Human Adenovirus Detection and Genetic Characterization in Irrigation Water from the Riyadh Region, Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
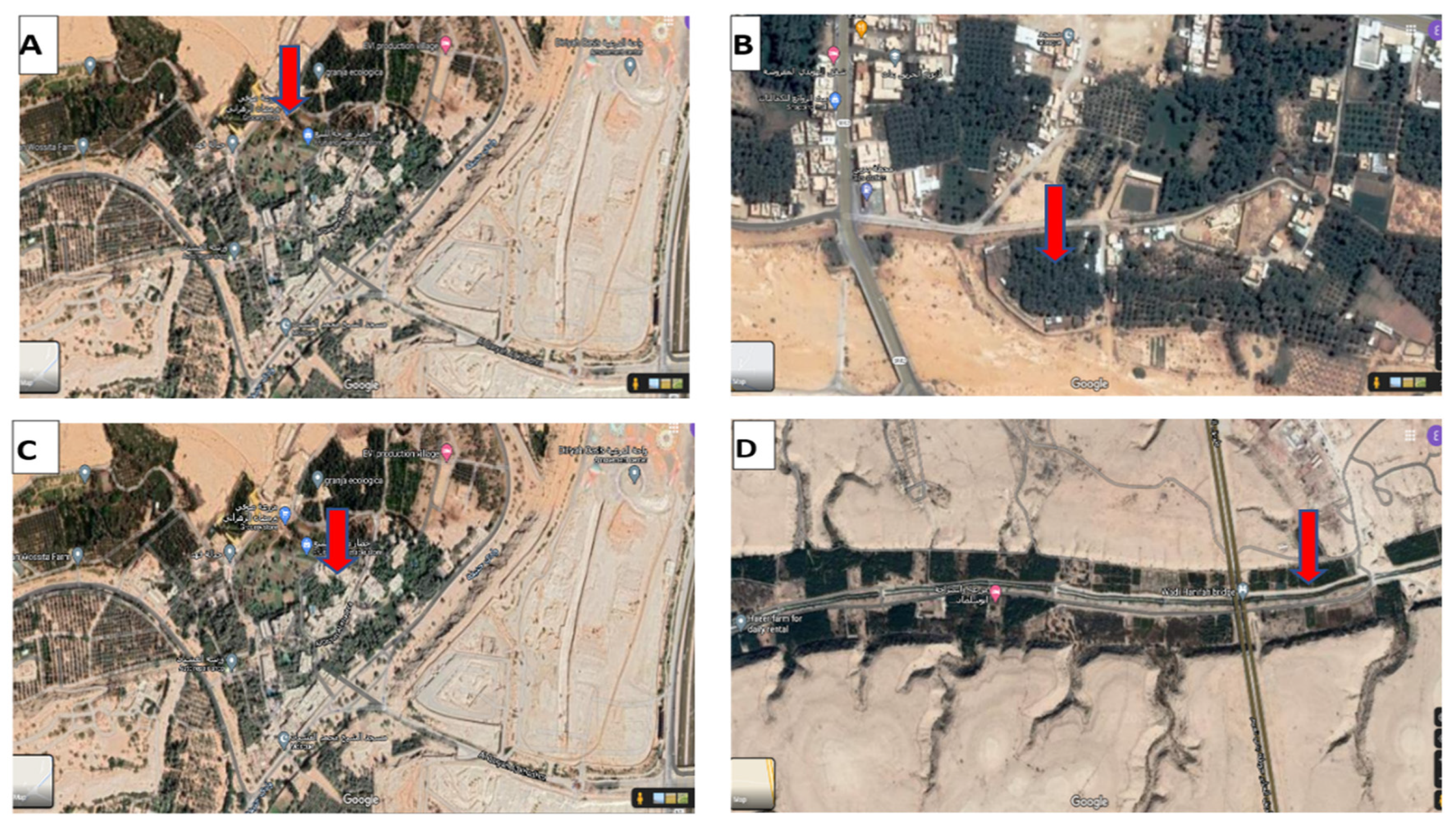
2.1. Samples Collection
2.2. Virus Concentration
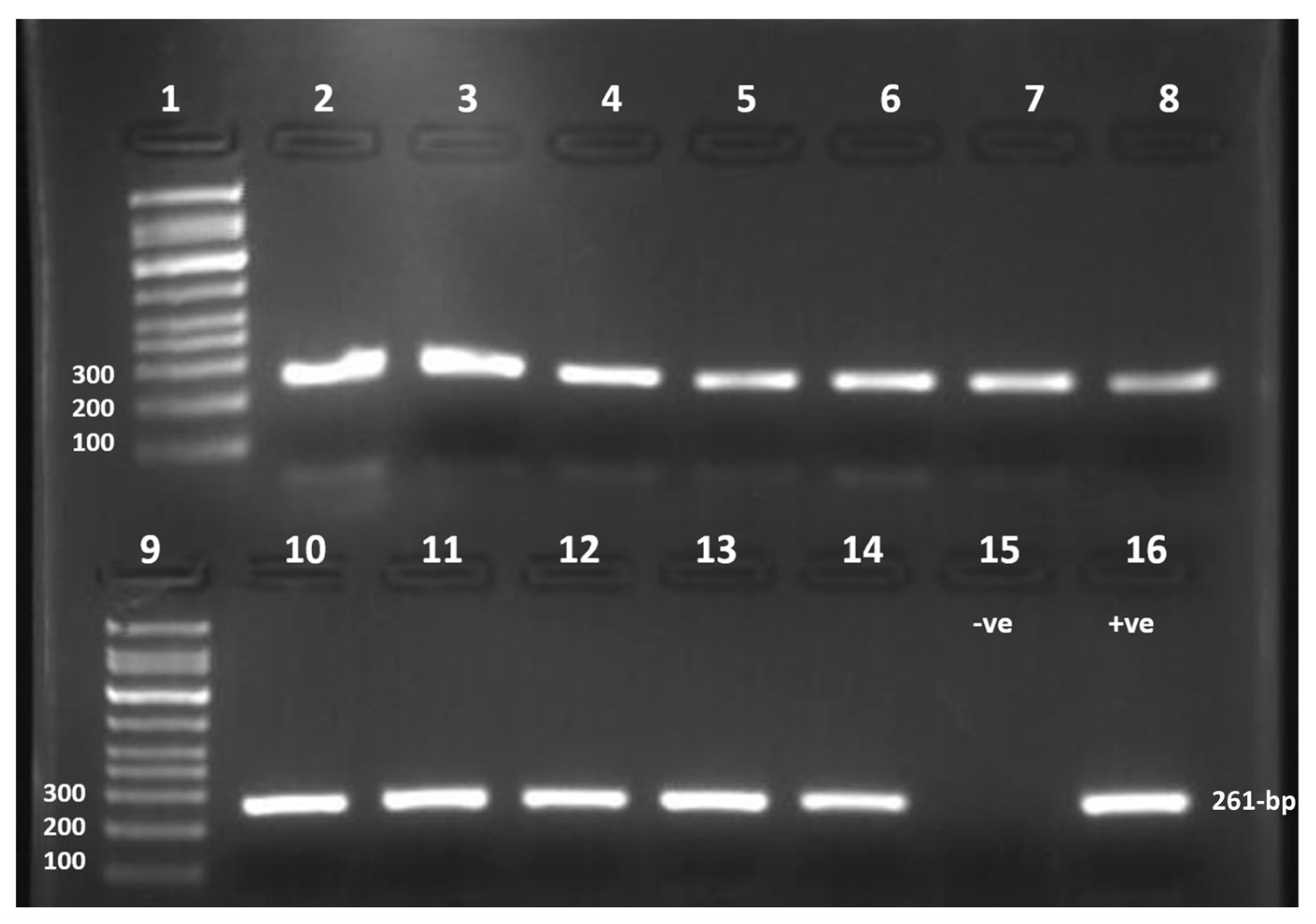
2.3. The Nucleic Acid Extraction and Amplification of the Specific Hexon Gene
2.4. Purification and Sanger Sequencing
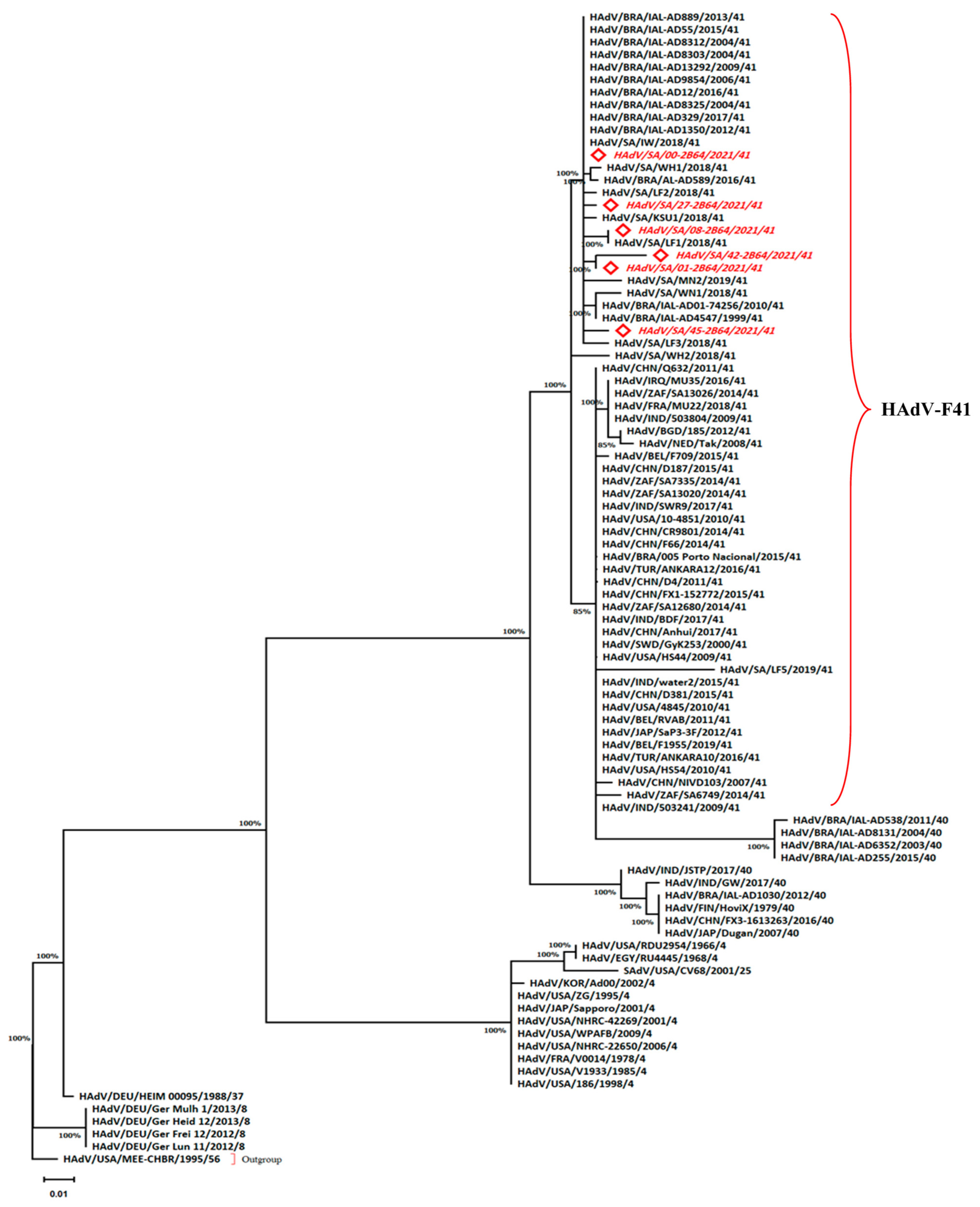
2.5. Phylogenetic Analysis
2.6. Prevalence
2.7. Statistical Analysis
3. Results
3.1. The Prevalence of Human Adenoviruses
3.2. The Predominance of Human Adenovirus F Serotype 41
3.3. The Distribution Isolates of Human Adenovirus F Serotype 41
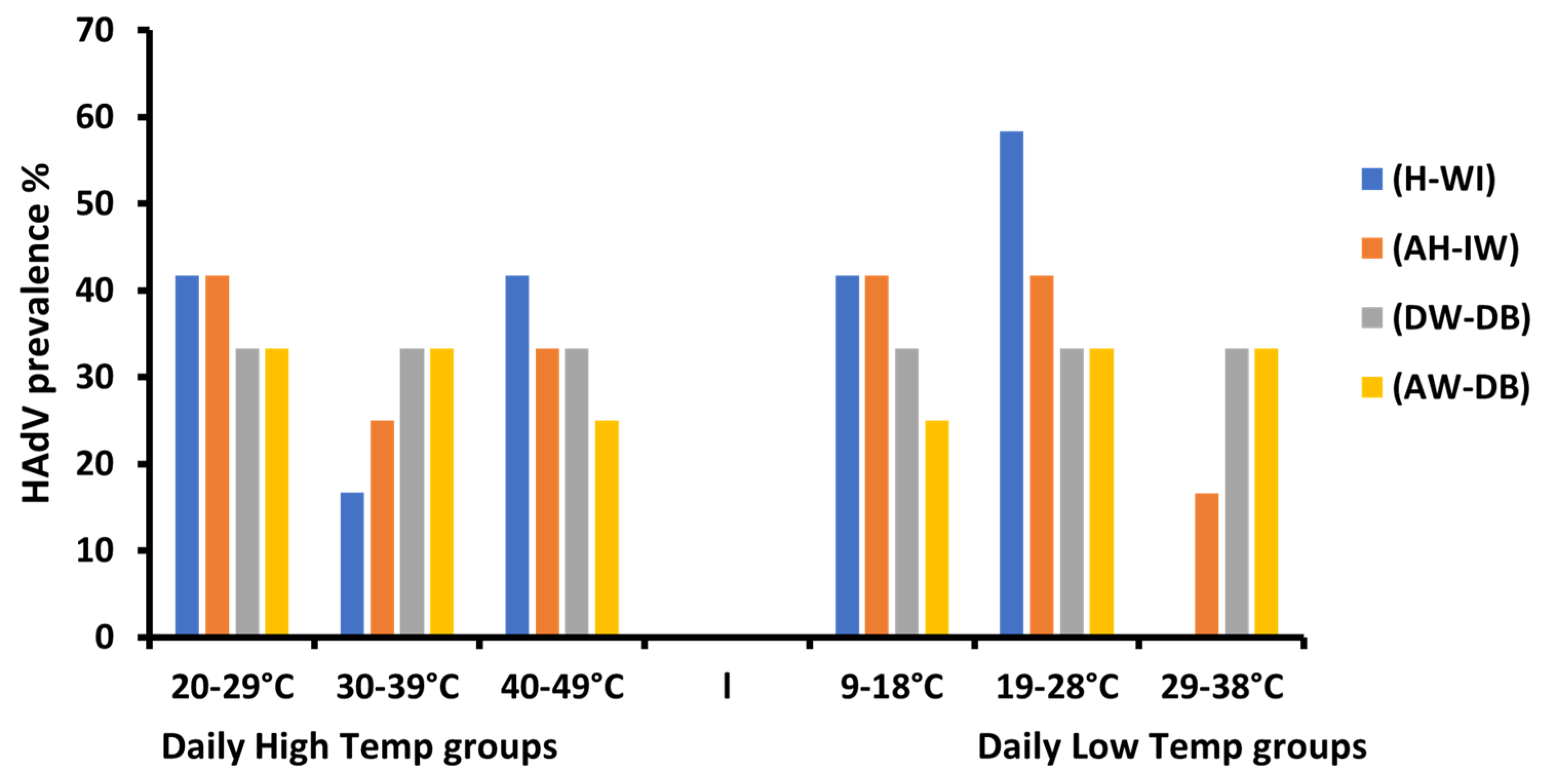
3.4. The Influence of Metrological Variation on HAdV Prevalence
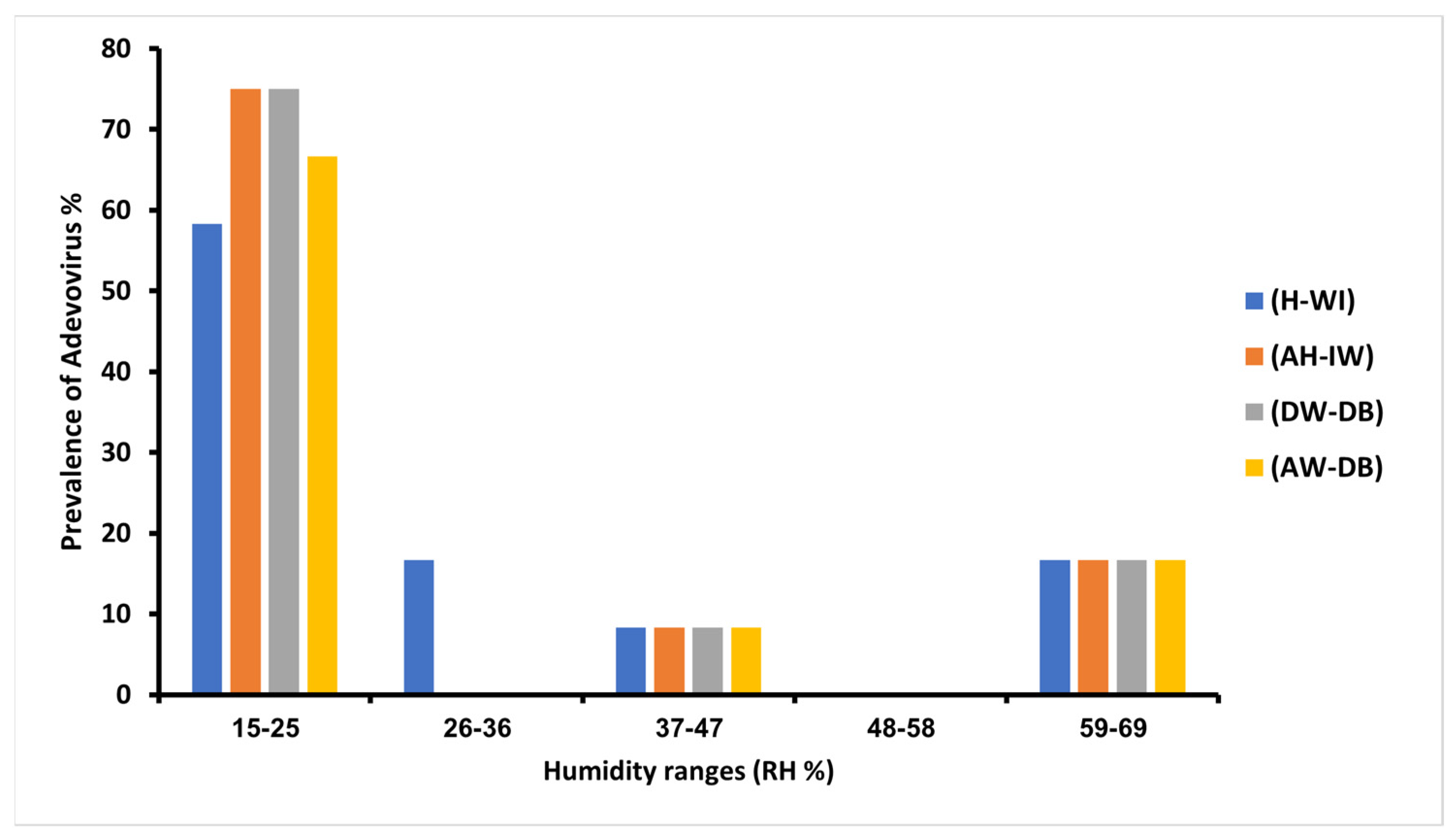
3.5. Humidity Variations’ Impact on Adenoviruses’ Prevalence
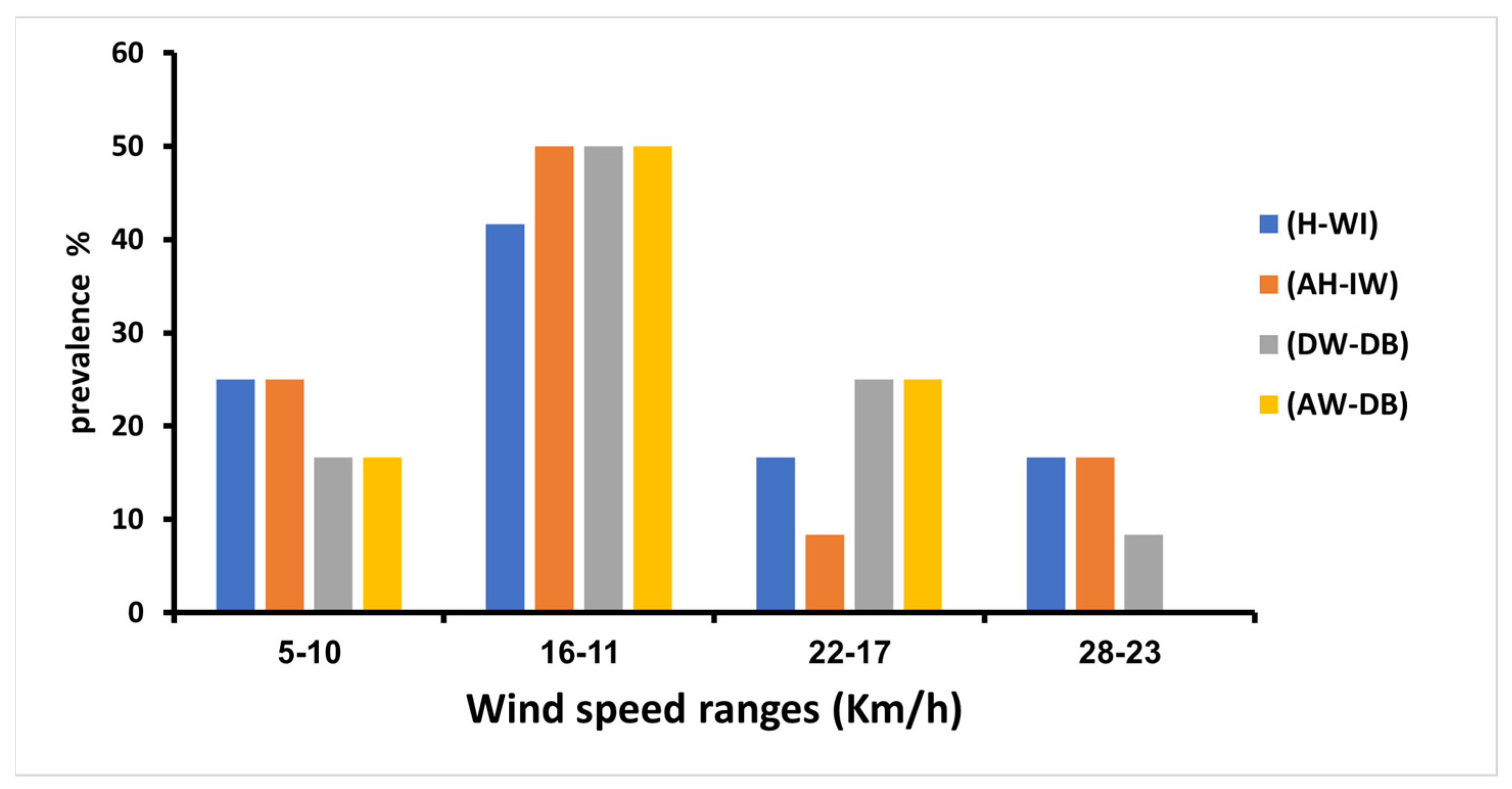
3.6. Wind Speed Impact on the Prevalence of Adenoviruses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokkinos, P.; Kozyra, I.; Lazic, S.; Söderberg, K.; Vasickova, P.; Bouwknegt, M.; Rutjes, S.; Willems, K.; Moloney, R.; de Roda Husman, A.M.; et al. Virological Quality of Irrigation Water in Leafy Green Vegetables and Berry Fruits Production Chains. Food Environ. Virol. 2017, 9, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.G.; Howe, A.; Rose, J.B. Metagenomic Approaches for Direct and Cell Culture Evaluation of the Virological Quality of Wastewater. J. Virol. Methods 2014, 210, 15–21. [Google Scholar] [CrossRef]
- Ebo Yahans Amuah, E.; Amanin-Ennin, P.; Antwi, K. Irrigation Water Quality in Ghana and Associated Implications on Vegetables and Public Health. A Systematic Review. J. Hydrol. 2022, 604, 127211. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef]
