Effect of Antibiotics and Thermophilic Pre-Treatment on Anaerobic Co-Digestion of Pig Manure and Corn Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Inoculum
2.2. The Physicochemical Characteristics of Organic Materials
2.3. Experiment Set-Up
2.4. Sampling and Physicochemical Analysis
3. Results and Discussions
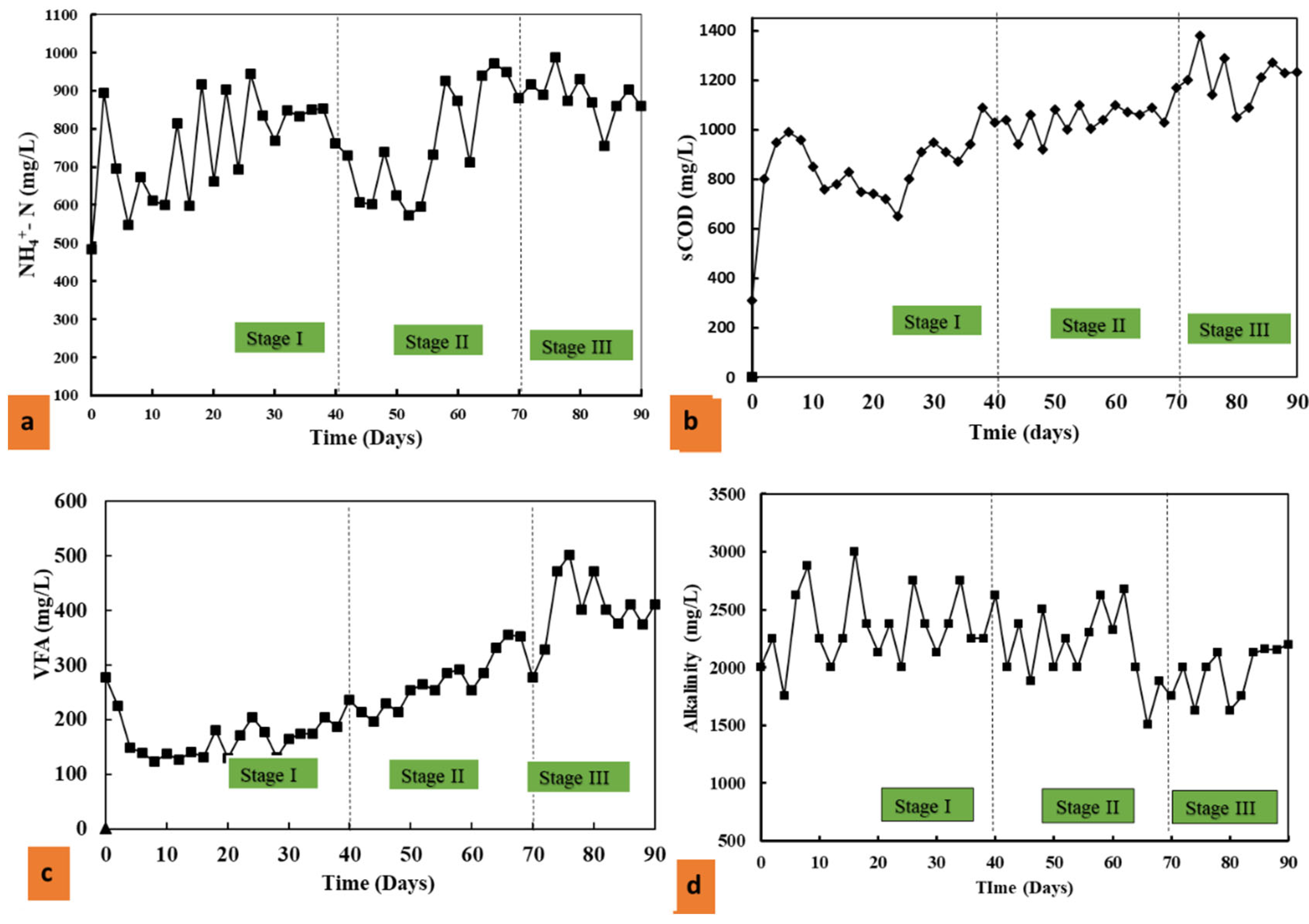
3.1. Effect of Antibiotics on Co-Digestion of Pig Manure and Corn Straw in Mesophilic AD
3.1.1. Effect on NH4+-N and sCOD on R-1
3.1.2. Effects on VFAs and Alkalinity
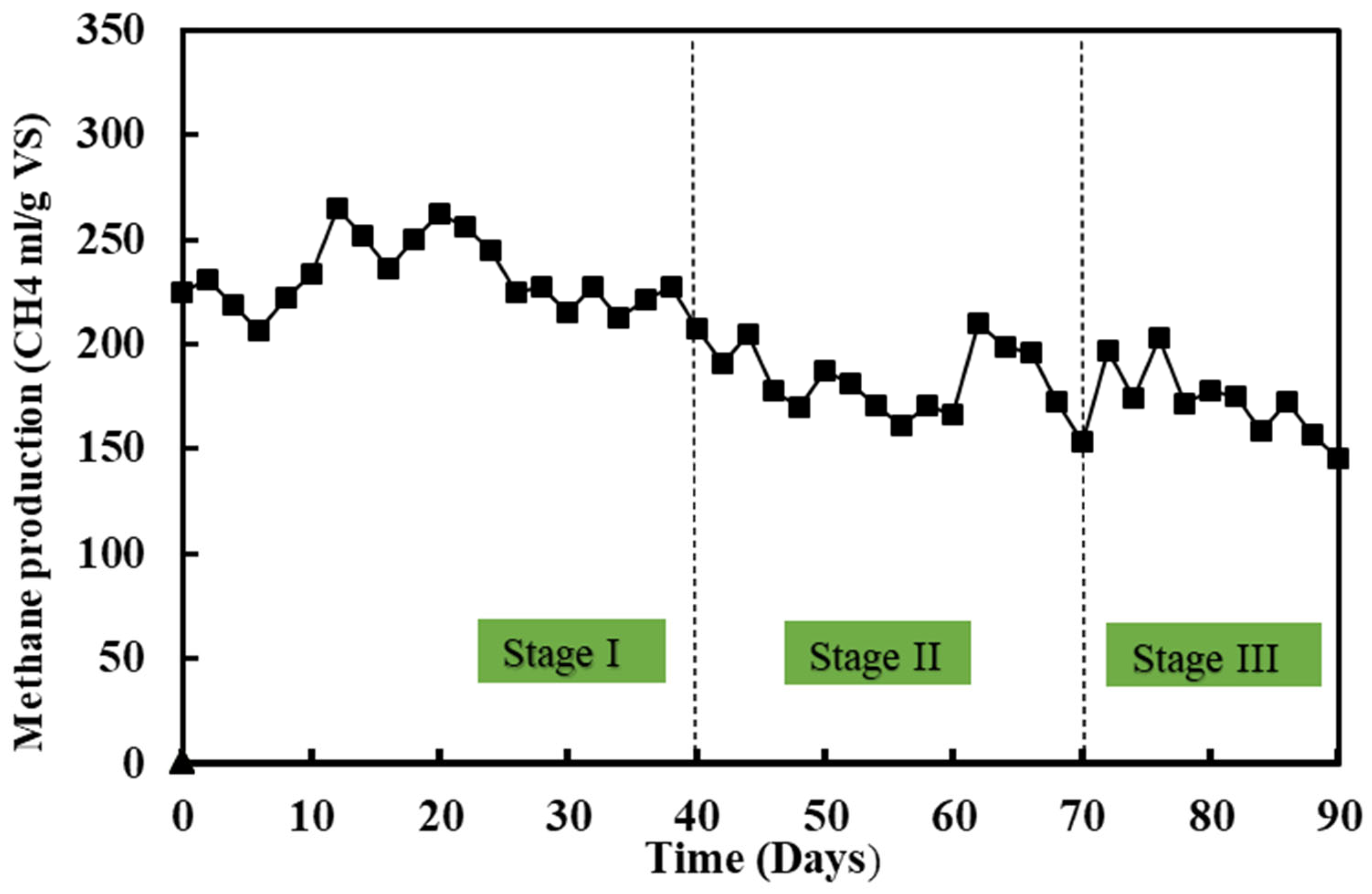
3.1.3. The Effects of Antibiotics on Methane Yield
3.1.4. Antibiotics Removal Efficiency on R-1
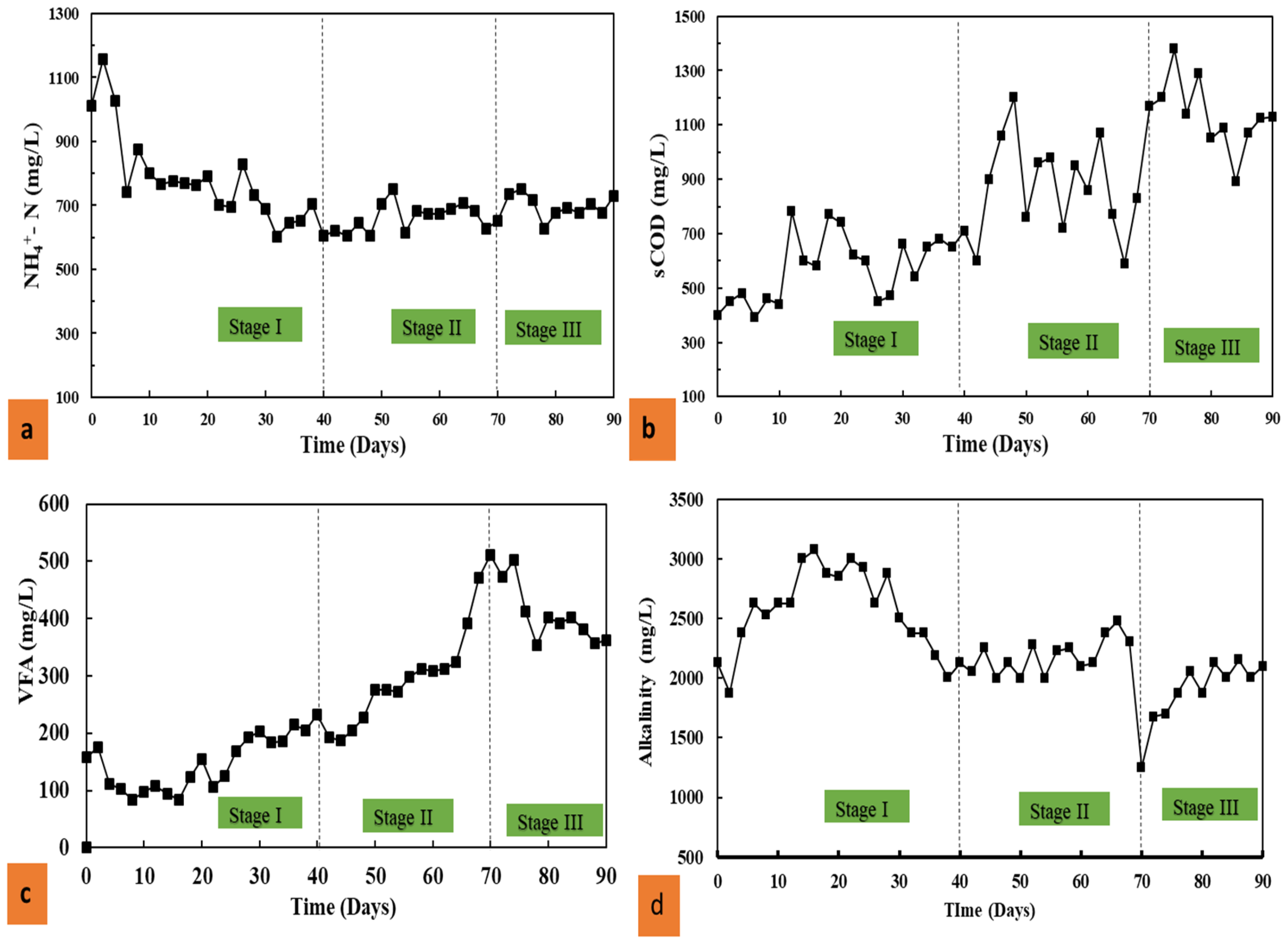
3.2. The Effects of Thermophilic Pre-Treatment on the Co-Digestion of Pig Manure and Corn Straw
3.2.1. Effects on NH4+-N and sCOD
3.2.2. Effects on VFAs and alkalinity
3.2.3. Effects on Methane Production
3.2.4. Antibiotics Removal Efficiency on R-2
4. Conclusions
- The addition of SMX and NOR had a negative effect on the digestion process;
- The CH4 production decreased by 20.48% in stage two and 37.56% in stage three;
- Thermophilic pretreatment increased the buffering capacity of the system and improved methane production by 15.57%;
- Thermal pretreatment also improved the degradation of SMX by 1.60% and 4.75% and NOR by 11.67% and 10.95% in the low and high antibiotic concentration stages;
- The presence of the antibiotics affects the CH4 production.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nan, S.H.A.N.; Hu, L.I.; LI, J.Z.; Ng, E.L.; Yan, M.A.; WANG, L.G.; Qing, C.H.E.N. A Major Pathway for Carbon and Nitrogen Losses—Gas Emissions during Storage of Solid Pig Manure in China. J. Integr. Agric. 2019, 18, 190–200. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste Biorefinery Models towards Sustainable Circular Bioeconomy: Critical Review and Future Perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Jiang, X.; Sommer, S.G.; Christensen, K.V. A Review of the Biogas Industry in China. Energy Policy 2011, 39, 6073–6081. