Abiotic–Biotic Interrelations in the Context of Stabilized Ecological Potential of Post-Mining Waters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Physicochemical Parameters: Sampling and Analyses
2.3. Biological Parameters: Sampling and Analyses
2.4. Biodiversity and Ecological and Trophic Indicators
2.5. Statistical Analyses
3. Results
3.1. Abiotic Parameters and Water Quality
3.2. Biotic Abundance-Based Indicators
3.3. Biotic Biomass-Based Indicators
3.4. Biodiversity and Ecological–Trophic Relations
3.5. Biotic–Abiotic Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schultze, M.; Pokrandt, K.-H.; Hille, W. Pit lakes of the Central German lignite mining district: Creation, morphometry and water quality aspects. Limnologica 2010, 40, 148–155. [Google Scholar] [CrossRef]
- Blanchette, M.L.; Lund, M.A. Pit lakes are a global legacy of mining: An integrated approach to achieving sustainable ecosystems and value for communities. Curr. Opin. Sustain. 2016, 23, 28–34. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Xu, Z.; Jiang, S.; Zhang, P.; Yang, B. Assessment of Abandoned Coal Mines as Urban Reservoirs. Mine Water Environ. 2019, 38, 215–225. [Google Scholar] [CrossRef]
- The European Parliament. Water Framework Directive (WFD) 2000/60/EC: Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy; The European Parliament: Strasbourg, France, 2000. [Google Scholar]
- Jawecki, B.; Dąbek, P.B.; Pawęska, K.; Wei, X. Estimating Water Retention in Post-mining Excavations Using LiDAR ALS Data for the Strzelin Quarry, in Lower Silesia. Mine Water Environ. 2018, 37, 744–753. [Google Scholar] [CrossRef]
- Stachowski, P.; Kraczkowska, K.; Liberacki, D.; Oliskiewicz-Krzywicka, A. Water Reservoirs as an Element of Shaping Water Resources of Post-Mining Areas. J. Ecol. Eng. 2018, 19, 217–225. [Google Scholar] [CrossRef]
- Skrzypczak, A.R.; Napiórkowska-Krzebietke, A. Identification of hydrochemical and hydrobiological properties of mine waters for use in aquaculture. Aquac. Rep. 2020, 18, 100460. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A.; Skrzypczak, A.R. A new charophyte habitat with a stabilized good ecological potential of mine water. Sci. Rep. 2021, 11, 14564. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: EU Biodiversity Strategy for 2030. Bringing Nature Back into Our Lives; COM/2020/380 Final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Claeys, G.; Tagliapietra, S.; Zachmann, G. How to Make the European Green Deal Work; Bruegel, JSTOR: New York, NY, USA, 2019; pp. 1–21. Available online: http://www.jstor.org/stable/resrep28626 (accessed on 1 December 2022).
- Hermoso, V.; Carvalho, S.B.; Giakoumi, S.; Goldsborough, D.; Katsanevakis, S.; Leontiou, S.; Markantonatou, V.; Rumes, B.; Vogiatzakis, I.N.; Yates, K.L. The EU Biodiversity Strategy for 2030: Opportunities and challenges on the path towards biodiversity recovery. Environ. Sci. Policy 2022, 127, 263–271. [Google Scholar] [CrossRef]
- Anderson, N.J.; Bennion, H.; Lotter, A.F. Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob. Chang. Biol. 2014, 20, 2741–2751. [Google Scholar] [CrossRef]
- Vishnu, D.; Dhandapani, B.; Kumar, K.S.; Balaji, G.; Mahadevan, S. Chapter 8—Removal of heavy metals from mine waters by natural zeolites. In New Trends in Removal of Heavy Metals from Industrial Wastewater; Shah, M.P., Couto, S.R., Kumar, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 161–175. [Google Scholar] [CrossRef]
- Mantero, J.; Thomas, R.; Holm, E.; Rääf, C.; Vioque, I.; Ruiz-Canovas, C.; García-Tenorio, R.; Forssell-Aronsson, E.; Isaksson, M. Pit lakes from Southern Sweden: Natural radioactivity and elementary characterization. Sci. Rep. 2020, 10, 13712. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F. Underground mining can contribute to freshwater biodiversity conservation: Allogenic succession forms suitable habitats for dragonfies. Biol. Conserv. 2012, 145, 109–117. [Google Scholar] [CrossRef]
- St-Gelais, N.F.; Jokela, A.; Beisner, B.E. Limited functional responses of plankton food webs in northern lakes following diamond mining. Can. J. Fish. Aquat. Sci. 2017, 75, 26–35. [Google Scholar] [CrossRef]
- Zdechlik, R.; Kania, J. Hydrogeochemical background and distribution of indicator ion concentrations in the region of the Bełchatów lignite deposit. Contemp. Probl. Hydrogeol. 2003, 11, 327–334. (In Polish) [Google Scholar]
- Pękala, A. The Mineral Character and Geomechanical Properties of the Transitional Rocks from the Mesozoic-Neogene Contact Zone in the Bełchatów Lignite Deposit. J. Sust. 2014, 13, 10–14. [Google Scholar] [CrossRef]
- Gogacz, M. The analysis of the quality of waters from mining plant open pit brown coal „Belchatow” joint—Stock company off the surface waterways. In Proceedings of the Mining Workshops from the Cycle “Natural Hazards in Mining”: Symposium Materials: Occasional Session: Problems of Natural Hazards in Brown Coal Mining, Bełchatów, Poland, 2–4 June 2004; Pilecka, E., Ed.; IGSMiE PAN, Cracow, Series: Symposia and Conferences. Volume 62, pp. 139–151. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1999; Available online: https://www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdf (accessed on 4 January 2019).
- Utermöhl, H. Guidance on the quantitative analysis of phytoplankton—Methods. Mitteilungen Int. Ver. Für Theor. Angew. Limnol. 1958, 9, 1–38. (In German) [Google Scholar]
- Napiórkowska-Krzebietke, A.; Kobos, J. Assessment of the cell biovolume of phytoplankton widespread in coastal and inland water bodies. Water Res. 2016, 104, 532–546. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 2013; Available online: http://www.algaebase.org (accessed on 12 July 2022).