- Hassou, N.; Bouseettine, R.; Abouchoaib, N.; Ennaji, M.M. Enteric Adenoviruses: Emerging of a Public Health Threat. In Emerging and Reemerging Viral Pathogens: Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens; Elsevier: Amsterdam, The Netherlands, 2019; pp. 879–905. ISBN 9780128194003. [Google Scholar]
- Yoshitomi, H.; Sera, N.; Gonzalez, G.; Hanaoka, N.; Fujimoto, T. First Isolation of a New Type of Human Adenovirus (Genotype 79), Species Human Mastadenovirus B (B2) from Sewage Water in Japan. J. Med. Virol. 2017, 89, 1192–1200. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Z.; Tang, L.; Wang, L.; Tan, X.; Yu, P.; Zhang, Y.; Tian, X.; Wang, J.; Zhang, Y.; et al. Genomic Analyses of Recombinant Adenovirus Type 11a in China. J. Clin. Microbiol. 2009, 47, 3082–3090. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Bakhrebah, M.A.; Nassar, M.S.; Natto, Z.S.; Al Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S.; et al. Suspected Adenovirus Causing an Emerging HEPATITIS among Children below 10 Years: A Review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef]
- Meqdam, M.M.; Thwiny, I.R. Prevalence of group a rotavirus, enteric adenovirus, norovirus and astrovirus infections among children with acute gastroenteritis in Al-Qassim, Saudi Arabia. Pak. J. Med. Sci. 2007, 23, 551–555. [Google Scholar]
- Saeed, M.; Al-Ayed, Z.; Asaad, A.M.; Qureshi, M.A.; Saeed, M.; Alayed, Z.; Morad Asaad, A.; Mahdi, A.A. Aetiology of Acute Gastroenteritis in Children in Najran Region, Saudi Arabia. J. Health Spec. 2013, 1, 84. [Google Scholar]
- Khalaf, R.J.; Fadhil, H.Y.; Auf, I.M.; Namdar, S.A. Molecular Diagnosis of Human Adenovirus in Children with Upper Respiratory Tract Infections. IOSR J. Pharm. Biol. Sci. 2017, 12, 9–13. [Google Scholar] [CrossRef]
- Rames, E.; Roiko, A.; Stratton, H.; Macdonald, J. Technical Aspects of Using Human Adenovirus as a Viral Water Quality Indicator. Water Res. 2016, 96, 308–326. [Google Scholar] [CrossRef]
