Pontastacus leptodactylus (Eschscholtz, 1823) and Faxonius limosus (Rafinesque, 1817) as New, Alternative Sources of Chitin and Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Biomass
2.1.2. Reagents
2.2. Chitin and Chitosan Isolation
2.2.1. Standard Chitin Extraction
Demineralization
Deproteinization
2.2.2. Standard Chitosan Extraction
Deacetylation
2.2.3. NADES Chitin Extraction
- The material was kept with NADES at 100 °C under mechanical stirring for 2 h. After filtration under vacuum, the obtained material was washed seven times with 25 mL of distilled water in a centrifuge at 4000 rpm (7 times for 5 min) until the neutral pH was obtained and dried in the oven at 80 °C for 48 h. Then, the extracted material was additionally treated with 20 mL of H2O2 at 10% for 2 h at 80 °C, washed with 25 mL of distilled water in a centrifuge at 4000 rpm (7 times for 5 min) until the neutral pH was obtained, and dried in the oven at 80 °C for 48 h.
- The material was pre-treated with 10 mL of C6H8O7 (10%, w/v) for 1 h at room temperature and then treated following the point above.
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Thermogravimetric Analysis (TG)
2.5. Scanning Electron Microscopy (SEM)
2.6. Molar Mass Distribution
2.7. Antimicrobial Assays
2.7.1. Chitosan Salification
2.7.2. Bacteria Culture
2.7.3. Evaluation of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.8. Cytotoxicity Assay (MTT)
2.8.1. Cell Culture
2.8.2. Assessment of metabolic activity
2.9. Statistical Analysis
3. Results
3.1. Chitin and Chitosan Recovery
3.1.1. Chitin and Chitosan Obtained Using Standard Chemical Procedures
3.1.2. Preliminary Extraction with NADES Mixture
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
Chitin and Chitosan Obtained Using Standard Extraction Characterisation
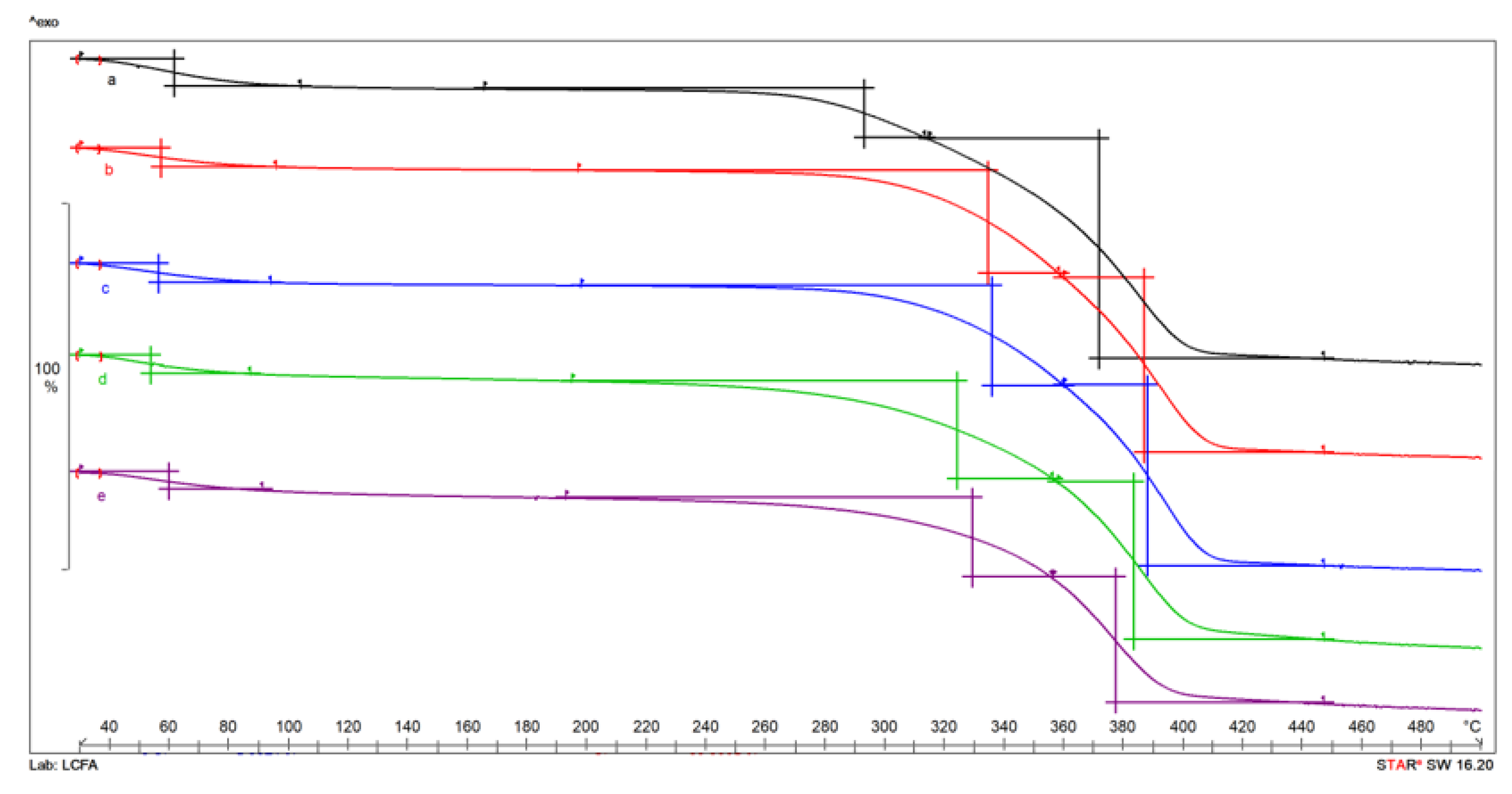
3.3. Thermogravimetric Analysis (TG) of Chitin and Chitosan Obtained Using Standard Extraction Characterisation
