRef]
- Evans, J.C.; Costa, M.J.; Cooley, B. The state-of-stress in soil-bentonite slurry trench cutoff walls. In Geoenvironment 2000: Characterization, Containment, Remediation, and Performance in Environmental Geotechnics; Acar, Y.N., Daniel, D.E., Eds.; American Society of Civil Engineers: Reston, VA, USA, 1995; pp. 1173–1191. [Google Scholar]
- Sharma, H.D.; Reddy, K.R. Geoenvironmental Engineering: Site Remediation, Waste Containment, and Emerging Waste Management Technologies; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Yeo, S.S.; Shackelford, C.D.; Evans, J.C. Consolidation and hydraulic conductivity of nine model soil-bentonite backfills. J. Geotech. Geoenviron. 2005, 131, 1189–1198. [Google Scholar] [CrossRef]
- Malusis, M.A.; McKeehan, M.D. Chemical compatibility of model soil-bentonite backfill containing multiswellable bentonite. J. Geotech. Geoenviron. 2013, 139, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.J.; Fan, R.D.; Reddy, K.R.; Liu, S.Y.; Yang, Y.L. Impacts of presence of lead contamination in clayey soil-calcium bentonite cutoff wall backfills. Appl. Clay Sci. 2015, 108, 111–122. [Google Scholar] [CrossRef]
- Lo, I.M.C.; Yang, X.Y. Use of organoclay as secondary containment for gasoline storage tanks. J. Environ. Eng. 2001, 127, 154–161. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ohtsubo, M.; Li, L.Y.; Higashi, T.; Park, J. Effect of salt of various concentrations on liquid limit, and hydraulic conductivity of different soil-bentonite mixtures. Environ. Geol. 2009, 57, 1145–1153. [Google Scholar] [CrossRef]
- Yong, R.N.; Ouhadi, V.R.; Goodarzi, A.R. Effect of Cu2+ ions and buffering capacity on smectite microstructure and performance. J. Geotech. Geoenviron. 2009, 135, 1981–1985. [Google Scholar] [CrossRef]
- Fu, X.L.; Shen, S.Q.; Reddy, K.R.; Yang, Y.L.; Du, Y.J. Hydraulic Conductivity of Sand/Biopolymer-Amended Bentonite Backfills in Vertical Cutoff Walls Permeated with Lead Solutions. J. Geotech. Geoenviron. 2021, 148, 04021186. [Google Scholar] [CrossRef]
- Yang, Y.L.; Reddy, K.R.; Zhan, H.B.; Fan, R.D.; Liu, S.Y.; Xue, Q.; Du, Y.J. Hydraulic conductivity of soil-bentonite backfill comprised of SHMP-amended Ca-bentonite to Cr(VI)-impacted groundwate. J. Contam. Hydrol. 2021, 242, 103856. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Z.; Li, J.G.; Tang, H.M.; Zhan, C.; Zhao, Y.S. Cutoff Phenol Polluted Groundwater with Activated Carbon Modified Soil-Bentonite Slurry Wall. J. Jilin Univ. Earth Sci. Ed. 2023, 53, 251–260. (In Chinese) [Google Scholar] [CrossRef]
- Ye, H.P.; Chen, F.Z.; Sheng, Y.Q.; Sheng, G.Y.; Fu, J.M. Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep. Purif. Technol. 2006, 50, 283–290. [Google Scholar] [CrossRef]
- Rusmin, R.; Sarkar, B.; Biswas, B.; Churchman, J.; Liu, Y.J.; Naidu, R. Structural, electrokinetic and surface properties of activated palygorskite for environmental application. Appl. Clay Sci. 2016, 134, 95–102. [Google Scholar] [CrossRef]