- Nour, I.; Hanif, A.; Ryan, M.; Eifan, S. Insights into Gastrointestinal Virome: Etiology and Public Exposure. Water 2021, 13, 2794. [Google Scholar] [CrossRef]
- Farkas, K.; Cooper, D.M.; McDonald, J.E.; Malham, S.K.; de Rougemont, A.; Jones, D.L. Seasonal and Spatial Dynamics of Enteric Viruses in Wastewater and in Riverine and Estuarine Receiving Waters. Sci. Total Environ. 2018, 634, 1174–1183. [Google Scholar] [CrossRef]
- Umuhoza, T.; Bulimo, W.D.; Oyugi, J.; Musabyimana, J.P.; Kinengyere, A.A.; Mancuso, J.D. Prevalence of Human Respiratory Syncytial Virus, Parainfluenza and Adenoviruses in East Africa Community Partner States of Kenya, Tanzania, and Uganda: A Systematic Review and Meta-Analysis (2007–2020). PLoS ONE 2021, 16, e0249992. [Google Scholar] [CrossRef]
- Elmahdy, E.M.; Shaheen, M.N.F.; Rizk, N.M.; Saad-Hussein, A. Quantitative Detection of Human Adenovirus and Human Rotavirus Group A in Wastewater and El-Rahawy Drainage Canal Influencing River Nile in the North of Giza, Egypt. Food Environ. Virol. 2020, 12, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; Almalki, R. Molecular Detection of Hepatitis A Virus and Rotavirus in Water Samples Collected from Albaha, Saudi Arabia. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2018, 10, 59–68. [Google Scholar]
- Waso, M.; Khan, S.; Khan, W. Microbial Source Tracking Markers Associated with Domestic Rainwater Harvesting Systems: Correlation to Indicator Organisms. Environ. Res. 2018, 161, 446–455. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: First Addendum to the Third Edition, Volume 1: Reccommendations; World Health Organization: Geneva, Switzerland, 2006; ISBN 9241546964.
- US EPA. Drinking Water Contaminant List 5—Final; Federal Register Notice: Drinking Water Contaminant Candidate List 5 2022, EPA 815-F-22-005; EPA: Washington, DC, USA, 2022.
- Silva, H.D.; García-Zapata, M.T.A.; Anunciação, C.E. Why the Use of Adenoviruses as Water Quality Virologic Marker? Food Environ. Virol. 2011, 3, 138–140. [Google Scholar] [CrossRef]