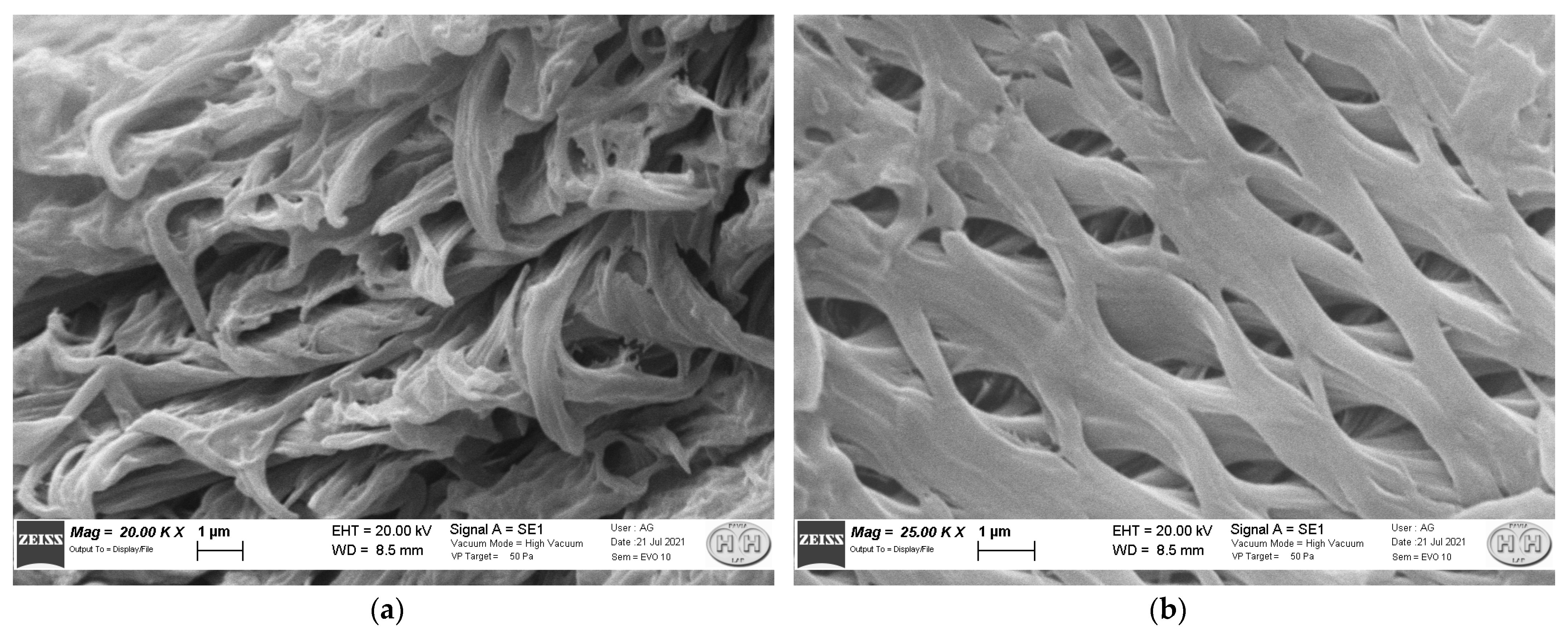
3.4. Scanning Electron Microscopy (SEM)
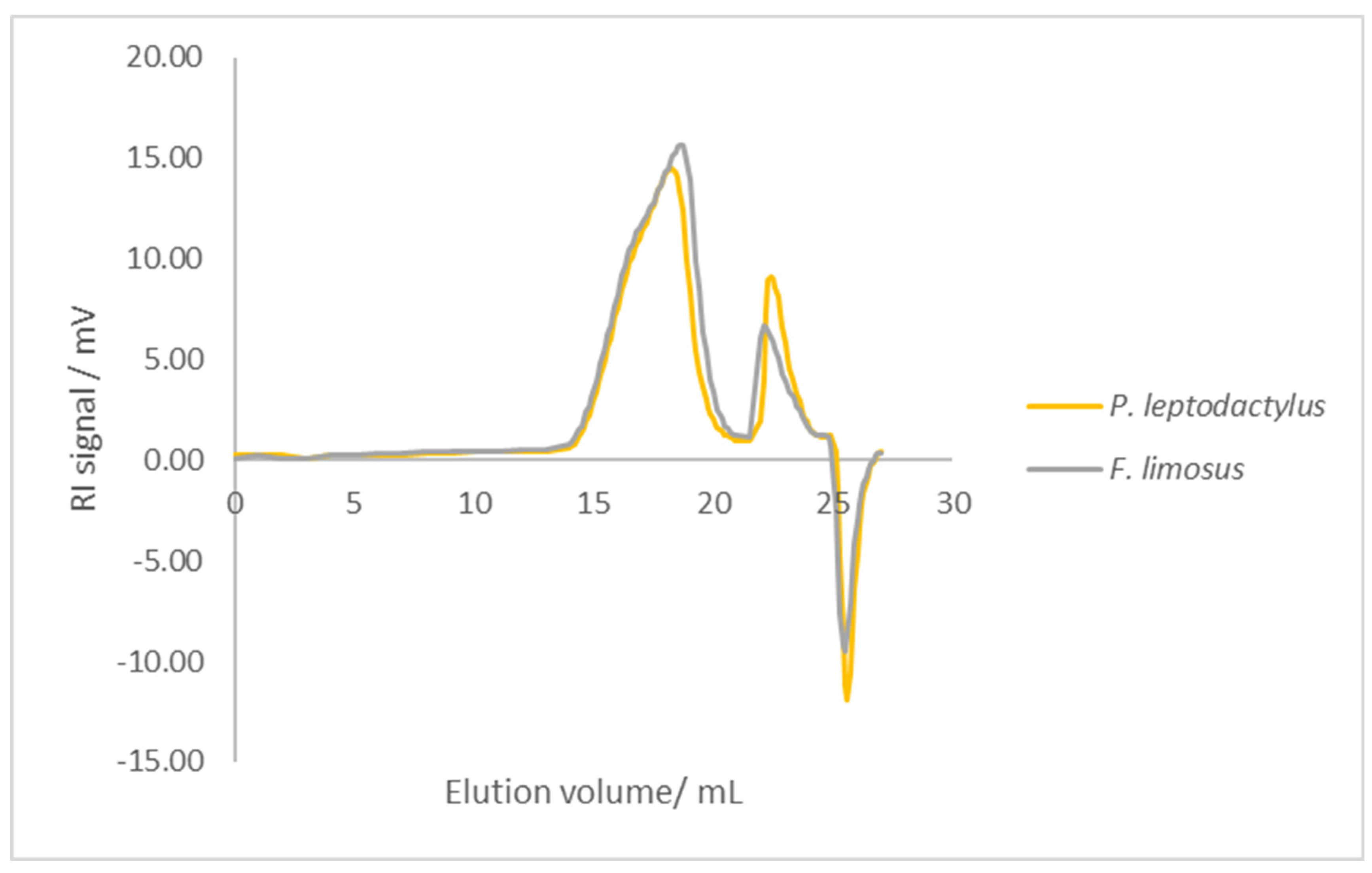
3.5. Molecular Weight (MW)
3.6. Antimicrobial Activity
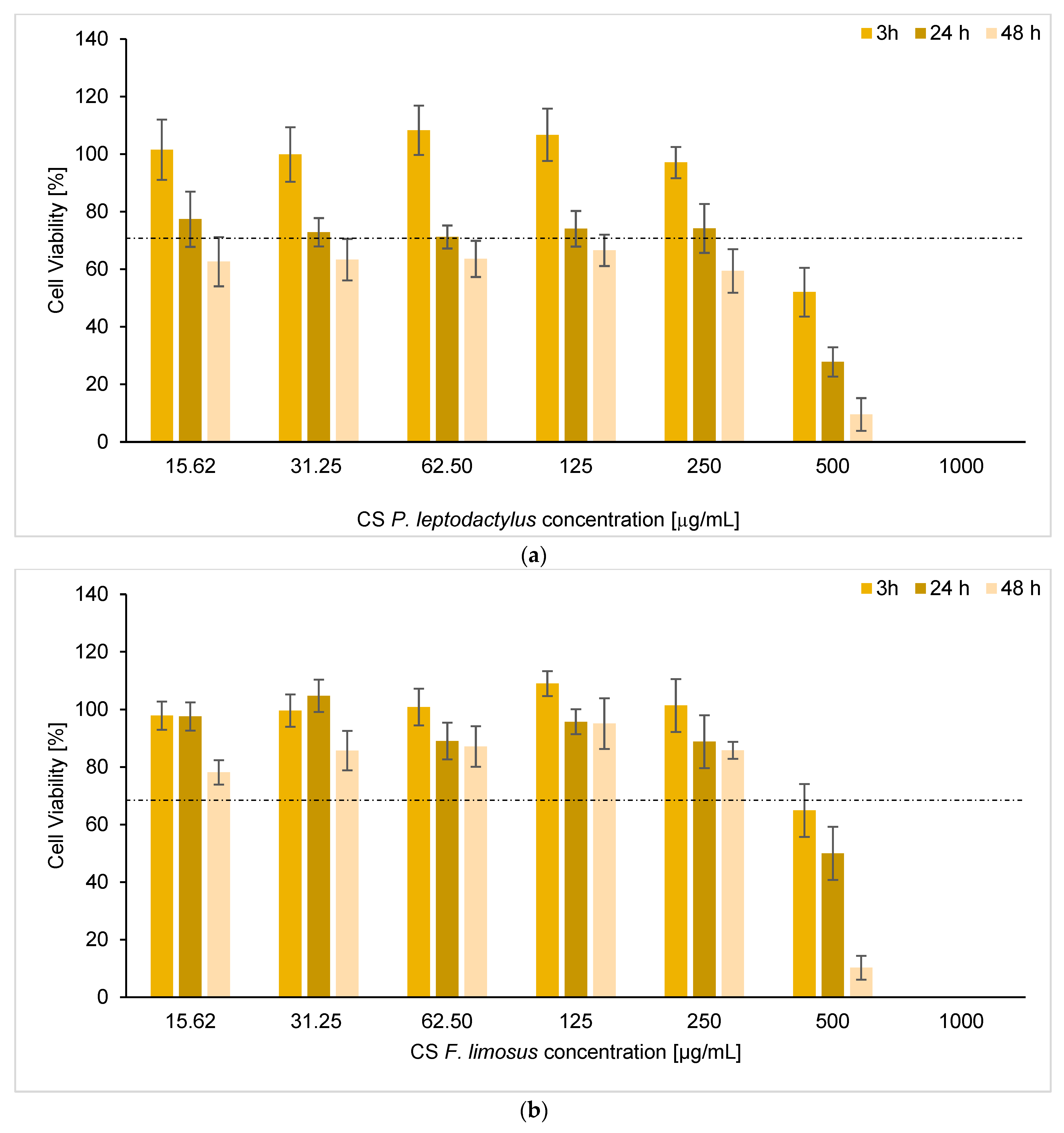
3.7. Determination of Cytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and chitosan: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar]
- Oyatogun, G.M.; Esan, T.A.; Akpan, E.I.; Adeosun, S.O.; Popoola, A.P.I.; Imasogie, B.I.; Akinluwade, K.J. Chitin, chitosan, marine to market. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–376. [Google Scholar]
- Atlas of Crayfish in Europe; Patrimoines Naturrels; Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. (Eds.) Muséum National d’Histoire Naturelle: Paris, France, 2006; Volume 64, p. 187. [Google Scholar]
- Kouba, A.; Petrusek, A.; Kozak, P. Continental-wide distribution of crayfish species in Europe. Update and maps. Knowl. Manag. Aquat. Ecosyst. 2014, 413, 5. [Google Scholar] [CrossRef]