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Chi, M.; Sun, Y.; Zhang, J.; Jiang, S.; Cui, Z. Effects of Co-Digestion of Cucumber Residues to Corn Stover and Pig Manure Ratio on Methane Production in Solid State Anaerobic Digestion. Bioresour. Technol. 2018, 250, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhuang, D.; Fu, J.; Huang, Y.; Wen, K. Bioenergy Potential from Crop Residues in China: Availability and Distribution. Renew. Sustain. Energy Rev. 2012, 16, 1377–1382. [Google Scholar] [CrossRef]
- Sara, P.; Giuliana, D.; Michele, P.; Maurizio, C.; Luca, C.; Fabrizio, A. Effect of Veterinary Antibiotics on Biogas and Bio-Methane Production. Int. Biodeterior. Biodegrad. 2013, 85, 205–209. [Google Scholar] [CrossRef]
- Van Epps, A.; Blaney, L. Antibiotic Residues in Animal Waste: Occurrence and Degradation in Conventional Agricultural Waste Management Practices. Curr. Pollut. Rep. 2016, 2, 135–155. [Google Scholar] [CrossRef]
- Kumar, K.; Gupta, C.S.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of Antibiotics from Wastewater or Animal Manure to Soil and Edible Crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef]
- Wang, J.; Lin, H.; Sun, W.; Xia, Y.; Ma, J.; Fu, J.; Zhang, Z.; Wu, H.; Qian, M. Variations in the Fate and Biological Effects of Sulfamethoxazole, Norfloxacin and Doxycycline in Different Vegetable-Soil Systems Following Manure Application. J. Hazard. Mater. 2016, 304, 49–57. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of Veterinary Antibiotics in Manures from Feedlot Livestock in Eight Provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Wohde, M.; Berkner, S.; Junker, T.; Konradi, S.; Schwarz, L.; Düring, R.A. Occurrence and Transformation of Veterinary Pharmaceuticals and Biocides in Manure: A Literature Review. Environ. Sci. Eur. 2016, 28, 23. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J.; Frear, C. The Effects of the Antibiotics Ampicillin, Florfenicol, Sulfamethazine, and Tylosin on Biogas Production and Their Degradation Efficiency during Anaerobic Digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the Anaerobic Digestion of Agricultural Resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, K.; Han, B.; Liang, J.; Zhai, Z.; Du, L. Anaerobic Co-Digestion of Pig Manure with Dried Maize Straw. BioResources 2016, 11, 8875–8889. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of Volatile Fatty Acid Concentrations on Methane Yield and Methanogenic Bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Zhang, L.; Loh, K.C.; Sarvanantharajah, S.; Tong, Y.W.; Wang, C.H.; Dai, Y. Mesophilic and Thermophilic Anaerobic Digestion of Soybean Curd Residue for Methane Production: Characterizing Bacterial and Methanogen Communities and Their Correlations with Organic Loading Rate and Operating Temperature. Bioresour. Technol. 2019, 288, 121597. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Liu, C.; Liu, X.; Wang, J.; Li, S.; Fan, G.; Zhang, L. Alkaline Pretreatment for Enhancement of Biogas Production from Banana Stem and Swine Manure by Anaerobic Codigestion. Bioresour. Technol. 2013, 149, 353–358. [Google Scholar] [CrossRef]
- Marcelino, R.B.P.; Leão, M.M.D.; Lago, R.M.; Amorim, C.C. Multistage Ozone and Biological Treatment System for Real Wastewater Containing Antibiotics. J. Environ. Manag. 2017, 195, 110–116. [Google Scholar] [CrossRef]
- Kawai, T.; Kazuhiko, I.; Takaya, N.; Yamaguchi, Y.; Kishii, R.; Kohno, Y.; Kurasaki, H. Sulfonamide-Based Non-Alkyne LpxC Inhibitors as Gram-Negative Antibacterial Agents. Bioorgan. Med. Chem. Lett. 2017, 27, 1045–1049. [Google Scholar] [CrossRef]
- Andriamanohiarisoamanana, F.J.; Saikawa, A.; Tarukawa, K.; Qi, G.; Pan, Z.; Yamashiro, T.; Iwasaki, M.; Ihara, I.; Nishida, T.; Umetsu, K. Anaerobic Co-Digestion of Dairy Manure, Meat and Bone Meal, and Crude Glycerol under Mesophilic Conditions: Synergistic Effect and Kinetic Studies. Energy Sustain. Dev. 2017, 40, 11–18. [Google Scholar] [CrossRef]