- Stern, R.T.; Shackelford, C.D. Permeation of sand-processed clay mixtures with calcium chloride solutions. J. Geotech. Geoenviron. 1998, 124, 231–241. [Google Scholar] [CrossRef]
- Day, S.R. The compatibility of slurry cutoff wall materials with contaminated groundwater. In Hydraulic Conductivity and Waste Contaminant Transport in Soils, ASTM STP 1142; Daniel, D.E., Trautwein, S.J., Eds.; American Society for Testing and Materials: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Broderick, G.P.; Daniel, D.E. Stabilizing compacted clay against chemical attack. J. Geotech. Eng. ASCE 1990, 116, 1549–1567. [Google Scholar] [CrossRef]
- Al-Rawas, A.A.; Mohamedzein, E.A.; Al-Shabibi, A.S.; Al-Katheiri, S. Sand-attapulgite clay mixtures as a landfill liner. Geotech. Geol. Eng. 2006, 24, 1365–1383. [Google Scholar] [CrossRef]
- Silva, G.; Almanza, R. Use of clays as liners in solar ponds. Sol. Energy 2009, 83, 905–919. [Google Scholar] [CrossRef]
- Tobin, W.R.; Wild, P.R. Attapulgite: A clay liner solution? Ovil Eng. ASCE N. Y. 1986, 56, 56–58. [Google Scholar]
- Zhang, W.B.; Rao, W.B.; Li, L.; Brusseau, M.L.; Liu, Y. Adsorption of cadmium onto sand-attapulgite cutoff wall backfill media. Water Air Soil Pollut. 2021, 232, 47. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Matlan, S.J.; Wang, H.; Pishro, A.A.; Zhang, L.L.; Gao, X.; Liang, Z.; Liu, X.Y.; Zhao, P.G. Geotechnical evaluation of loess modifications as the sustainable compacted soil liner material in solid waste landfill. Materials 2022, 15, 4982. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.R. Soil-bentonite cutoff walls. In Geotechnical Practice for Waste Disposal’87; Woods, R.D., Ed.; American Society of Civil Engineers: New York, NY, USA, 1987; pp. 182–204. [Google Scholar]
- Zhu, W.; Xu, H.Q.; Wang, S.W.; Fan, X.H. Hydraulic conductivity of model clay-based cut-off wall backfills. In Proceedings of the 7th International Congress on Environmental Geotechnics, Melbourne, Australia, 10–14 November 2014; pp. 1516–1523. [Google Scholar]
- Zhu, W.; Xu, H.Q.; Wang, S.W.; Fan, X.H. Influence of CaCl2 solution on the permeability of different clay-based cutoff walls. Rock Soil Mech. 2016, 37, 1224–1230. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.B.; Rao, W.B.; Li, L.; Liu, Y.; Wang, S.; Jin, K.; Zheng, F.W. Compressibility and hydraulic conductivity of sand-attapulgite cut-off wall backfills. J. Zhejiang Univ.-Sci. A 2019, 20, 218–228. [Google Scholar] [CrossRef]
- Shackelford, C.D.; Sample-Lord, K.M. Hydraulic conductivity and compatibility of bentonite for hydraulic containment barriers. Soil Behav. Fundam. Innov. Geotech. Eng. Honor. Roy E. Olson 2014, 370–387. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Control. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- ASTM D4318-10; ASTM (American Society for Testing and Materials) Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: Philadelphia, PA, USA, 2010.