- MEWA. Statistics Book 2021; Ministry of Environment Water and Agriculture: Riyadh, Saudi Arabia, 2021.
- Mena, K.D.; Gerba, C.P. Waterborne Adenovirus. Rev. Environ. Contam. Toxicol. 2009, 198, 133–167. [Google Scholar]
- Beaudeau, P.; de Valk, H.; Vaillant, V.; Mannschott, C.; Tillier, C.; Mouly, D.; Ledrans, M. Lessons Learned from Ten Investigations of Waterborne Gastroenteritis Outbreaks, France, 1998–2006. J. Water Health 2008, 6, 491–503. [Google Scholar] [CrossRef]
- Hjelmsø, M.H.; Hellmér, M.; Fernandez-Cassi, X.; Timoneda, N.; Lukjancenko, O.; Seidel, M.; Elsässer, D.; Aarestrup, F.M.; Löfström, C.; Bofill-Mas, S.; et al. Evaluation of Methods for the Concentration and Extraction of Viruses from Sewage in the Context of Metagenomic Sequencing. PLoS ONE 2017, 12, e0170199. [Google Scholar] [CrossRef]
- Maniah, K.; Nour, I.; Hanif, A.; Yassin, M.T.; Alkathiri, A.; Al-Ashkar, I.; Eifan, S. Molecular Identification of Human Adenovirus Isolated from Different Wastewater Treatment Plants in Riyadh, Saudi Arabia: Surveillance and Meteorological Impacts. Water 2023, 15, 1367. [Google Scholar] [CrossRef]
- Dey, R.S.; Ghosh, S.; Chawla-Sarkar, M.; Panchalingam, S.; Nataro, J.P.; Sur, D.; Manna, B.; Ramamurthy, T. Circulation of a Novel Pattern of Infections by Enteric Adenovirus Serotype 41 among Children below 5 Years of Age in Kolkata, India. J. Clin. Microbiol. 2011, 49, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Bertrand, I.; Schijven, J.F.; Sánchez, G.; Wyn-Jones, P.; Ottoson, J.; Morin, T.; Muscillo, M.; Verani, M.; Nasser, A.; de Roda Husman, A.M.; et al. The Impact of Temperature on the Inactivation of Enteric Viruses in Food and Water: A Review. J. Appl. Microbiol. 2012, 112, 1059–1074. [Google Scholar] [CrossRef]