- Flössner, D. Krebstiere, Crustacea. In Kiemen- und Blattfüsser, Branchiopoda, Fischläuse, Branchiura; VEB Gustav Fischer Verlag: Jena, Germany, 1972. [Google Scholar]
- Ejsmont-Karabin, J.; Radwan, S.; Bielańska-Grajner, I. Rotifers. Monogononta—Atlas of species. In Polish Freshwater Fauna; University of Łódź: Łódź, Poland, 2004. (In Polish) [Google Scholar]
- Błędzki, L.A.; Rybak, J.I. Freshwater crustacean zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida). In Key to Species Identification with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Bottrell, H.H.; Duncan, A.; Gliwicz, Z.M.; Grygierek, E.; Herzig, A.; Hillbricht-Ilkowska, A.; Kurasawa, H.; Larsson, P.; Weglenska, T. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976, 24, 419–456. [Google Scholar]
- Ruttner-Kolisko, A. Suggestions for biomass calculation of planktonic rotifers. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1977, 8, 71–76. [Google Scholar]
- Ejsmont-Karabin, J. Empirical equations for biomass calculation of planktonic rotifers. Pol. Arch. Hydr. 1998, 45, 513–522. [Google Scholar]
- BS EN 14757:2005; Water Quality—Sampling of Fish with Multimesh Gillnets. European Committee for Standardization: Brussels, Belgium, 2005. [CrossRef]
- Deceliere-Vergẻs, C.; Argillier, C.; Lanoiselée, C.; De Bortoli, J.; Guillard, J. Stability and precision of the fish metrics obtained using CEN multi-mesh gillnets in natural and artificial lakes in France. Fish. Res. 2009, 99, 17–25. [Google Scholar] [CrossRef]
- Olin, M.; Tiainen, J.; Kurkilahti, M.; Rask, M.; Lehtonen, H. An evaluation of gillnet CPUE as an index of perch density in small forest lakes. Fish. Res. 2015, 173, 20–25. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The mathematical theory of communication. Urbana 1949, 1–117. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Minister of Infrastructure. Regulation of the Minister of Infrastructure of 25 June 2021 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classification of the Status of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances. J. Laws 2021, item 1475. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 1 December 2022).