- Szaniawska, A.; Dobrzycka-Krahel, A.; Jaszczołt, J. Spiny-cheek crayfish Orconectes limosus (Rafinesque, 1817) on its way to the open coastal waters of the Baltic Sea. Oceanol. Hydrobiol. Stud. 2017, 46, 451–463. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Fidalgo, M.L. Euryhalinity and geographical origin aid global crayfish invasion. Water 2023, 15, 569. [Google Scholar] [CrossRef]
- Hessen, D.O.; Skurdal, J. Food consumption, turnover rates and assimilation in the noble crayfish (Astacus astacus). Freshw. Crayfish 1987, 7, 309–317. [Google Scholar]
- D’Agaro, E.; Renai, B.; Gherardi, F. Evaluation of the American waterweed (Elodea canadensis Michx.) as supplemental food for the noble crayfish, Astacus astacus. BFPP-Bull. Fr. Peche Piscic. 2004, 372–373, 439–445. [Google Scholar] [CrossRef]
- Vojkovská, R.; Horká, I.; Ďuriš, Z. The diet of the spiny-cheek crayfish Orconectes limosus in the Czech Republic. Cent. Eur. J. Biol. 2014, 9, 58–69. [Google Scholar] [CrossRef]
- Harlioğlu, M.M. The present situation of freshwater crayfish, Astacus leptodactylus (Eschscholtz, 1823) in Turkey. Aquaculture 2004, 230, 181–187. [Google Scholar] [CrossRef]
- Crandall, K.A.; De Grave, S. An updated classification of the freshwater crayfishes (Decapoda: Astacidea) of the world, with a complete species list. J. Crust. Biol. 2017, 37, 615–653. [Google Scholar] [CrossRef]
- Cai, J.; Galli, G. Top 10 Species Groups in Global Aquaculture 2018; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Özturan, S.; Gülgün, F.; Ünal Şengör, G.G.U. Effects of cooking methods on the quality and safety of crayfish (Astacus leptodactylus Eschscholtz, 1823) during chilled storage. J. Food Process. Preserv. 2022, 46, e16887. [Google Scholar] [CrossRef]
- CABI. 2023 CABI Compendium. Available online: https://www.cabi.org (accessed on 30 March 2023).
