Unsaturated Vertical Flow Constructed Wetland for Chlorothalonil Remediation with Target Application in Ethiopian Floriculture Industry
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
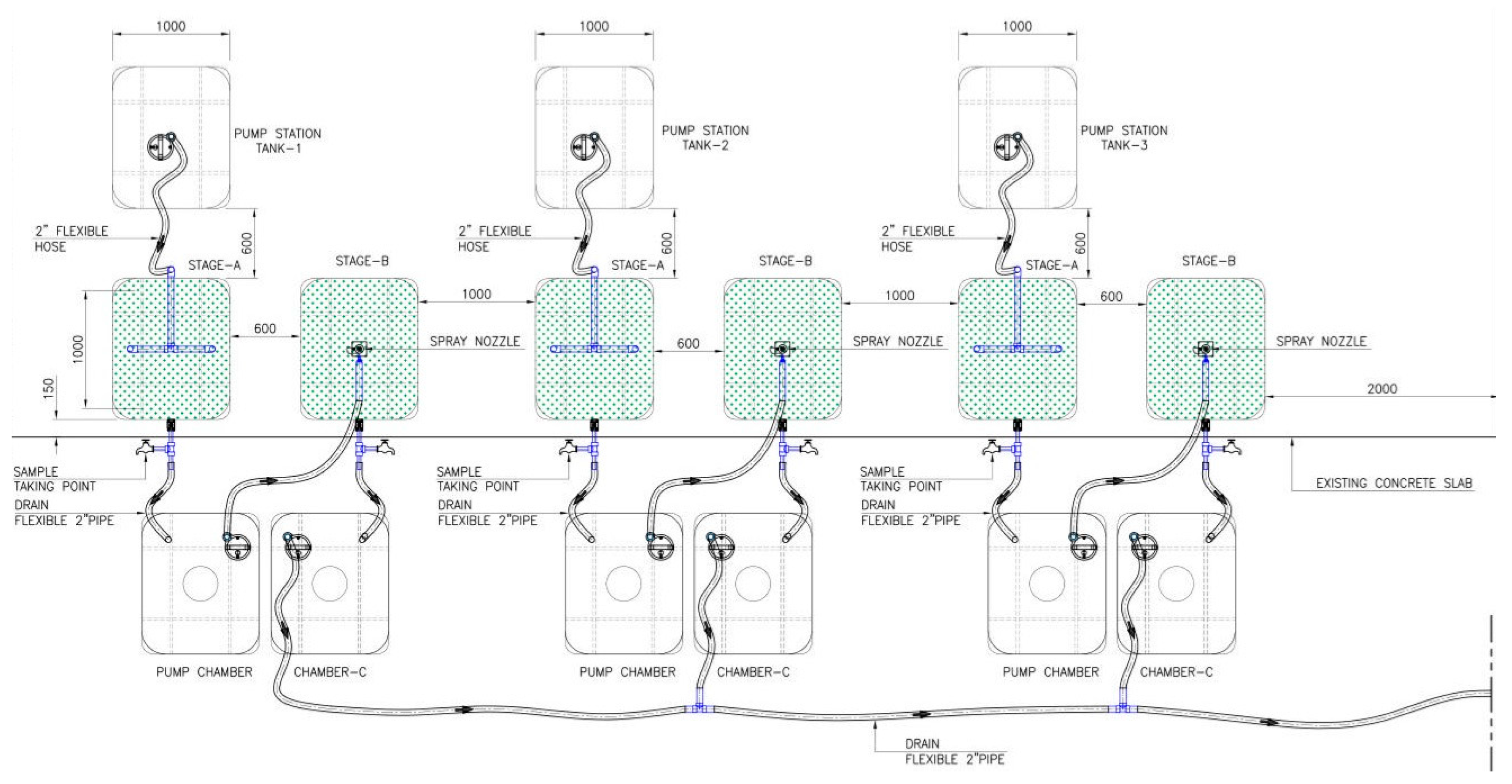
2.2. Experimental Setup
2.3. Analytical Methods
2.3.1. Chlorothalonil Concentration Analysis
2.3.2. Comparison of Water Parameters with Different Water Saturation Conditions
2.3.3. Removal Mechanisms for Chlorothalonil
2.4. Calculation Methods
2.5. Data Analysis
3. Results and Discussion
3.1. Chlorothalonil Removal
3.2. Water Parameters
3.3. Chlorothalonil Removal Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Lin, J.; Xie, T.; Zou, H.; Shen, Y. Determination of Residual Chlorothalonil in Textiles. J. Mater. Sci. Chem. Eng. 2020, 8, 106–114. [Google Scholar] [CrossRef]
- List of Registered Pesticides in Ethiopia. Available online: https://www.plantwise.org/FullTextPDF/2018/20187800405.pdf (accessed on 13 July 2023).