- Napiórkowska-Krzebietke, A.; Chybowski, Ł.; Prus, P.; Adamczyk, M. Assessment criteria and ecological classification of Polish lakes and rivers: Limitations and current state. In Polish River Basins and Lakes—Part II Biological Status and Water Management: The Handbook of Environmental Chemistry; Korzeniewska, E., Harnisz, M., Eds.; Springer: Cham, Switzerland, 2020; Volume 87, pp. 295–325. [Google Scholar]
- Burns, N.; McIntosh, J.; Scholes, P. Strategies for Managing the Lakes of the Rotorua District. New Zealand. Lake Reserv. Manag. 2005, 21, 61–72. [Google Scholar] [CrossRef]
- Napiórkowska-Krzebietke, A. Phytoplankton response to fish-induced environmental changes in a temperate shallow pond-type lake. Arch. Pol. Fish. 2017, 25, 211–264. [Google Scholar] [CrossRef]
- Malina, G.; Niezgoda, G. Redevelopment concept of open pits following lignite exploitation in the area of Belchatow. Ochrona Środowiska 2017, 39, 19–30. [Google Scholar]
- Sobolev, D.; Moore, K.; Morris, A.L. Nutrients and light limitation of phytoplankton biomass in a Turbid Southeastern reservoir. Implic. Water Qual. Southeast Nat. 2009, 8, 255–266. [Google Scholar] [CrossRef]
- Laurenceau-Cornec, E.C.; Trull, T.W.; Davies, D.M.; Bray, S.G.; Doran, J.; Planchon, F.; Carlotti, F.; Jouandet, M.-P.; Cavagna, A.-J.; Waite, A.M.; et al. The relative importance of phytoplankton aggregates and zooplankton fecal pellets to carbon export: Insights from free drifting sediment trap deployments in naturally iron-fertilized waters near the Kerguelen Plateau. Biogeosciences 2015, 12, 1007–1027. [Google Scholar] [CrossRef]
- Maggi, F. Biological flocculation of suspended particles in nutrient-rich aqueous ecosystems. J. Hydrol. 2009, 376, 116–125. [Google Scholar] [CrossRef]
