Re-Establishing Naturally Reproducing Sturgeon Populations in the Caspian Basin: A Wicked Problem in the Ural River
Abstract
:1. Introduction
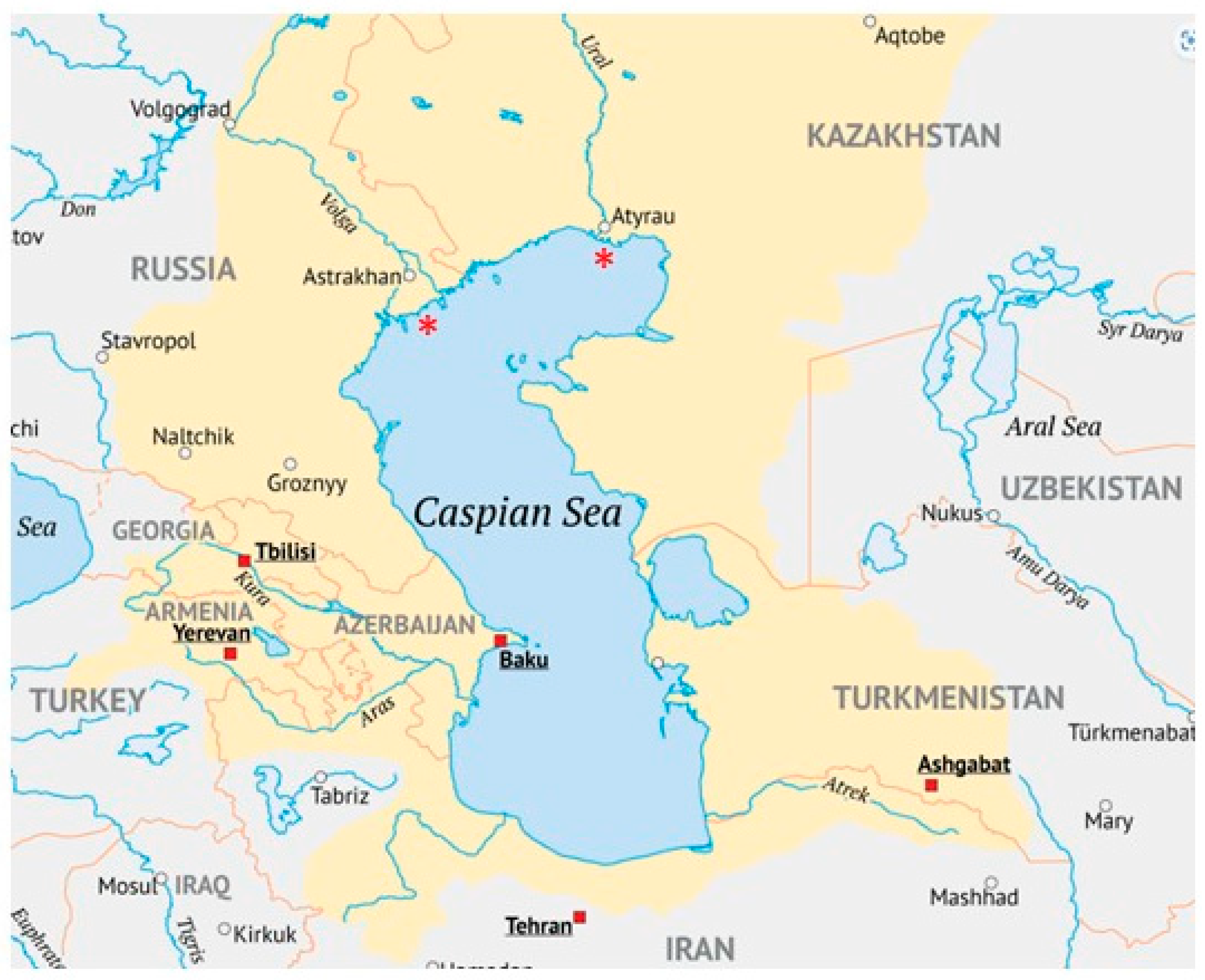
2. The Caspian Basin as an Environment for Sturgeon
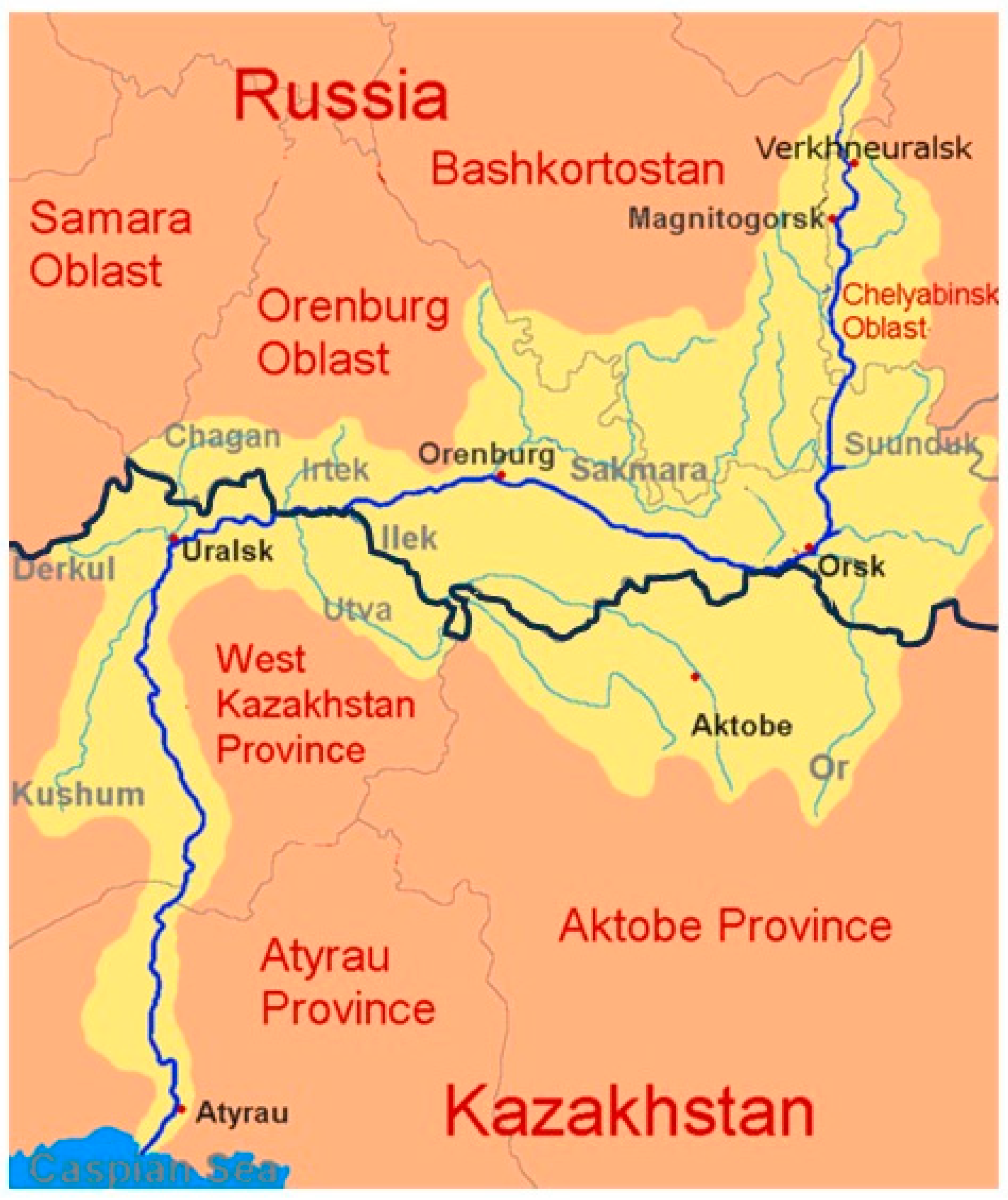
3. Preservation of Naturally Reproducing Sturgeon in the Caspian Basin: The Ural River
3.1. Habitat
3.2. Overfishing
3.3. Stocking
3.4. Transboundary Cooperation
4. The Wicked Nature of Re-Establishing Sturgeon in the Ural River
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D. Species, distributions and conservation of Acipenseriformes. Tsinghua Sci. Technol. 2002, 7, 416–420. [Google Scholar]
- Beamesderfer, R.C.P.; Farr, R.A. Alternatives for the protection and restoration of sturgeons and their habitat. Environ. Biol. Fishes 1997, 48, 407–417. [Google Scholar] [CrossRef]
- Saffron, I. Caviar; Broadway Books: New York, NY, USA, 2002. [Google Scholar]
- Birstein, V.J.; Waldman, J.R.; Bemis, W.E. (Eds.) Sturgeon Biodiversity and Conservation; Kluwer: New York, NY, USA, 2002. [Google Scholar]
- Pikitch, E.K.; Doukakis, P.; Lauck, L.; Chakrabarty, P.; Erickson, D.L. Status, trends and management of sturgeon and paddlefish fisheries. Fish Fish. 2005, 6, 233–265. [Google Scholar] [CrossRef]
- van Uhm, D.; Siegel, D. The illegal trade in black caviar. Trends Org. Crime 2016, 19, 67–87. [Google Scholar] [CrossRef]