- California Department of Pesticide Regulation. Potential for Chlorothalonil Movement to California Groundwater as a Result of Agricultural Use. Available online: https://www.cdpr.ca.gov/docs/emon/pubs/ehapreps/analysis_memos/2626_chlorothalonil_hha_042519.pdf (accessed on 17 January 2023).
- van Scoy, A.R.; Tjeerdema, R.S. Environmental Fate and Toxicology of Chlorothalonil. Rev. Environ. Contam. Toxicol. 2014, 232, 89–105. [Google Scholar] [CrossRef]
- European Commission Notification Detail. Available online: https://ec.europa.eu/growth/tools-databases/tbt/en/search/?tbtaction=search.detail&Country_ID=EU&num=625&dspLang=en&basdatedeb=&basdatefin=&baspays=&basnotifnum=&basnotifnum2=&bastypepays=ANY&baskeywords=chlorothalonil# (accessed on 18 May 2023).
- Beyond Pesticides France’s Drinking Water Contaminated with Toxic Fungicide Chlorothalonil, Banned in EU but Widely Used in U.S. Available online: https://beyondpesticides.org/dailynewsblog/2023/04/frances-drinking-water-contaminated-with-toxic-fungicide-chlorothalonil-banned-in-eu-but-widely-used-in-u-s/ (accessed on 13 July 2023).
- Pesticides Remediation Technologies from Water and Wastewater, 1st ed.; Dehghani, M.H.; Karri, R.R.; Anastopoulos, I. (Eds.) Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323908948. [Google Scholar]
- Malakootian, M.; Shahesmaeili, A.; Faraji, M.; Amiri, H.; Silva Martinez, S. Advanced Oxidation Processes for the Removal of Organophosphorus Pesticides in Aqueous Matrices: A Systematic Review and Meta-Analysis. Process Saf. Environ. Prot. 2020, 134, 292–307. [Google Scholar] [CrossRef]
- Affam, A.C.; Chaudhuri, M.; Kutty, S.R. Comparison of Five Advanced Oxidation Processes for Degradation of Pesticide in Aqueous Solution. Bull. Chem. React. Eng. Catal. 2018, 13, 179. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Hashmi, Z.; Adriyani, R.; Yuniarto, A.; Mazari, S.A.; Akhter, F.; Mubarak, N.M. Recent Trends and Future Challenges of Pesticide Removal Techniques—A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 105571. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Degradation of Traditional and New Emerging Pesticides in Water by Nanomaterials: Recent Trends and Future Recommendations. Int. J. Environ. Sci. Technol. 2018, 15, 1347–1380. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Treatment of Industrial Wastewaters: A Review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Liu, T.; Xu, S.; Lu, S.; Qin, P.; Bi, B.; Ding, H.; Liu, Y.; Guo, X.; Liu, X. A Review on Removal of Organophosphorus Pesticides in Constructed Wetland: Performance, Mechanism and Influencing Factors. Sci. Total Environ. 2019, 651, 2247–2268. [Google Scholar] [CrossRef]
- Rana, V.; Maiti, S.K. Municipal and Industrial Wastewater Treatment Using Constructed Wetlands. In Concepts and Strategies in Plant Sciences; Springer: Cham, Switzerland, 2020; pp. 329–367. [Google Scholar]
- Engida, T.; Alemu, T.; Wu, J.; Xu, D.; Zhou, Q.; Wu, Z. Analysis of Constructed Wetlands Technology Performance Efficiency for the Treatment of Floriculture Industry Wastewater, in Ethiopia. J. Water Process Eng. 2020, 38, 101586. [Google Scholar] [CrossRef]
- Sherrard, R.M.; Bearr, J.S.; Murray-Gulde, C.L.; Rodgers, J.H.; Shah, Y.T. Feasibility of Constructed Wetlands for Removing Chlorothalonil and Chlorpyrifos from Aqueous Mixtures. Environ. Pollut. 2004, 127, 385–394. [Google Scholar] [CrossRef]
- Ríos-Montes, K.A.; Peñuela-Mesa, G.A. Degradacià del clorotalonilo por un consorcio microbiano aislado de humedales construidos en ensayos de laboratorio. Actual. Biol. 2015, 37, 255–265. [Google Scholar]