- Ming, Y.; Gao, L. Flocculation of suspended particles during estuarine mixing in the Changjiang estuary-East China Sea. J. Mar. Syst. 2022, 233, 103766. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Deng, Z.; He, Q.; Safar, Z.; Chassagne, C. The role of algae in fine sediment flocculation: In-situ and laboratory measurements. Mar. Geol. 2019, 413, 71–84. [Google Scholar] [CrossRef]
- Li, Y.; Meng, J.; Zhang, C.; Ji, S.; Kong, Q.; Wang, R.; Liu, J. Bottom-up and top-down effects on phytoplankton communities in two freshwater lakes. PLoS ONE 2020, 15, e0231357. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, A. Role of constant nutrient input and mortality rate of planktivorous fish in plankton community ecosystem with instantaneous nutrient recycling. Can. Appl. Math. Q. 2012, 20, 179–207. [Google Scholar]
- Pal, S.; Chatterjee, A. Dynamics of the interaction of plankton and planktivorous fish with delay. Cogent Math. 2015, 2, 1074337. [Google Scholar] [CrossRef]
- Makler-Pick, V.; Hipsey, M.R.; Zohary, T.; Carmel, Y.; Gal, G. Intraguild Predation Dynamics in a Lake Ecosystem Based on a Coupled Hydrodynamic-Ecological Model: The Example of Lake Kinneret (Israel). Biology 2017, 6, 22. [Google Scholar] [CrossRef]
- Wissel, B.; Freier, K.; Müller, B.; Koop, J.; Benndorf, J. Moderate planktivorous fish biomass stabilizes biomanipulation by suppressing large invertebrate predators of Daphnia. Fundam. Appl. Limnol. 2000, 149, 177–192. [Google Scholar] [CrossRef]
- Reissig, M.; Queimaliños, C.; Modenutti, B.; Balseiro, E. Prey C:P ratio and phosphorus recycling by a planktivorous fish: Advantages of fish selection towards pelagic cladocerans. Ecol. Freshw. Fish 2014, 24, 214–224. [Google Scholar] [CrossRef]