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Richardson, D.M. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Śmietana, N.; Panicz, R.; Sobczak, M.; Śmietana, P.; Nędzarek, A. Spiny-cheek crayfish, Faxonius limosus (Rafinesque, 1817), as an alternative food source. Animals 2020, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and their Isolation. In Chitin and Chitosan: Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- El-Naggar, M.M.; Abou-Elmagd, W.S.; Suloma, A.; El-Shabaka, H.A.; Khalil, M.T.; Abd El-Rahman, F.A. Optimization and physicochemical characterization of chitosan and chitosan nanoparticles extracted from the crayfish Procambarus clarkii wastes. J. Shellfish. Res. 2019, 38, 385–395. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents–solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Mišan, A.; Nađpal, J.; Stupar, A.; Pojić, M.; Mandić, A.; Verpoorte, R.; Choi, Y.H. The perspectives of natural deep eutectic solvents in agri-food sector. Crit. Rev. Food Sci. Nutr. 2020, 60, 2564–2592. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, M.; Vatanpour, V.; Castro-Muñoz, R.; Boczkaj, G. Chitin and derivative chitosan-based structures—Preparation strategies aided by deep eutectic solvents: A review. Carbohydr. Polym. 2022, 275, 118702. [Google Scholar] [CrossRef] [PubMed]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ehrlich, H. 3D chitin scaffolds of marine demosponge origin for biomimetic mollusk hemolymph-associated biomineralization ex-vivo. Mar. Drugs 2020, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, H.; Li, S.; Huang, L.; Zhang, R.; Zhang, L.; Duan, B. Polyphenol-driving assembly for constructing chitin-polyphenol-metal hydrogel as wound dressing. Carbohydr. Polym. 2022, 290, 119444. [Google Scholar] [CrossRef]
- Lv, S.H. High-performance superplasticizer based on chitosan. In Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials; Woodhead Publishing: Sawston, UK, 2016; pp. 131–150. [Google Scholar] [CrossRef]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Wedmore, I.; McManus, J.G.; Pusateri, A.E.; Holcomb, J.B. A special report on the chitosan-based hemostatic dressing: Experience in current combat operations. J. Trauma Acute Care Surg. 2006, 60, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Dionísio, M.; Remunan Lopez, C.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Rodriguez-Veiga, I.; Acosta, N.; Aranaz, I.; Dobrzycka-Krahel, A. Exploring Saduria entomon (Crustacea Isopoda) as a New Source for Chitin and Chitosan Isolation. Int. J. Mol. Sci. 2022, 23, 16125. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.L.; Craig, W.A.; Nadle, H.; Reller, L.B.; Sanders, C.C.; Swenson, J.M. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19, p. 18. [Google Scholar]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard M7-A6, 6th ed.; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity 2009. International Organization for Standardization: Geneva, Switzerland, 2009.
- Ferrero, F.; Periolatto, M. Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.M.; Kuhlmann, F.M. Enterotoxigenic Escherichia coli infections. Curr. Infect. Dis. Rep. 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Ford, C.A.; Hurford, I.M.; Cassat, J.E. Antivirulence strategies for the treatment of Staphylococcus aureus infections: A mini review. Front. Microbiol. 2021, 11, 632706. [Google Scholar] [CrossRef]
- Meyers, S.P.; Chen, H.M.; No, H.K.; Lee, K.S. An integrated approach to recovery and utilization of Louisiana crawfish processing wastes. In Making Profits out of Seafood Wastes; Keller, S., Ed.; University of Alaska Sea Grant: Anchorage, AK, USA, 1990; pp. 161–171. [Google Scholar]