- Manikas, I.; Malindretos, G.; Abeliotis, K. Sustainable Cities through Alternative Urban Farming: The Case of Floriculture. J. Int. Food Agribus. Mark. 2020, 32, 295–311. [Google Scholar] [CrossRef]
- Lv, T.; Carvalho, P.N.; Zhang, L.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H. Functionality of Microbial Communities in Constructed Wetlands Used for Pesticide Remediation: Influence of System Design and Sampling Strategy. Water Res. 2017, 110, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, D.; Sun, Z.; Yang, Q.; Liu, Y.; Cao, T.; Chen, R.; Dzakpasu, M.; Wang, X.C. Stereoselective Degradation Pathway of Amide Chiral Herbicides and Its Impacts on Plant and Bacterial Communities in Integrated Vertical Flow Constructed Wetlands. Bioresour. Technol. 2022, 351, 126997. [Google Scholar] [CrossRef]
- Lyu, T.; Zhang, L.; Xu, X.; Arias, C.A.; Brix, H.; Carvalho, P.N. Removal of the Pesticide Tebuconazole in Constructed Wetlands: Design Comparison, Influencing Factors and Modelling. Environ. Pollut. 2018, 233, 71–80. [Google Scholar] [CrossRef]
- Wang, G.; Liang, B.; Li, F.; Li, S. Recent Advances in the Biodegradation of Chlorothalonil. Curr. Microbiol. 2011, 63, 450–457. [Google Scholar] [CrossRef]
- Tang, L.; Dong, J.; Ren, L.; Zhu, Q.; Huang, W.; Liu, Y.; Lu, D. Biodegradation of Chlorothalonil by Enterobacter Cloacae TUAH-1. Int. Biodeterior. Biodegrad. 2017, 121, 122–130. [Google Scholar] [CrossRef]
- Aregu, M.B.; Asfaw, S.L.; Khan, M.M. Developing Horizontal Subsurface Flow Constructed Wetland Using Pumice and Chrysopogon Zizanioides for Tannery Wastewater Treatment. Environ. Syst. Res. 2021, 10, 33. [Google Scholar] [CrossRef]
- Mburu, N.; Tebitendwa, S.M.; van Bruggen, J.J.A.; Rousseau, D.P.L.; Lens, P.N.L. Performance Comparison and Economics Analysis of Waste Stabilization Ponds and Horizontal Subsurface Flow Constructed Wetlands Treating Domestic Wastewater: A Case Study of the Juja Sewage Treatment Works. J. Environ. Manag. 2013, 128, 220–225. [Google Scholar] [CrossRef]
- DWA-A 262E (2017); Principles for Dimensioning, Construction and Operation of Wastewater Treatment Plants with Planted and Unplanted Filters for Treatment of Domestic and Municipal Wastewater. DWA Set of Rules, German Association for Water, Wastewater and Waste (DWA): Hennef, Germany, 2017; ISBN 978-3-88721-642-9.
- Reed Bed Reed Bed Services. Available online: https://reedbed.ae/reed-bed-services/ (accessed on 20 January 2023).
- Wehbe, S.; Zewge, F.; Inagaki, Y.; Sievert, W.; Uday Kumar, N.T.; Deshpande, A. Performance of Carbendazim Removal Using Constructed Wetlands for the Ethiopian Floriculture Industry. Water Sci. Technol. 2022, 86, 142–151. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information PubChem Annotation Record for CHLOROTHALONIL, Source: Hazardous Substances Data Bank (HSDB). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chlorothalonil#section=Solubility (accessed on 27 December 2022).
- US EPA Method 8081B. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/8081b.pdf (accessed on 17 March 2022).
- Ji, Z.; Tang, W.; Pei, Y. Constructed Wetland Substrates: A Review on Development, Function Mechanisms, and Application in Contaminants Removal. Chemosphere 2022, 286, 131564. [Google Scholar] [CrossRef] [PubMed]
- Gikas, G.D.; Parlakidis, P.; Mavropoulos, T.; Vryzas, Z. Particularities of Fungicides and Factors Affecting Their Fate and Removal Efficacy: A Review. Sustainability 2022, 14, 4056. [Google Scholar] [CrossRef]
- Sanders, J. Veusz: Scientific plotting package (version 3.4.0.1). Mac. Garching. Github.io. 2021. [Google Scholar]
- Microsoft Corporation. Microsoft Excel for Mac (version 16.76), Mac. Microsoft Corporation: Redmond, WA, USA, 2023.