- Vanni, M.J.; Bowling, A.M.; Dickman, E.M.; Hale, R.S.; Higgins, K.A.; Horgan, M.J.; Knoll, L.B.; Renwick, W.H.; Stein, R.A. Nutrient cycling by fish supports relatively more primary production as lake productivity increases. Ecology 2006, 87, 1696–1709. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Flecker, A.S.; Vanni, M.J.; Hood, J.M.; Taylor, B.W.; Thomas, S.A. Fish distributions and nutrient cycling in streams: Can fish create biogeochemical hotspots? Ecology 2008, 89, 2335–2346. [Google Scholar] [CrossRef]
- Bao, Q.; Liu, Z.; Zhao, M.; Hu, Y.; Li, D.; Han, C.; Zeng, C.; Chen, B.; Wei, Y.; Ma, S.; et al. Role of carbon and nutrient exports from different land uses in the aquatic carbon sequestration and eutrophication process. Sci. Total Environ. 2022, 813, 151917. [Google Scholar] [CrossRef]
- Vanni, M.J.; Boros, G.; McIntyre, P.B. When are fish sources vs. sinks of nutrients in lake ecosystems? Ecology 2013, 94, 2195–2206. [Google Scholar] [CrossRef]
- Kazama, T.; Urabe, J.; Yamamichi, M.; Tokita, K.; Yin, X.; Katano, I.; Doi, H.; Yoshida, T.; Hairston, N.G., Jr. A unified framework for herbivore-to-producer biomass ratio reveals the relative influence of four ecological factors. Commun. Biol. 2021, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Goździejewska, A.M.; Skrzypczak, A.R.; Koszałka, J.; Bowszys, M. Effects of recreational fishing on zooplankton communities of drainage system reservoirs at an open-pit mine. Fish. Manag. Ecol. 2020, 27, 279–291. [Google Scholar] [CrossRef]
- Lyche, A.; Faafeng, B.A.; Brabrand, Å. Predictability and possible mechanisms of plankton response to reduction of planktivorous fish. Hydrobiologia 1990, 200, 251–261. [Google Scholar] [CrossRef]
- Maszczyk, P.; Bartosiewicz, M.; Jurkowski, J.E.; Wyszomirski, T. Interference competition in a planktivorous fish (Rutilus rutilus) at different prey densities and temperatures. Limnology 2014, 15, 155–162. [Google Scholar] [CrossRef]



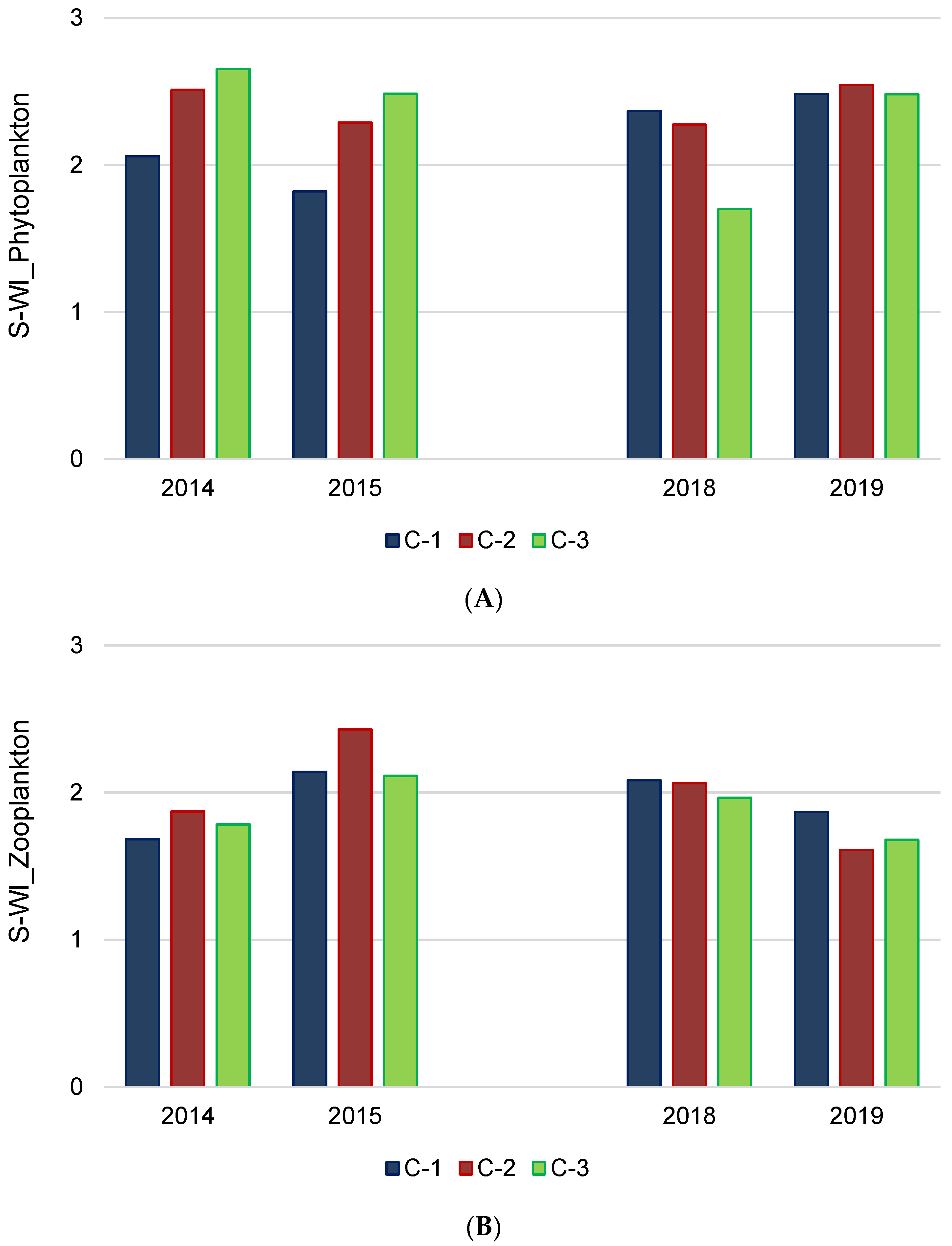
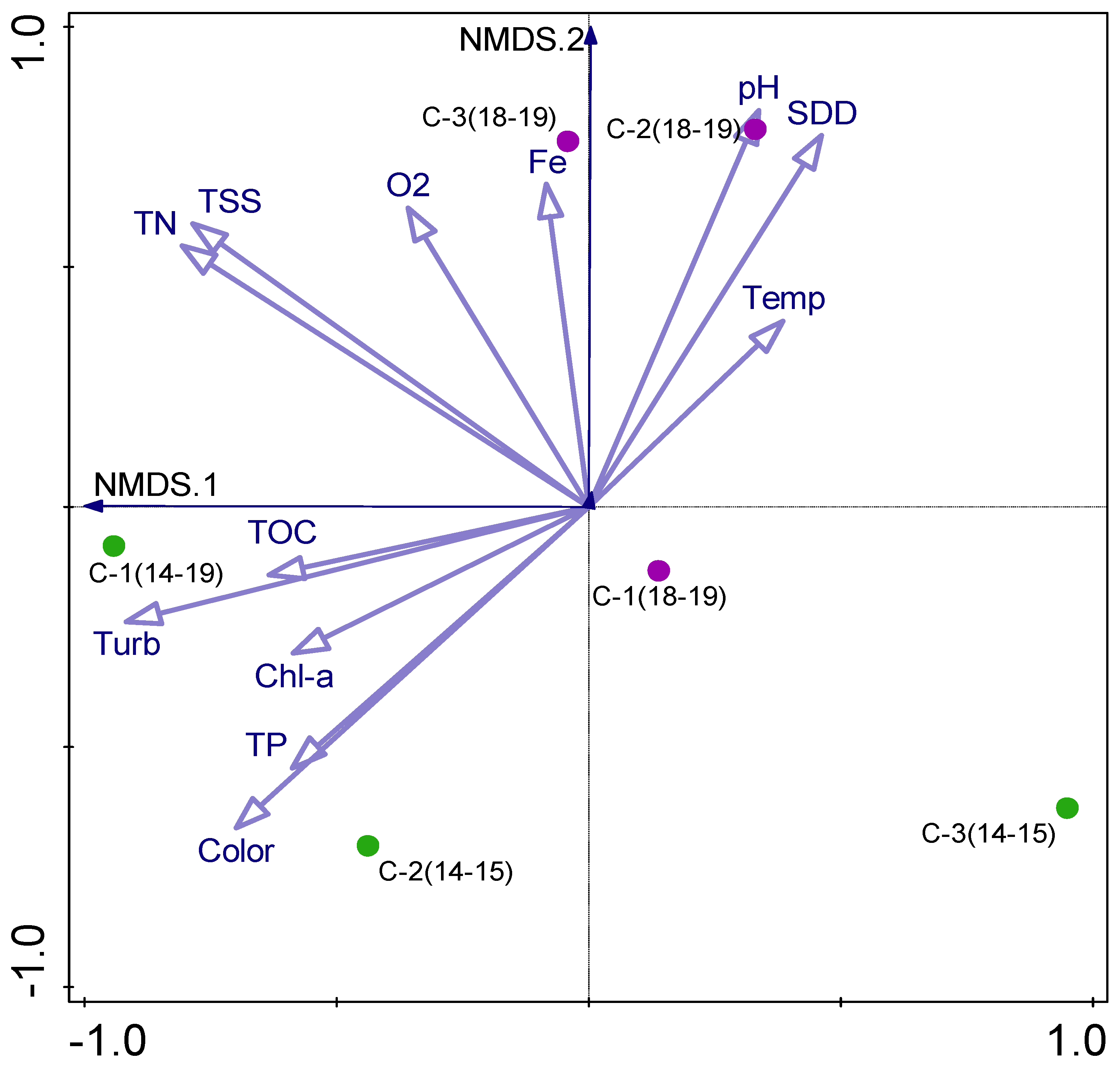