- Iaconelli, M.; Muscillo, M.; Della Libera, S.; Fratini, M.; Meucci, L.; De Ceglia, M.; Giacosa, D.; La Rosa, G. One-Year Surveillance of Human Enteric Viruses in Raw and Treated Wastewaters, Downstream River Waters, and Drinking Waters. Food Environ. Virol. 2017, 9, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, Y.; Pang, X.L.; Ashbolt, N.J. Spiked Virus Level Needed to Correctly Assess Enteric Virus Recovery in Water Matrices. Appl. Environ. Microbiol. 2019, 85, e00111-19. [Google Scholar] [CrossRef]
- Jumat, M.R.; Hasan, N.A.; Subramanian, P.; Heberling, C.; Colwell, R.R.; Hong, P.Y. Membrane Bioreactor-Basedwastewater Treatment Plant in Saudi Arabia: Reduction of Viral Diversity, Load, and Infectious Capacity. Water 2017, 9, 534. [Google Scholar] [CrossRef]
- Silva, D.; Luz, D.; der Sand, V. First Description of Adenovirus, Enterovirus, Rotavirus and Torque Teno Virus in Water Samples Collected from the Arroio Dilúvio, Porto Alegre, Brazil. Braz. J. Biol. 2012, 72, 323–329. [Google Scholar]
- Nour, I.; Hanif, A.; Zakri, A.M.; Al-Ashkar, I.; Alhetheel, A.; Eifan, S. Human Adenovirus Molecular Characterization in Various Water Environments and Seasonal Impacts in Riyadh, Saudi Arabia. Int. J. Environ. Res. Public Health 2021, 18, 4773. [Google Scholar] [CrossRef]
- Pang, X.; Gao, T.; Qiu, Y.; Caffrey, N.; Popadynetz, J.; Younger, J.; Lee, B.E.; Neumann, N.; Checkley, S. The Prevalence and Levels of Enteric Viruses in Groundwater of Private Wells in Rural Alberta, Canada. Water Res. 2021, 202, 117425. [Google Scholar] [CrossRef] [PubMed]
- Sedji, M.I.; Varbanov, M.; Meo, M.; Colin, M.; Mathieu, L.; Bertrand, I. Quantification of Human Adenovirus and Norovirus in River Water in the North-East of France. Environ. Sci. Pollut. Res. 2018, 25, 30497–30507. [Google Scholar] [CrossRef] [PubMed]