- Aghilinejhad, S.M.; Gorgin, S.; van Uhm, D.; Joolaie, R.; Ghorbani, R.; Paighambari, S.Y.; Mohammadi, J.; Jalali, A. What are the drivers of the occurrence of illegal fishing and conservation barriers of sturgeons in the Caspian Sea? Aquat. Conserv. 2018, 28, 690–701. [Google Scholar] [CrossRef]
- Couto, T.B.A.; Julian, D.O. Global proliferation of small hydropower plants–science and policy. Front. Ecol. Environ. 2018, 16, 91–100. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Frenken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef]
- Thieme, M.L.; Tickner, D.; Grill, G.; Carvallo, J.P.; Goichot, M.; Hartmann, J.; Higgins, J.; Lehner, B.; Mulligan, M.; Nilsson, C. Navigating trade-offs between dams and river conservation. Glob. Sust. 2021, 4, e17. [Google Scholar] [CrossRef]
- Jungwirth, M. River continuum and fish migration-going beyond the longitudinal river corridor in understanding ecological integrity. In Fish Migration and Fish Bypasses; Jungwirth, M., Schmutz, S., Weiss, S., Eds.; Fishing News Books: Oxford, UK, 1998; pp. 19–32. [Google Scholar]
- Friedrich, T. Danube sturgeons: Past and future. In Riverine Ecosystem Management; Schmutz, S., Sendzimir, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 507–518. [Google Scholar] [CrossRef]