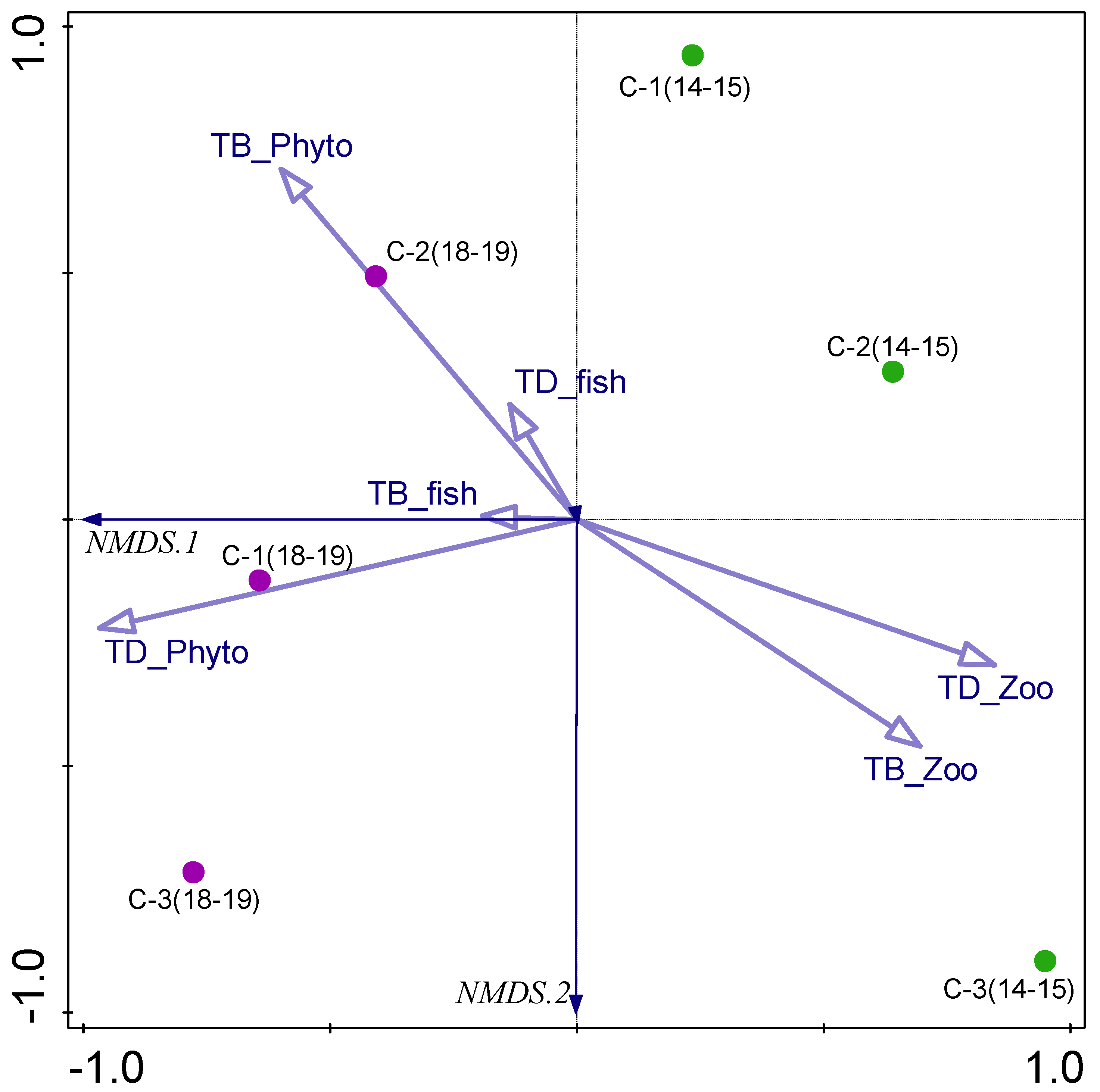
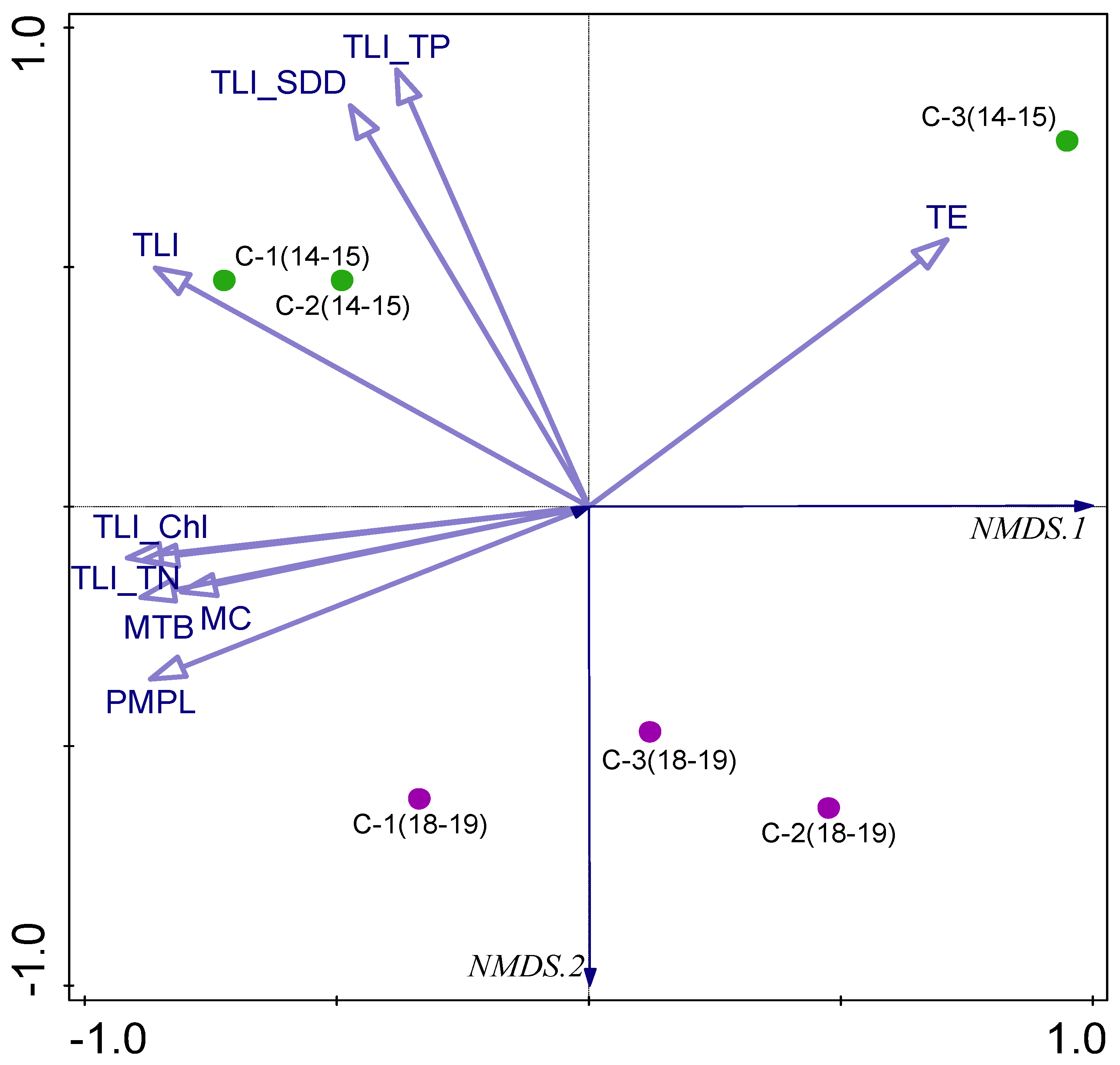
| Parameter | 2014–2015 | 2018–2019 | ||||||
|---|---|---|---|---|---|---|---|---|
| Inflow | C-1 | C-2 | C-3 | Inflow | C-1 | C-2 | C-3 | |
| 1 DO (mg L−1) | 7.78 A ±0.51 | 8.91 AB ±0.52 | 8.59 AB ±0.49 | 8.34 AB ±1.05 | 8.36 AB ±1.09 | 9.50 B ±1.30 | 9.51 B ±1.17 | 9.45 AB ±1.38 |
| 2 pH | 7.51 A ±0.16 | 7.57 AB ±0.13 | 7.54 AB ±0.14 | 7.58 AB ±0.17 | 7.61 AB ±0.11 | 7.78 B ±0.12 | 7.79 B ±0.13 | 7.79 B ±0.09 |
| 3 TDS (mg L−1) | 664.2 AB ±7.9 | 659.5 AB ±11.7 | 659.1 AB ±3.7 | 655.6 A ±9.1 | 707.5 AB ±58.4 | 702.3 AB ±40.1 | 719.1 B ±45.2 | 710.0 AB ±47.2 |
| 4 ISS (mg L−1) | 9.1 A ±4.5 | 10.6 A ±5.0 | 11.2 A ±3.9 | 6.4 AB ±4.2 | 6.0 AB ±3.6 | 4.2 AB ±2.9 | 4.5 AB ±2.6 | 3.7 B ±2.1 |
| 5 TOC (mg L−1) | 2.02 AB ±0.70 | 3.35 B ±1.33 | 2.52 AB ±0.59 | 2.57 AB ±0.49 | 1.65 A ±0.54 | 3.10 B ±0.63 | 2.58 AB ±0.62 | 2.81 B ±0.67 |
| 6 Chl a (µg L−1) | 2.20 AB ±2.31 | 6.10 A ±4.37 | 4.93 AB ±2.56 | 3.22 AB ±2.26 | 1.47 B ±1.13 | 4.65 AB ±3.91 | 2.49 AB ±1.86 | 3.67 AB ±2.20 |
| 7 TP (mg L−1) | 0.132 A ±0.039 | 0.141 A ±0.049 | 0.123 A ±0.029 | 0.106 A ±0.046 | 0.089 B ±0.026 | 0.074 B ±0.040 | 0.074 B ±0.026 | 0.076 B ±0.032 |
| 8 HCO3− (mg L−1) | 239.6 AB ±12.5 | 216.0 A ±15.2 | 231.4 AB ±14.7 | 228.0 AB ±18.5 | 247.9 B ±33.2 | 213.5 AB ±34.9 | 228.1 AB ±36.4 | 200.5 AB ±46.4 |
| 9 SO4− (mg L−1) | 169.8 A ±29.2 | 157.0 A ±44.9 | 153.9 A ±29.2 | 141.3 A ±28.7 | 256.7 B ±75.2 | 238.8 B ±58.5 | 235.9 B ±70.1 | 236.2 B ±63.3 |
| 10 Ca2+ (mg L−1) | 123.0 AB ±17.2 | 107.3 A ±19.0 | 114.7 A ±21.0 | 112.9 A ±24.5 | 157.5 B ±55.1 | 153.9 B ±56.9 | 151.4 B ±54.0 | 137.6 B ±50.3 |
| Parameter | 2014–2015 | 2018–2019 | |||||
|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | C-1 | C-2 | C-3 | ||
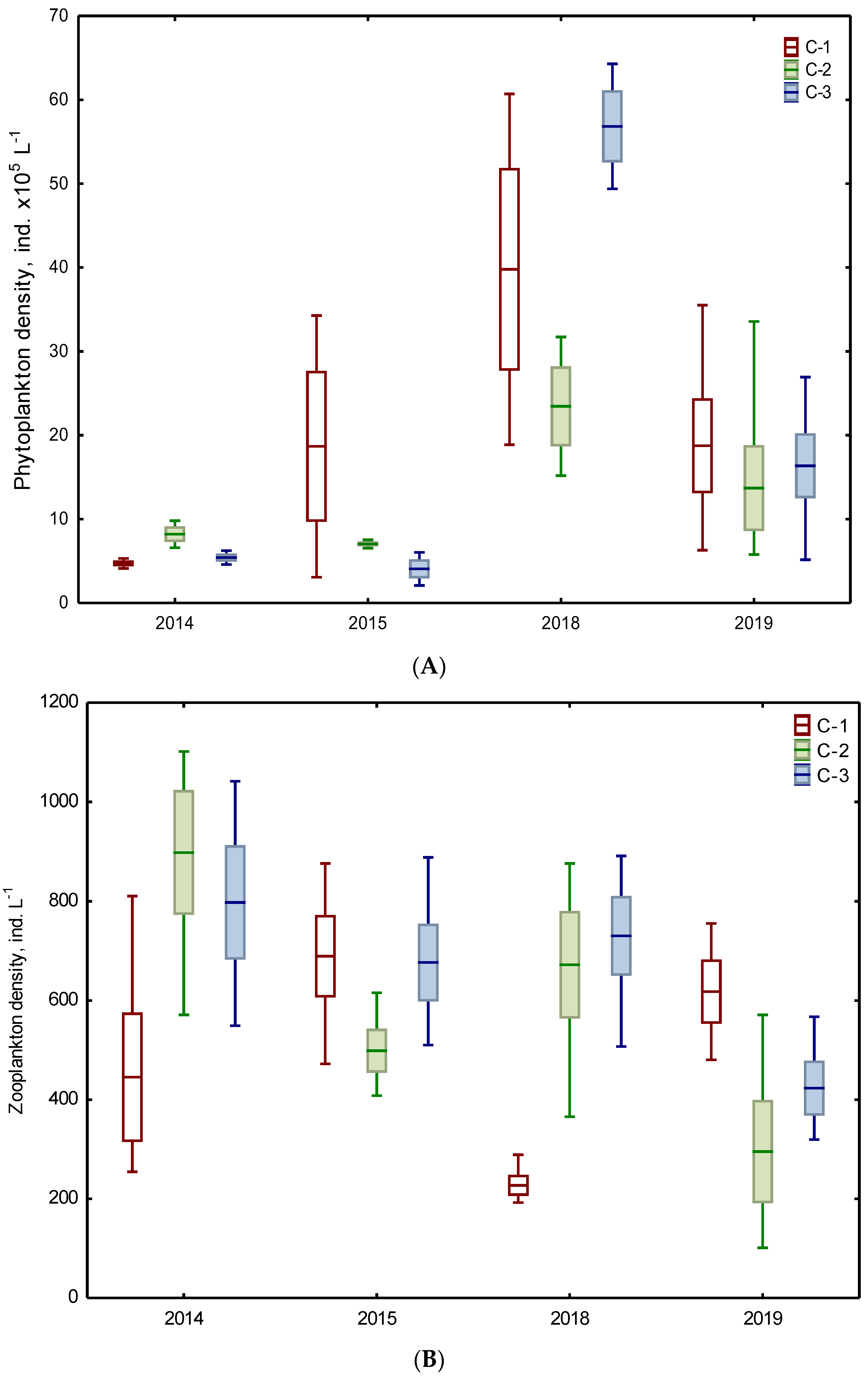
| Phytoplankton | 1 density ind. x105 L−1 | 11.69 ABC ±12.49 | 7.61 ABC ±1.24 | 4.73 A ±1.54 | 26.63 B ±18.30 | 17.36 ABC ±10.74 | 31.54 BC ±22.30 |