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A Review on Recent Progress in the Detection Methods and Prevalence of Human Enteric Viruses in Water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.K.; El-Senousy, W.M.; Sayed, E.S.T.A.E.S.; AlKhazindar, M. Infectious Pepper Mild Mottle Virus and Human Adenoviruses as Viral Indices in Sewage and Water Samples. Food Environ. Virol. 2022, 14, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Mallick, J.; Singh, C.K.; Almesfer, M.K.; Singh, V.P.; Alsubih, M. Groundwater Quality Studies in the Kingdom of Saudi Arabia: Prevalent Research and Management Dimensions. Water 2021, 13, 1266. [Google Scholar] [CrossRef]
- Tayeb, H.T.; Dela Cruz, D.M.; Al-Qahtani, A.; Al-Ahdal, M.N.; Carter, M.J. Enteric Viruses in Pediatric Diarrhea in Saudi Arabia. J. Med. Virol. 2008, 80, 1919–1929. [Google Scholar] [CrossRef]
- da Graça Pedrosa de Macena, L.; Baur Vieira, C.; Gonçalves Maranhão, A.; César Ferreira, F.; Sampaio Lemos, E.R.; Miagostovich, M.P. Genetic Diversity and Quantification of Human Adenoviruses and JC Polyomaviruses in Wastewater Samples. Int. J. Plant Anim. Environ. Sci. 2021, 11, 614–626. [Google Scholar] [CrossRef]
- Khraif, R.M.; Salam, A.A.; Nair, P.S.; Elsegaey, I. Migration in Saudi Arabia: Present and Prospects. In India’s Low-Skilled Migration to the Middle East: Policies, Politics and Challenges; Palgrave Macmillan: Singapore, 2019. [Google Scholar]
- Fong, T.T.; Phanikumar, M.S.; Xagoraraki, I.; Rose, J.B. Quantitative Detection of Human Adenoviruses in Wastewater and Combined Sewer Overflows Influencing a Michigan River. Appl. Environ. Microbiol. 2010, 76, 715–723. [Google Scholar] [CrossRef]
- Wang, Z.; Shin, H.; Jung, S.; Yeo, D.; Park, H.; Shin, S.; Seo, D.J.; Park, K.H.; Choi, C. Effects of Weather and Environmental Factors on the Seasonal Prevalence of Foodborne Viruses in Irrigation Waters in Gyeonggi Province, Korea. Microorganisms 2020, 8, 1224. [Google Scholar] [CrossRef]
- Shaheen, M.N.F.; Elmahdy, E.M.; Chawla-Sarkar, M. Quantitative PCR-Based Identification of Enteric Viruses Contaminating Fresh Produce and Surface Water Used for Irrigation in Egypt. Environ. Sci. Pollut. Res. 2019, 26, 21619–21628. [Google Scholar] [CrossRef]