- Kaya, M.; Baran, T.; Karaarslan, M. A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat. Prod. Res. 2015, 29, 1477–1480. [Google Scholar] [CrossRef]
- Ghannam, H.E.; Talab, A.S.; Dolgano, N.V.; Husse, A.; Abdelmagui, N.M. Characterization of chitosan extracted from different crustacean shell wastes. J. Appl. Sci. 2016, 16, 454–461. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Küçükgülmez, A. Extraction of chitin from crayfish (Astacus leptodactylus) shell waste. Alinteri J. Agric. Sci. 2018, 33, 99–104. [Google Scholar] [CrossRef]
- Gadgey, K.K.; Bahekar, A. Studies On Extraction Methods of Chitin From CRAB Shell and Investigation of Its Mechanical Properties. Int. J. Mech. Eng. Technol. 2017, 8, 220–231. [Google Scholar]
- Pan, S.Y.; Fan, C.; Lin, Y.P. Development and deployment of green technologies for sustainable environment. Environments 2019, 6, 114. [Google Scholar] [CrossRef]
- Huang, W.C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, W.C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.C.; Liu, C.; Liu, X.; Lu, Y.; Zhou, G.; Mei, C. Microwave-assisted deep eutectic solvent extraction of chitin from crayfish shell wastes for 3D printable inks. Ind. Crops Prod. 2023, 194, 116325. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef]
- Morgan, K.; Conway, C.; Faherty, S.; Quigley, C. A Comparative Analysis of Conventional and Deep Eutectic Solvent (DES)-Mediated Strategies for the Extraction of Chitin from Marine Crustacean Shells. Molecules 2021, 26, 7603. [Google Scholar] [CrossRef]
- Austin, P.R.; Brine, C.J.; Castle, J.E.; Zikakis, J.P. Chitin: New facets of research. Science 1981, 212, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods—A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kaya, M.; Baran, T.; Mentes, A. Extraction and Characterization of α-Chitin and Chitosan from Six Different Aquatic Invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.V.; Forte, T.C.M.; Tavares, S.J.S.; Andrade, F.K.; Vieira, R.S.; Lima, V. The effects of the molecular weight of chitosan on the tissue inflammatory response. J. Biomed. Mater. Res. Part A 2021, 109, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Whu, S.W.; Tsai, C.L.; Wu, Y.H.; Chen, H.W.; Hsieh, K.H. Chitosan as scaffold materials: Effects of molecular weight and degree of deacetylation. J. Polym. Res. 2004, 11, 141–147. [Google Scholar] [CrossRef]
- Yang, H.C.; Hon, M.H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009, 92, 87–91. [Google Scholar] [CrossRef]
- Shrivastava, A. Polymerization. In Introduction to Plastics Engineering; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 17–48. ISBN 9780323395007. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Erman, A.; Hergouth, V.K.; Blango, M.G.; Kos, M.K.; Mulvey, M.A.; Veranič, P. Repeated treatments with chitosan in combination with antibiotics completely eradicate uropathogenic Escherichia coli from infected mouse urinary bladders. J. Infect. Dis. 2017, 216, 375–381. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Palacios, J.L.; Barahona, T.; Mosi-Roa, Y.; Anguita-Barrales, F.; Bustamante, M. Synthesis of New Chitosan from an Endemic Chilean Crayfish Exoskeleton (Parastacus pugnax): Physicochemical and Biological Properties. Polymers 2021, 13, 2304. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Fan, Y.T.; Zhou, T.J.; Gong, J.H.; Cui, L.H.; Cho, K.-H.; Cho, C.S. Chemical Modification of Chitosan for Efficient Vaccine Delivery. Molecules 2018, 23, 229. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Chalitangkoon, J.; Wongkittisin, M.; Monvisade, P. Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol. 2020, 159, 194–203. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and cytotoxicity of chitosan molecules and nanoparticles: Effects of molecular weight and degree of deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef]