- Yang, H.; Paruch, L.; Chen, X.; van Eerde, A.; Skomedal, H.; Wang, Y.; Liu, D.; Clarke, J.L. Antibiotic Application and Resistance in Swine Production in China: Current Situation and Future Perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic Bioconversion of Food Waste into Energy: A Critical Review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of Pretreatment Strategies for Enhancing Sewage Sludge Disintegration and Subsequent Anaerobic Digestion: Current Advances, Full-Scale Application and Future Perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A Comprehensive Review on Food Waste Anaerobic Digestion: Research Updates and Tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Yeh, D.H.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Enhanced Mesophilic Anaerobic Digestion of Food Waste by Thermal Pretreatment: Substrate versus Digestate Heating. Waste Manag. 2015, 46, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Lee, J.; Loh, K.C.; Dai, Y.; Tong, Y.W. Enhancement of Biogas Production in Anaerobic Co-Digestion of Food Waste and Waste Activated Sludge by Biological Co-Pretreatment. Energy 2017, 137, 479–486. [Google Scholar] [CrossRef]
- Gaworski, M.; Jabłoński, S.; Pawlaczyk-Graja, I.; Ziewiecki, R.; Rutkowski, P.; Wieczyńska, A.; Gancarz, R.; Łukaszewicz, M. Enhancing Biogas Plant Production Using Pig Manure and Corn Silage by Adding Wheat Straw Processed with Liquid Hot Water and Steam Explosion. Biotechnol. Biofuels 2017, 10, 259. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, L.; Zhao, Y.; Huang, L.; Chen, Z. Insight into Effects of Antibiotics on Reactor Performance and Evolutions of Antibiotic Resistance Genes and Microbial Community in a Membrane Reactor. Chemosphere 2018, 197, 420–429. [Google Scholar] [CrossRef]
- Tian, H.; Duan, N.; Lin, C.; Li, X.; Zhong, M. Anaerobic Co-Digestion of Kitchen Waste and Pig Manure with Different Mixing Ratios. J. Biosci. Bioeng. 2015, 120, 51–57. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic Co-Digestion of Pig Manure and Crude Glycerol: Process Performance and Digestate Stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yaohari, H.