- Wei, J.; Kniel, K.E. Pre-Harvest Viral Contamination of Crops Originating from Fecal Matter. Food Environ. Virol. 2010, 2, 195–206. [Google Scholar] [CrossRef]
- Jung, J.H.; Yoo, C.H.; Koo, E.S.; Kim, H.M.; Na, Y.; Jheong, W.H.; Jeong, Y.S. Occurrence of Norovirus and Other Enteric Viruses in Untreated Groundwaters of Korea. J. Water Health 2011, 9, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.; Lee, C.; Song, S.W.; Choi, W.C.; Lee, C.H.; Kim, S.J. Enteric Viruses in Raw Vegetables and Groundwater Used for Irrigation in South Korea. Appl. Environ. Microbiol. 2009, 75, 7745–7751. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Microbial Pollution and Food Safety. AIMS Microbiol. 2018, 4, 377–396. [Google Scholar] [CrossRef] [PubMed]
| Month | (H-WI) | (AH-IW) | (DW-DB) | (AW-DB) |
|---|---|---|---|---|
| January | + | + | + | + |
| February | + | + | + | + |
| March | + | + | + | + |
| April | + | + | + | + |
| May | + | + | + | − |
| June | + | + | + | + |
| July | + | + | + | + |
| August | + | + | + | + |
| September | + | + | + | + |
| October | + | + | + | + |
| November | + | + | + | + |
| December | + | + | + | + |
| Isolates | Accession Numbers | Location | Month | High Temp (°C) | Low Temp (°C) | Wind Speed (km/h) | Humidity (%) |
|---|---|---|---|---|---|---|---|
| 00-2B64 | OP784585 | All sites | From January to December | 20~49 | 9~38 | 5~28 | 15~36–>58 |
| 01-2B64 | OP784580 | AH-IW | January | 23 | 9 | 12 | 48 |
| 08-2B64 | OP784581 | AH-IW | February | 37 | 25 | 12 | 12 |
| 27-2B64 | OP784582 | AW-DB | August | 44 | 29 | 20 | 15 |
| 42-2B64 | OP784583 | H-IW | September | 40 | 22 | 22 | 7 |
| 45-2B64 | OP784584 | AH-IW | November | 26 | 14 | 14 | 23 |
| Sampling Area | Meteorological Factor | R2 | RMSE | Equation |
|---|---|---|---|---|