| 2 biomass mg L−1 | 4.61 A ±4.68 | 2.11 A ±0.72 | 1.19 A ±0.21 | 3.33 A ±3.62 | 4.20 A ±6.92 | 2.32 A ±2.64 | |
| Zooplankton | 3 density ind. L−1 | 567.38 A ±241.01 | 698.56 A ±275.80 | 737.13 A ±193.80 | 422.63 A ±227.17 | 483.69 A ±281.61 | 576.81 A ±208.23 |
| 4 biomass mg L−1 | 0.28 A ±0.09 | 0.36 A ±0.24 | 0.43 A ±0.23 | 0.20 A ±0.09 | 0.28 A ±0.18 | 0.37 A ±0.28 | |
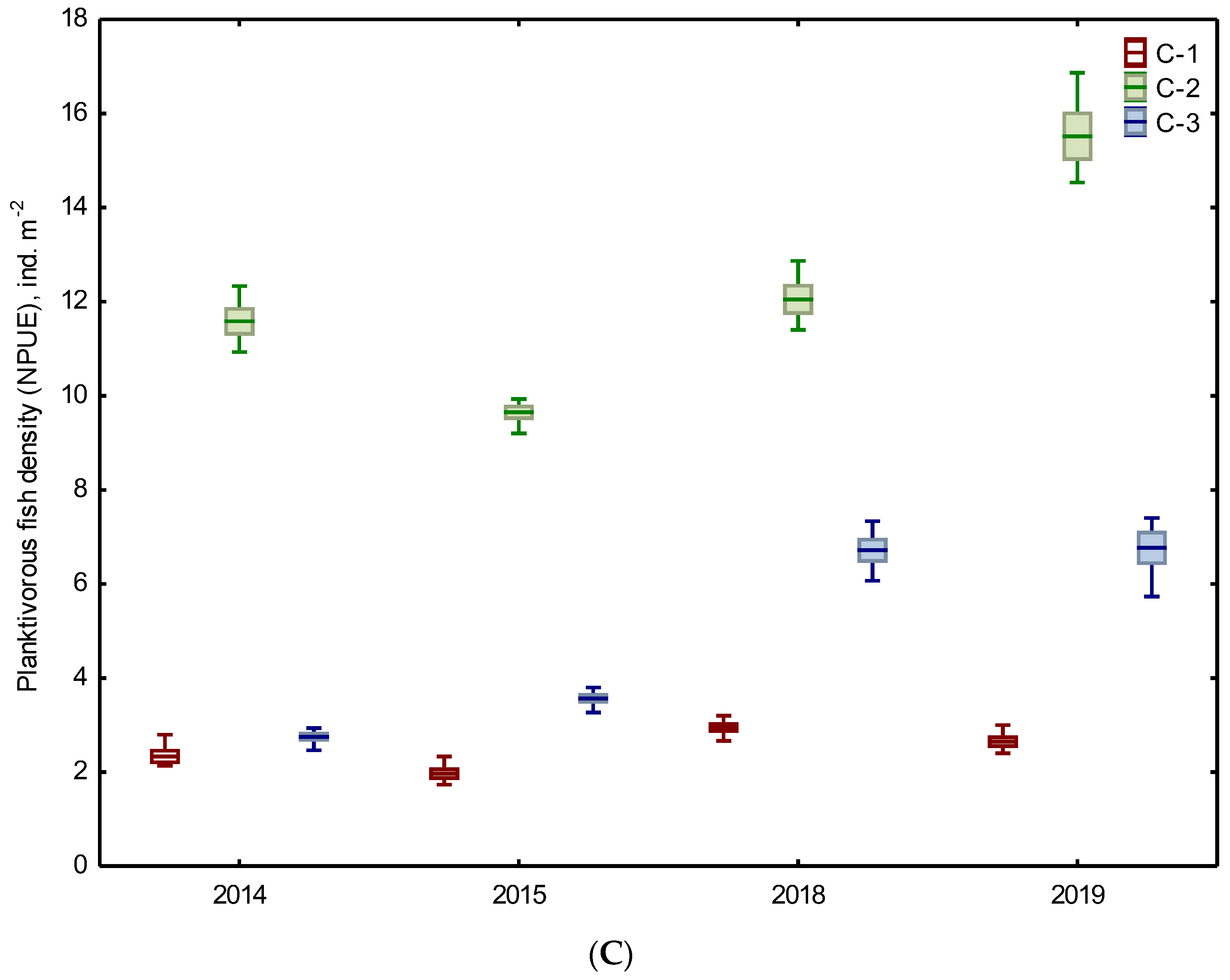
| Planktivorous fish | 5 NPUE ind. m−2 | 2.15 A ±0.33 | 10.6 BC ±1.12 | 3.16 ABC ±0.48 | 2.80 A ±0.27 | 13.78 BC ±2.02 | 6.74 BC ±0.59 |
| 6 WPUE g m−2 | 9.78 A ±1.46 | 48.19 BC ±3.86 | 18.26 BC ±3.8 | 12.72 A ±1.28 | 68.05 BC ±7.55 | 43.86 BC ±5.13 | |
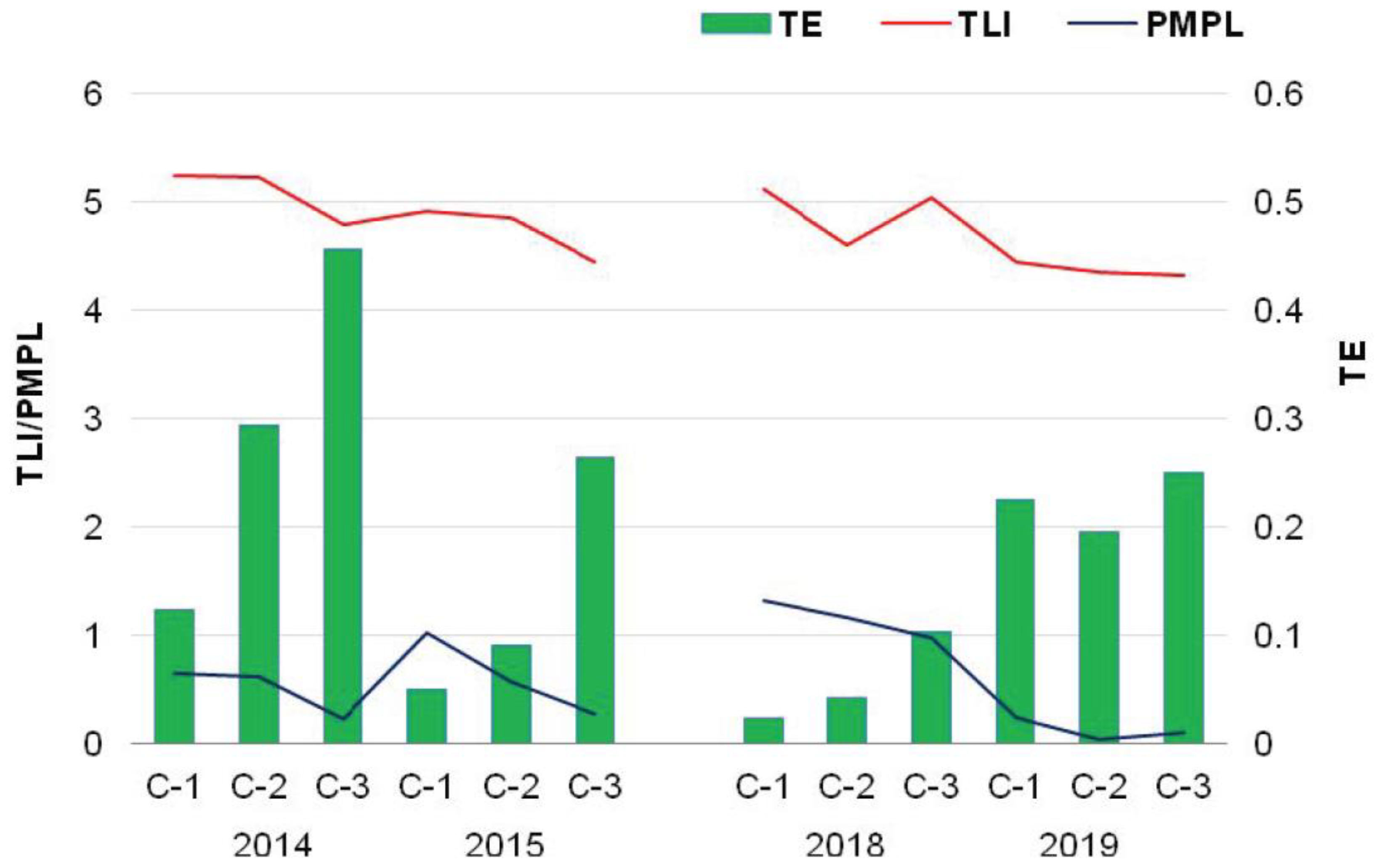
| Year | Chamber | Trophic Level Index | ||||
|---|---|---|---|---|---|---|
| TLI-TP | TLI-TN | TLI-SDD | TLI-Chl | TLI | ||
| 2014 | C-1 | 6.8 | 4.4 | 5.6 | 4.2 | 5.3 |
| C-2 | 6.7 | 4.0 | 5.8 | 4.4 | 5.2 | |
| C-3 | 6.6 | 3.9 | 5.5 | 3.2 | 4.8 | |
| 2015 | C-1 | 6.1 | 4.2 | 5.8 | 3.6 | 4.9 |
| C-2 | 6.1 | 4.0 | 5.9 | 3.4 | 4.9 | |
| C-3 | 5.8 | 3.3 | 5.6 | 3.1 | 4.5 | |
| 2018 | C-1 | 5.9 | 4.3 | 5.7 | 4.6 | 5.1 |
| C-2 | 5.8 | 3.8 | 5.5 | 3.3 | 4.6 | |
| C-3 | 5.9 | 4.6 | 5.6 | 4.1 | 5.1 | |
| 2019 | C-1 | 5.6 | 3.7 | 5.0 | 3.5 | 4.5 |
| C-2 | 5.5 | 3.8 | 5.0 | 3.1 | 4.4 | |
| C-3 | 5.4 | 3.6 | 5.0 | 3.3 | 4.3 | |
| Year | Chamber | MTB 1 | MCB 2 | MC 3 | PMPL 4 | Ecological Classification | |
|---|---|---|---|---|---|---|---|
| Class | Potential | ||||||
| 2014 | C-1 | 1.13 | 0.10 | 0.43 | 0.65 | I | maximum |
| C-2 | 0.93 | 0.10 | 0.58 | 0.62 | I | maximum | |
| C-3 | 0.50 | 0.10 | 0.00 | 0.22 | I | maximum | |
| 2015 | C-1 | 2.49 | 0.10 | 0.00 | 1.02 | II | good |
| C-2 | 1.38 | 0.10 | 0.00 | 0.57 | I | maximum | |
| C-3 | 0.62 | 0.10 | 0.00 | 0.27 | I | maximum | |
| 2018 | C-1 | 2.45 | 0.10 | 0.81 | 1.32 | II | good |
| C-2 | 2.84 | 0.10 | 0.00 | 1.16 | II | good | |