- Jo, H.Y.; Katsumi, T.; Benson, C.H.; Edil, T.B. Hydraulic conductivity and swelling of nonprehydrated GCLs permeated with single-species salt solutions. J. Geotech. Geoenviron. 2001, 127, 557–567. [Google Scholar] [CrossRef]
- Katsumi, T.; Kamon, M.; Inui, T.; Araki, S. Hydraulic barrier performance of SBM cut-off wall constructed by the trench cutting and re-mixing deep wall method. GeoCongress 2008, 628–635. [Google Scholar] [CrossRef]
- Bohnhoff, G.L.; Shackelford, C.D. Hydraulic conductivity of polymerized bentonite-amended backfills. J. Geotech. Geoenviron. 2014, 140, 04013028. [Google Scholar] [CrossRef]
- Shear, D.L.; Olsen, H.W.; Nelson, K.R. Effects of Desiccation on the Hydraulic Conductivity Versus Void Ratio Relationship for a Natural Clay; National Academy Press: Washington, DC, USA, 1993; pp. 1365–1370. [Google Scholar]
- Zhang, P.; Fang, Y.G.; Yan, X.Q.; He, Z.W. Study of different dry methods for drying remolded bentonite sample with mercury intrusion test. Rock Soil Mech. 2011, 32, 388–391. (In Chinese) [Google Scholar] [CrossRef]
- Qu, Z.K.; Wang, H.J.; Xun, W.F.; Liu, F.S.; Bian, H.B. Study on permeability characteristics of modified soil based on mercury intrusion. S-N Water Transf. Water Sci. Technol. 2017, 15, 172–178. (In Chinese) [Google Scholar] [CrossRef]
- Yanful, E.K.; Shikatani, K.S.; Quirt, D.H. Hydraulic conductivity of natural soils permeated with acid mine drainage. Can. Geotech. J. 1995, 32, 624–646. [Google Scholar] [CrossRef]
- Gouy, G. Constitution of the electric charge at the surface of an electrolyte. J. Phys. Radium 1910, 9, 457–467. [Google Scholar]