- Passeport, E.; Tournebize, J.; Chaumont, C.; Guenne, A.; Coquet, Y. Pesticide Contamination Interception Strategy and Removal Efficiency in Forest Buffer and Artificial Wetland in a Tile-Drained Agricultural Watershed. Chemosphere 2013, 91, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Passeport, E.; Tournebize, J.; Jankowfsky, S.; Prömse, B.; Chaumont, C.; Coquet, Y.; Lange, J. Artificial Wetland and Forest Buffer Zone: Hydraulic and Tracer Characterization. Vadose Zone J. 2010, 9, 73. [Google Scholar] [CrossRef]
- Rìos-Montes, K.A.; Casas-Zapata, J.C.; Briones-Gallardo, R.; Peñuela, G. Optimal Conditions for Chlorothalonil and Dissolved Organic Carbon in Horizontal Subsurface Flow Constructed Wetlands. J. Environ. Sci. Health B 2017, 52, 274–281. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Liu, X.; Huang, S.; Yang, Y.; Liu, Z. The Effect of Dissolved Oxygen Concentration on Long-Term Stability of Partial Nitrification Process. J. Environ. Sci. (China) 2020, 90, 343–351. [Google Scholar] [CrossRef] [PubMed]
- EPA Ethiopia. The United Nations Industrial Development Organization. In Guideline Ambient Environment Standards for Ethiopia; EPA Ethiopia: Addis Ababa, Ethiopia, 2003. [Google Scholar]
- Voulvoulis, N. Water Reuse from a Circular Economy Perspective and Potential Risks from an Unregulated Approach. Curr. Opin. Environ. Sci. Health 2018, 2, 32–45. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, L.; Pan, L.; Zhang, X.; Zou, J.-J. Rational Design, Synthesis, Adsorption Principles and Applications of Metal Oxide Adsorbents: A Review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut, J.; Stein, O.; von Sperling, M. Treatment Wetlands; IWA Publishing: London, UK, 2021; Volume 7, ISBN 9781780408774. [Google Scholar]
- Kulshreshtha, N.M.; Verma, V.; Soti, A.; Brighu, U.; Gupta, A.B. Exploring the Contribution of Plant Species in the Performance of Constructed Wetlands for Domestic Wastewater Treatment. Bioresour. Technol. Rep. 2022, 18, 101038. [Google Scholar] [CrossRef]
- Kurzbaum, E. The Partial Contribution of Constructed Wetland Components (Roots, Gravel, Microorganisms) in the Removal of Phenols: A Mini Review. Water 2022, 14, 626. [Google Scholar] [CrossRef]
- Climate Change Knowledge Portal Ethiopia—Current Climate. Available online: https://climateknowledgeportal.worldbank.org/country/ethiopia/climate-data-historical (accessed on 31 December 2022).
- Wang, Z.; Yang, L.; Cheng, P.; Yu, Y.; Zhang, Z.; Li, H. Adsorption, Degradation and Leaching Migration Characteristics of Chlorothalonil in Different Soils. Eur. J. Remote Sens. 2021, 54, 238–247. [Google Scholar] [CrossRef]


| Scale of Study | Type of CW | Saturation | Influent Load | Chlorothalonil Removal Efficiency | HRT (h) | Reference |
|---|---|---|---|---|---|---|
| Pilot | UVF-CW | Unsaturated | 100 µg L−1 | >99.9% | 2 | Present study |
| Pilot | UVF-CW | Unsaturated | 500 µg L−1 | >99.9% | 2 | Present study |
| Pilot | HSS-CW | Partially saturated | 148 µg L−1 | 94% | 24 | [16] |
| Pilot | HSS-CW | Partially saturated | 326 µg L−1 | >99.9% | 30 | [16] |
| Pilot | HSS-CW | Partially saturated | 296 µg L−1 | >99.9% | 24 | [16] |
| Field | HSS-CW | Partially saturated | 11.8 g | 78% | 66.5 | [35,36] |
| pH | DO (%) | EC (µS cm−1) | Water Temperature (°C) | ||
|---|---|---|---|---|---|
| Control | Influent | 7.57 ± 0.13 | 21.4 ± 2.2 | 427 ± 14 | 34.5 ± 1.7 |
| Effluent | 8.26 ± 0.11 | 55.9 ± 3.2 | 544 ± 20 | 35.2 ± 1.8 | |
| 100 µg L−1 | Influent | 7.56 ± 0.16 | 21.9 ± 2.2 | 421 ± 9 | 33.5 ± 1.8 |