- Gessner, J.; Tautenhahn, M.; Spratte, S.; Arndt, G.M.; von Nordheim, H. Development of a German action plan for the restoration of the European sturgeon Acipenser sturio L.—Implementing international commitments on a national scale. J. Appl. Ichthyol. 2011, 27, 192–198. [Google Scholar] [CrossRef]
- Sandu, C.; Reinartz, R.; Bloesch, J. (Eds.) “Sturgeon 2020”: A Program for the Protection and Rehabilitation of Danube Sturgeons. Danube Sturgeon Task Force (DSTF) and EU Strategy for the Danube River (EUSDR). Available online: researchgate.net/publication/292328373_Sturgeon_2020_A_program_for_the_protection_and_rehabilitation_of_Danube_sturgeons (accessed on 29 June 2023).
- Pollock, M.S.; Carr, M.; Kreitals, N.M.; Phillips, I.D. Review of a species in peril: What we do not know about lake sturgeon may kill them. Environ. Rev. 2014, 23, 30–43. [Google Scholar] [CrossRef]
- Dadswell, M.J. A review of the status of Atlantic sturgeon in Canada, with comparisons to populations in the United States and Europe. Fisheries 2006, 31, 218–229. [Google Scholar] [CrossRef]
- Li, J.; Du, H.; Wu, J.; Zhang, H.; Shen, L.; Wie, Q. Foundation and prospects of wild population reconstruction of Acipenser dabryanus. Fishes 2021, 6, 55. [Google Scholar] [CrossRef]
- China Continues to Strengthen Protection of Chinese Sturgeon. Available online: english.news.cn/20220410/1b113fbb9ae84fdf9c1a73cd1c4a98bf/c.html (accessed on 29 June 2023).