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Removal of Selected Pharmaceuticals in an Anaerobic Membrane Bioreactor (AnMBR) with/without Powdered Activated Carbon (PAC). Chem. Eng. J. 2017, 321, 335–345. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, Y.; Gao, Y.; Tian, Z.; Yang, M. Anaerobic Treatment of Antibiotic Production Wastewater Pretreated with Enhanced Hydrolysis: Simultaneous Reduction of COD and ARGs; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 110, ISBN 8610629198. [Google Scholar]
- Razaviarani, V.; Buchanan, I.D. Anaerobic Co-Digestion of Biodiesel Waste Glycerin with Municipal Wastewater Sludge: Microbial Community Structure Dynamics and Reactor Performance. Bioresour. Technol. 2015, 182, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.Q.; Zhang, L.; Tang, K.; Chen, G.; Kumar Khanal, S.; Lu, H. Removal of Sulfamethoxazole (SMX) in Sulfate-Reducing Flocculent and Granular Sludge Systems. Bioresour. Technol. 2019, 288, 121592. [Google Scholar] [CrossRef] [PubMed]
- Pilli, S.; More, T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Anaerobic Digestion of Thermal Pre-Treated Sludge at Different Solids Concentrations—Computation of Mass-Energy Balance and Greenhouse Gas Emissions. J. Environ. Manag. 2015, 157, 250–261. [Google Scholar] [CrossRef]
- Ge, H.; Jensen, P.D.; Batstone, D.J. Pre-Treatment Mechanisms during Thermophilic-Mesophilic Temperature Phased Anaerobic Digestion of Primary Sludge. Water Res. 2010, 44, 123–130. [Google Scholar] [CrossRef]
- Anjum, M.; Khalid, A.; Mahmood, T.; Aziz, I. Anaerobic Co-Digestion of Catering Waste with Partially Pretreated Lignocellulosic Crop Residues. J. Clean. Prod. 2016, 117, 56–63. [Google Scholar] [CrossRef]
- Cetecioglu, Z.; Ince, B.; Orhon, D.; Ince, O. Anaerobic Sulfamethoxazole Degradation Is Driven by Homoacetogenesis Coupled with Hydrogenotrophic Methanogenesis. Water Res. 2016, 90, 79–89. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhang, Z.; Ma, D.; Xu, G. Alkaline Thermal Pretreatment at Mild Temperatures for Biogas Production from Anaerobic Digestion of Antibiotic Mycelial Residue. Bioresour. Technol. 2016, 208, 49–57. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, K. Antibiotic Residues May Stimulate or Suppress Methane Yield and Microbial Activity during High-Solid Anaerobic Digestion. Chem. Eng. J. 2019, 359, 1303–1315. [Google Scholar] [CrossRef]