- Chapman, D.L. A contribution to the theory of electrocapillanty. Philos. Mag. 1913, 25, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.K.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley and Sons: New York, NY, USA, 2005. [Google Scholar]



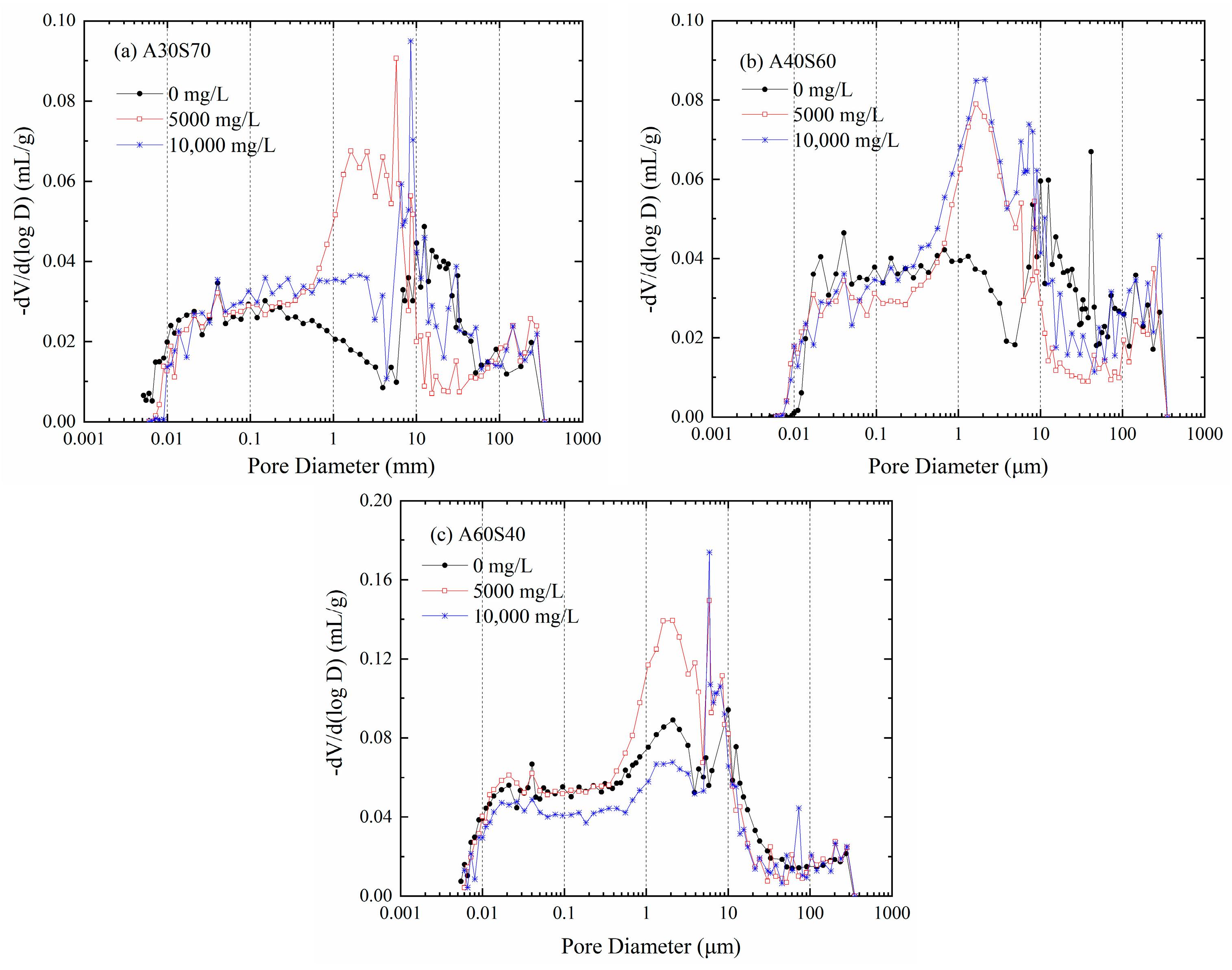
| Ca2+ Concentration (mg/L) | Pore Size Distribution (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A30S70 | A40S60 | A60S40 | |||||||
| 0 | 5000 | 10,000 | 0 | 5000 | 10,000 | 0 | 5000 | 10,000 | |
| Macropores (d > 30 µm) | 16.41 | 12.21 | 16.03 | 18.96 | 13.09 | 14.96 | 7.75 | 6.48 | 9.44 |
| Mesopores (5 µm < d ≤ 30 µm) | 22.56 | 14.29 | 20.02 | 17.65 | 10.73 | 19.49 | 18.10 | 15.14 | 22.66 |
| Small pores (25 µm ≤ d ≤ 5 µm) | 23.27 | 46.14 | 32.76 | 31.71 | 48.39 | 41.81 | 38.71 | 47.87 | 34.92 |
| Micropores (0.05 µm ≤ d ≤ 0.25 µm) | 18.52 | 15.03 | 18.25 | 18.16 | 14.64 | 13.52 | 17.28 | 14.69 | 15.64 |
| Extremely microporous (d < 0.05 µm) | 19.24 | 12.32 | 12.94 | 13.52 | 13.14 | 10.21 | 18.16 | 15.82 | 17.33 |
| Small pores + micropores + extremely microporous (d ≤ 5 µm) | 61.03 | 73.49 | 63.95 | 63.39 | 76.17 | 65.54 | 74.15 | 78.38 | 67.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Rao, W.; Ye, H.; Lv, Y.; Zhou, L.; Xiong, X. The Chemical Compatibility of Sand–Attapulgite Cut-Off Walls for Landfills. Water 2023, 15, 2940. https://doi.org/10.3390/w15162940
Zhang W, Rao W, Ye H, Lv Y, Zhou L, Xiong X. The Chemical Compatibility of Sand–Attapulgite Cut-Off Walls for Landfills. Water. 2023; 15(16):2940. https://doi.org/10.3390/w15162940
Chicago/Turabian StyleZhang, Wenbing, Wenbo Rao, Hong Ye, Yiyan Lv, Lipei Zhou, and Xin Xiong. 2023. "The Chemical Compatibility of Sand–Attapulgite Cut-Off Walls for Landfills" Water 15, no. 16: 2940. https://doi.org/10.3390/w15162940