- Secor, D.H.; Areflev, V.; Nikolaev, A.; Sharov, A. Restoration of sturgeons: Lessons from the Caspian Sea sturgeon ranching programme. Fish Fish. 2000, 1, 215–230. [Google Scholar] [CrossRef]
- Leroy, S.A.G.; Lahijani, H.A.K.; Crétaux, J.-F.; Aladin, N.V.; Plotnikov, I.S. Past and current changes in the largest lake in the world: The Caspian Sea. In Large Asian Lakes in a Changing World; Mischke, S., Ed.; Springer: Cham, Switzerland, 2020; pp. 65–108. [Google Scholar] [CrossRef]
- The IUCN Red List of Threatened Species, Version 2021-3. Available online: www.iucnredlist.org/ (accessed on 9 July 2022).
- Babushkin, N.Y.; Borzenko, M.P. Sturgeons of the Caspian Sea; Pishchepromizdat: Moscow, Soviet Union, 1951; p. 67. (In Russian) [Google Scholar]
- Korobochkina, Z.S. Main stages of development of sturgeon fishery in the Caspian basin. Trudy VNIRO 1964, 52, 59–86. (In Russian) [Google Scholar]
- Abbasov, R.; Karimov, R.; Jafarova, N. The Caspian Sea and its values in Azerbaijan. In Ecosystem Services in Azerbaijan; Springer: Cham, Switzerland, 2022; pp. 1–28. [Google Scholar] [CrossRef]
- MacGregor, J.; Karousakis, K.; Groom, B. Using Economic Incentives to Conserve CITES-Listed Species; Earthprint Ltd.: Stevenage, UK, 2004. [Google Scholar]
- Sánchez, W.A. Kazakhstan’s green strategy: Keeping the steppe green. SAIS Rev. Int. Aff. 2023, 43, 23–38. [Google Scholar] [CrossRef]
- Ural River Delta and Adjacent Caspian Sea Coast. Available online: https://rsis.ramsar.org/ris/1856 (accessed on 7 May 2023).
- Lagutov, V.; Lagutov, V. The Ural River sturgeons: Population dynamics, catch, reasons for decline and restoration strategies. In Rescue of Sturgeon Species in the Ural River Basin; Lagutov, V., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 193–276. [Google Scholar]
- Sicuro, B. The future of caviar production on the light of social changes: A new dawn for caviar? Rev. Aquacult. 2018, 11, 204–219. [Google Scholar] [CrossRef]
- Khodorevskaya, R.P.; Ruban, G.I.; Pavlov, D.S. Behaviour, Migrations, Distribution, and Stocks of Sturgeons in the Volga-Caspian Basin; Books on Demand GmbH: Norderstedt, Germany, 2009. [Google Scholar]
- Eremkina, T.V.; Yarushina, M.I. Ural River basin. In Rivers of Europe, 2nd ed.; Tockner, K., Zarfl, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 883–899. [Google Scholar] [CrossRef]
- Bronzi, P.; Rosenthal, H.; Gessner, J. Global sturgeon aquaculture production: An overview. J. Appl. Ichthyol. 2011, 27, 169–175. [Google Scholar] [CrossRef]
- Shadrina, E. The great Caspian caviar game. Secur. Index 2012, 13, 55–78. [Google Scholar] [CrossRef]
- Pouncy, C.J. (Ed.) The Domostroi; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Derzhavin, A.N. Reproduction of Sturgeon Stocks; Izd. an Azerbaidzhanskoi SSR: Baku, Soviet Union, 1947; p. 243. (In Russian) [Google Scholar]
- Borodine, N. The Ural Cossacks and their fisheries. Pop. Sci. Mon. 1893, 43, 767–779. [Google Scholar]
- Hartley, J.M. The Volga; Yale University Press: New Haven, CT, USA, 2021. [Google Scholar]