- Franca, R.; Mbeh, D.A.; Samani, T.D.; Le Tien, C.; Mateescu, M.A.; Yahia, L.H.; Sacher, E. The effect of ethylene oxide sterilization on the surface chemistry and in vitro cytotoxicity of several kinds of chitosan. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1444–1455. [Google Scholar] [CrossRef]
- Tan, G.; Kaya, M.; Tevlek, A.; Sargin, I.; Baran, T. Antitumor activity of chitosan from mayfly with comparison to commercially available low, medium and high molecular weight chitosans. Vitr. Cell. Dev. Biol.-Anim. 2018, 54, 366–374. [Google Scholar] [CrossRef]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Macleod, G.S.; Fell, J.T.; Collett, J.H.; Sharma, H.L.; Smith, A.M. Selective drug delivery to the colon using pectin: Chitosan: Hydroxypropyl methylcellulose film coated tablets. Int. J. Pharm. 1999, 187, 251–257. [Google Scholar] [CrossRef]




| Species | Treatment | Chitin Recovery [%] | Chitosan Recovery from Chitin [%] | Chitosan Recovery from Shells [%] |
|---|---|---|---|---|
| P. leptodactylus | standard extraction | 22 ± 2.7 a | 70 ± 13.0 b | 15 ± 0.3 b |
| P. leptodactylus | NADES | 24 c | N/D | N/D |
| P. leptodactylus | NADES with citric acid pretreatment | 29 c | N/D | N/D |
| F. limosus | standard extraction | 20 ± 3.4 d | 76 ± 9.0 b | 14 ± 4.2 b |
| F. limosus | NADES | 31 c | N/D | N/D |
| F. limosus | NADES with citric acid pretreatment | 25 c | N/D | N/D |
| Functional Group | Commercial Chitin Wavenumber [cm−1] | P. leptodactylus Wavenumber [cm−1] | F. limosus Wavenumber [cm−1] | P. leptodactylus (NADES) Wavenumber [cm−1] | F. limosus (NADES) Wavenumber [cm−1] |
|---|---|---|---|---|---|
| Amide I | 1622 | 1633 | 1628 | 1622 | 1635 |
| Amide II | 1551 | 1553 | 1552 | 1551 | 1549 |
| -C-H | 2875 | 2879 | 2890 | 2883 | 2874 |
| -C-O-C- | 1070 | 1063 | 1067 | 1071 | 1068 |
| 1026 | 1026 | 1026 | 1011 | 1027 |
| Functional Group | Commercial Chitosan Wavenumber [cm−1] | P. leptodactylus Wavenumber [cm−1] | F. limosus Wavenumber [cm−1] |
|---|---|---|---|
| Amide I | 1647 | 1654 | 1650 |
| Amide II | 1564 | 1590 | 1590 |
| -C-H | 2872 | 2874 | 2864 |
| -C-O-C- | 1063 | 1054 | 1059 |
| 1027 | 1027 | 1026 |
| Species | MIC (E. coli) | MIC (S. aureus) | MBC (E. coli) | MBC (S. aureus) |
|---|---|---|---|---|
| P. leptodactylus | 1.19 | 1.59 | 1.58 | 2.48 |
| F. limosus | 2.48 | 1.96 | 4.90 | 4.90 |
| Chitosan s.* | 1.15 | 2.30 | >9.20 | >9.20 |
| Ampicilin | 5 × 10−3 | 0.5 × 10−3 | 10 × 10−3 | 2 × 10−3 |
| Species | IC50 (3 h) | IC50 (24 h) | IC50 (48 h) |
|---|---|---|---|
| P. leptodactylus | 0.51 | 0.24 | 0.14 |
| F. limosus | 0.52 | 0.49 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuc, Z.; Brusotti, G.; Catenacci, L.; Grenha, A.; Pontes, J.F.; Pinto da Silva, J.; Rosa da Costa, A.M.; Moro, P.; Milanese, C.; Grisoli, P.; et al. Pontastacus leptodactylus (Eschscholtz, 1823) and Faxonius limosus (Rafinesque, 1817) as New, Alternative Sources of Chitin and Chitosan. Water 2023, 15, 3024. https://doi.org/10.3390/w15173024
Nuc Z, Brusotti G, Catenacci L, Grenha A, Pontes JF, Pinto da Silva J, Rosa da Costa AM, Moro P, Milanese C, Grisoli P, et al. Pontastacus leptodactylus (Eschscholtz, 1823) and Faxonius limosus (Rafinesque, 1817) as New, Alternative Sources of Chitin and Chitosan. Water. 2023; 15(17):3024. https://doi.org/10.3390/w15173024
Chicago/Turabian StyleNuc, Zofia, Gloria Brusotti, Laura Catenacci, Ana Grenha, Jorge F. Pontes, Joana Pinto da Silva, Ana Maria Rosa da Costa, Paola Moro, Chiara Milanese, Pietro Grisoli, and et al. 2023. "Pontastacus leptodactylus (Eschscholtz, 1823) and Faxonius limosus (Rafinesque, 1817) as New, Alternative Sources of Chitin and Chitosan" Water 15, no. 17: 3024. https://doi.org/10.3390/w15173024