| DW-DB | Wind speed | 0.193 | 2.133 | %Prev = 5.33 − 0.166 × W |
| Humidity | 0.571 | 1.788 | %Prev = 7.581 − 0.145 × H | |
| Temperature | 0.230 | 13.873 | %Prev = 1.99 + 0.09 × T | |
| AW-DB | Wind speed | 0.128 | 2.857 | %Prev = 4.4 − 0.1 × W |
| Humidity | 0.571 | 1.788 | %Prev = 7.581 − 0.145 × H | |
| Temperature | 0.230 | 13.873 | %Prev = 1.99 + 090 × T | |
| AH-IW | Wind speed | 0.300 | 2.017 | %Prev = 4.4 − 0.1 × W |
| Humidity | 0.500 | 1.897 | %Prev = 6.236 − 0.109 × H | |
| Temperature | 0.183 | 14.293 | %Prev = 2.77 + 0.070 × T | |
| H-IW | Wind speed | 0.300 | 1.293 | %Prev = 4.4 − 0.10 × W |
| Humidity | 0.644 | 1.294 | %Prev = 5.50 − 0.10 × H | |
| Temperature | 0.041 | 15.482 | %Prev = 3.35 + 0.050 × T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkathiri, A.; Eifan, S.; Hanif, A.; Nour, I.; Al-Anazi, A.E.; Maniah, K.; Alotaibi, R.; Alharbi, Y. Human Adenovirus Detection and Genetic Characterization in Irrigation Water from the Riyadh Region, Saudi Arabia. Water 2023, 15, 3318. https://doi.org/10.3390/w15183318
Alkathiri A, Eifan S, Hanif A, Nour I, Al-Anazi AE, Maniah K, Alotaibi R, Alharbi Y. Human Adenovirus Detection and Genetic Characterization in Irrigation Water from the Riyadh Region, Saudi Arabia. Water. 2023; 15(18):3318. https://doi.org/10.3390/w15183318
Chicago/Turabian StyleAlkathiri, Abdulrahman, Saleh Eifan, Atif Hanif, Islam Nour, Abdullah E. Al-Anazi, Khalid Maniah, Riyadh Alotaibi, and Yazeed Alharbi. 2023. "Human Adenovirus Detection and Genetic Characterization in Irrigation Water from the Riyadh Region, Saudi Arabia" Water 15, no. 18: 3318. https://doi.org/10.3390/w15183318
APA StyleAlkathiri, A., Eifan, S., Hanif, A., Nour, I., Al-Anazi, A. E., Maniah, K., Alotaibi, R., & Alharbi, Y. (2023). Human Adenovirus Detection and Genetic Characterization in Irrigation Water from the Riyadh Region, Saudi Arabia. Water, 15(18), 3318. https://doi.org/10.3390/w15183318