| Effluent | 8.35 ± 0.1 | 54.6 ± 3.5 | 562 ± 34 | 34.5 ± 1.8 | |
| 500 µg L−1 | Influent | 7.68 ± 0.13 | 21.6 ± 2.0 | 417 ± 10 | 33.7 ± 2.1 |
| Effluent | 8.27 ± 0.16 | 55.4 ± 2.4 | 559 ± 27 | 34.6 ± 2.1 | |
| Nitrate Concentration (mgL−1) | Phosphate Concentration (mgL−1) | BOD (mgL−1) | COD (mgL−1) | TOC (mgL−1) | ||
|---|---|---|---|---|---|---|
| Control | Influent | 0.05 | 0.34 | <12 | 12 | 0.62 |
| Effluent | 0.05 | <0.02 | <12 | <10 | 0.25 | |
| 500 µg L−1 | Influent | 0.05 | 0.25 | <12 | 1394 | 276 |
| Effluent | 0.06 | <0.02 | <12 | 12 | 5.46 | |
| Source of Variation | SS | df | MS | F | p-Value | F Crit | |
|---|---|---|---|---|---|---|---|
| ΔpH | Sample | 0.12 | 2 | 0.059 | 1.340 | 0.271 | 3.168 |
| Saturation | 20.31 | 1 | 20.312 | 464.945 | 0.000 | 4.020 | |
| Cin × Saturation | 0.36 | 2 | 0.178 | 4.069 | 0.023 | 3.168 | |
| Within | 2.36 | 54 | 0.044 | ||||
| Total | 23.14 | 59 | |||||
| ΔDO | Sample | 46.03 | 2 | 23.017 | 1.535 | 0.225 | 3.168 |
| Saturation | 7370.42 | 1 | 7370.417 | 491.665 | 0.000 | 4.020 | |
| Cin × Saturation | 4.63 | 2 | 2.317 | 0.155 | 0.857 | 3.168 | |
| Within | 809.50 | 54 | 14.991 | ||||
| Total | 8230.58 | 59 | |||||
| ΔEC | Sample | 2465.63 | 2 | 1232.817 | 1.396 | 0.256 | 3.168 |
| Saturation | 933,005.40 | 1 | 933,005.400 | 1056.707 | 0.000 | 4.020 | |
| Cin × Saturation | 1647.10 | 2 | 823.550 | 0.933 | 0.400 | 3.168 | |
| Within | 47,678.60 | 54 | 882.937 | ||||
| Total | 984,796.73 | 59 | |||||
| ΔT | Sample | 5.73 | 2 | 2.867 | 2.674 | 0.078 | 3.168 |
| Saturation | 3.75 | 1 | 3.750 | 3.497 | 0.067 | 4.020 | |
| Cin × Saturation | 2.80 | 2 | 1.400 | 1.306 | 0.279 | 3.168 | |
| Within | 57.90 | 54 | 1.072 | ||||
| Total | 70.18 | 59 |
| Plant Uptake | |
| Total amount of chlorothalonil added to experimental Group 3 (mg) | 5016 |
| Amount of biomass harvested (kg) | 6.12 |
| Amount of chlorothalonil present in plant sample (mg kg−1) | 0.696 |
| Percentage removal of chlorothalonil by plant uptake (%) | 0.085 |
| Substrate Sorption | |
| Total amount of chlorothalonil added to experimental Group 3 (mg) | 5016 |
| Estimated amount of substrate present in top-layer stage A and stage B (kg) | 1890 |
| Amount of chlorothalonil present in substrate sample (mg kg−1) | 39.46 |
| Theoretical removal capacity of chlorothalonil using the substrate (mg) | 74,579.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wehbe, S.; Zewge, F.; Inagaki, Y.; Sievert, W.; Nutakki, T.U.K.; Deshpande, A. Unsaturated Vertical Flow Constructed Wetland for Chlorothalonil Remediation with Target Application in Ethiopian Floriculture Industry. Water 2023, 15, 3282. https://doi.org/10.3390/w15183282
Wehbe S, Zewge F, Inagaki Y, Sievert W, Nutakki TUK, Deshpande A. Unsaturated Vertical Flow Constructed Wetland for Chlorothalonil Remediation with Target Application in Ethiopian Floriculture Industry. Water. 2023; 15(18):3282. https://doi.org/10.3390/w15183282
Chicago/Turabian StyleWehbe, Stan, Feleke Zewge, Yoshihiko Inagaki, Wolfram Sievert, Tirumala Uday Kumar Nutakki, and Akshay Deshpande. 2023. "Unsaturated Vertical Flow Constructed Wetland for Chlorothalonil Remediation with Target Application in Ethiopian Floriculture Industry" Water 15, no. 18: 3282. https://doi.org/10.3390/w15183282
APA StyleWehbe, S., Zewge, F., Inagaki, Y., Sievert, W., Nutakki, T. U. K., & Deshpande, A. (2023). Unsaturated Vertical Flow Constructed Wetland for Chlorothalonil Remediation with Target Application in Ethiopian Floriculture Industry. Water, 15(18), 3282. https://doi.org/10.3390/w15183282