| C-3 | 2.05 | 0.10 | 0.35 | 0.98 | I | maximum | |
| 2019 | C-1 | 0.53 | 0.10 | 0.00 | 0.23 | I | maximum |
| C-2 | 0.02 | 0.10 | 0.00 | 0.03 | I | maximum | |
| C-3 | 0.19 | 0.10 | 0.00 | 0.10 | I | maximum | |
| Parameter | TB_Phyto | TD_Phyto | TB_Zoo | TD_Zoo | TB_Fish | TD_Fish |
|---|---|---|---|---|---|---|
| SDD | −0.575 | n.s. | n.s. | n.s. | n.s. | n.s. |
| TSS | 0.520 | 0.467 | n.s. | n.s. | n.s. | n.s. |
| Turbidity | 0.594 | 0.417 | n.s. | n.s. | n.s. | n.s. |
| Color | 0.592 | n.s. | n.s. | n.s. | n.s. | n.s. |
| pH | n.s. | 0.706 | n.s. | n.s. | n.s. | n.s. |
| TP | 0.452 | n.s. | n.s. | n.s. | n.s. | n.s. |
| Porg | 0.447 | n.s. | n.s. | n.s. | n.s. | n.s. |
| TN | 0.483 | n.s. | n.s. | n.s. | n.s. | n.s. |
| NH43− | 0.507 | n.s. | n.s. | n.s. | n.s. | n.s. |
| NO3− | 0.624 | n.s. | n.s. | n.s. | n.s. | n.s. |
| TOC | n.s. | n.s. | n.s. | n.s. | −0.496 | −0.504 |
| Fe | 0.552 | n.s. | n.s. | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napiórkowska-Krzebietke, A.; Skrzypczak, A.R.; Kicińska, A. Abiotic–Biotic Interrelations in the Context of Stabilized Ecological Potential of Post-Mining Waters. Water 2023, 15, 3328. https://doi.org/10.3390/w15193328
Napiórkowska-Krzebietke A, Skrzypczak AR, Kicińska A. Abiotic–Biotic Interrelations in the Context of Stabilized Ecological Potential of Post-Mining Waters. Water. 2023; 15(19):3328. https://doi.org/10.3390/w15193328
Chicago/Turabian StyleNapiórkowska-Krzebietke, Agnieszka, Andrzej R. Skrzypczak, and Alicja Kicińska. 2023. "Abiotic–Biotic Interrelations in the Context of Stabilized Ecological Potential of Post-Mining Waters" Water 15, no. 19: 3328. https://doi.org/10.3390/w15193328
APA StyleNapiórkowska-Krzebietke, A., Skrzypczak, A. R., & Kicińska, A. (2023). Abiotic–Biotic Interrelations in the Context of Stabilized Ecological Potential of Post-Mining Waters. Water, 15(19), 3328. https://doi.org/10.3390/w15193328