- Pandit, K.S.; Chavan, P.V.; Desai, U.V.; Kulkarni, M.A.; Wadgaonkar, P.P. Tris-Hydroxymethylaminomethane (THAM): A Novel Organocatalyst for a Environmentally Benign Synthesis of Medicinally Important Tetrahydrobenzo[b]Pyrans and Pyran-Annulated Heterocycles. New J. Chem. 2015, 39, 4452–4463. [Google Scholar] [CrossRef]


| Substrate | TS (%) | VS (%) | MC (%) | C (%) | N (%) |
|---|---|---|---|---|---|
| Pig manure | 23.5 | 17.4 | 76.5 | 46.26 | 4.25 |
| Corn straw | 82.4 | 64.8 | 17.6 | 45.17 | 0.63 |
| Reactor | Sampling Day | Antibiotics Type | Antibiotics Concentration (mg L−1) | Removal Efficiency (%) |
|---|---|---|---|---|
| R-1 | 64 | SMX | 5.81 ± 0.115 | 71.10 ± 0.58 |
| NOR | 7.846 ± 0.234 | 60.79 ± 1.17 | ||
| 88 | SMX | 15.864 ± 0.215 | 73.56 ± 1.08 | |
| NOR | 20.768 ± 0.156 | 65.39 ±0.75 | ||
| R-2 | 64 | SMX | 4.523 ± 0.155 | 72.19 ± 0.69 |
| NOR | 5.79 ± 1.22 | 73.28 ± 0.59 | ||
| 88 | SMX | 11.428 ± 0.201 | 77.39± 0.78 | |
| NOR | 16.681 ± 0.137 | 80.95 ± 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Q.; Asfaw, S.T.; Yang, S.; Chen, Z.; Ahmed, S.A.; Li, Y.; Shiferaw, H. Effect of Antibiotics and Thermophilic Pre-Treatment on Anaerobic Co-Digestion of Pig Manure and Corn Straw. Water 2023, 15, 3223. https://doi.org/10.3390/w15183223
Wen Q, Asfaw ST, Yang S, Chen Z, Ahmed SA, Li Y, Shiferaw H. Effect of Antibiotics and Thermophilic Pre-Treatment on Anaerobic Co-Digestion of Pig Manure and Corn Straw. Water. 2023; 15(18):3223. https://doi.org/10.3390/w15183223
Chicago/Turabian StyleWen, Qinxue, Solomon Tsegay Asfaw, Shuo Yang, Zhiqiang Chen, Salman Abdu Ahmed, Yixin Li, and Henok Shiferaw. 2023. "Effect of Antibiotics and Thermophilic Pre-Treatment on Anaerobic Co-Digestion of Pig Manure and Corn Straw" Water 15, no. 18: 3223. https://doi.org/10.3390/w15183223