- Pianciola, N. Cossacks and sturgeons: Fisheries, colonization, and science around the Aral Sea (1873–1906). J. Econ. Soc. Hist. Orient 2019, 62, 626–673. [Google Scholar] [CrossRef]
- Ivanov, V.P.; Belyaeva, V.N.; Vlasenko, A.D. Regional distribution of resources of the Caspian Sea. Fishery 1995, 2, 18–21. (In Russian) [Google Scholar]
- Kozhin, N.I. Sturgeons of the USSR and their reproduction. Trudy VNIRO 1964, 52, 21–58. (In Russian) [Google Scholar]
- Ruban, G.I.; Khodorevskaya, R.P. Caspian Sea sturgeon fishery: A historic overview. J. Appl. Ichthyol. 2011, 27, 199–208. [Google Scholar] [CrossRef]
- Golovanov, F. Caspian sturgeon. Rybn. Hozâjstvo 1940, 2, 19–21. (In Russian) [Google Scholar]
- Khodorevskaya, R.P.; Kalmykov, B.A.; Zhilkin, A.A. Present state of Caspian sturgeon reserves and measures on its conservation. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2012, 1, 99–106. (In Russian) [Google Scholar]
- Khodorevskaya, R.P.; Dovgopol, G.F.; Zhuravleva, O.L.; Vlasenko, A.D. Present status of commercial stocks of sturgeons in the Caspian Sea basin. Environ. Biol. Fishes 1997, 48, 209–218. [Google Scholar] [CrossRef]
- Maltsev, S.A. Conservation of sturgeon fish in lower Volga. In Biology, Conservation and Sustainable Development of Sturgeons; Carmona, R., Domezain, A., Garcia-Gallego, M., Hernando, J.A., Rodríguez, F., Ruiz-Rejόn, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 265–273. [Google Scholar]
- Raspopov, V.M.; Vershchev, P.V.; Novikova, A.S. The relationship between sturgeon fecundity and reproduction in the Volga River. In Proceedings of the 2nd International Symposium, Moscow, Russia, 6–11 September 1993; Gershanovich, A.D., Smith, T.I.J., Eds.; VNIRO Publishing: Moscow, Russia, 1995; pp. 158–163. (In Russian). [Google Scholar]
- Shylov, V.I.; Khazov, Y.K.; Ivoilova, N.K. Specific weight of sturgeons in ichthyofauna of Saratov and Volgograd water bodies, catch structure and allocation. Sci. Rep. Saratov Branch. GosNIORKH 1971, 11, 5–51. (In Russian) [Google Scholar]
- Kotenev, B.N. Hydrological and production characteristics of the main basins for reproduction and fattening of sturgeons. In Biology, Conservation and Sustainable Development of Sturgeons; Carmona, R., Domezain, A., Garcia-Gallego, M., Hernando, J.A., Rodríguez, F., Ruiz-Rejόn, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 345–357. [Google Scholar]
- Ruban, G.I.; Khodorevskaya, R.P.; Shatunovsky, M.I. On the impact of dam construction in the Volga-Caspian basin on the state of sturgeon populations and on measures to conserve their numbers. Prob. Fish. 2018, 19, 139–149. (In Russian) [Google Scholar]
- Shilov, V.I.; Khazov, I.K. Reproduction of sturgeons in Volgograd and Saratov reservoirs after construction of Saratov hydroelectric power plant. In Proceedings of the Joint Session CNIORKh and AzNIIRKh, Astrakhan, Soviet Union, 1971; pp. 123–124. (In Russian). [Google Scholar]
- Lagutov, V. The Mechanism of Extermination of Fish Stocks in South Russia and Possibilities of Their Restoration; Donskaya Rech: Novocherkassk, Russia, 1995. (In Russian) [Google Scholar]
- Tokareva, A.A. Changes in the Water-Salt Regime of Natural Complexes of the Lower Volga. Ph.D. Thesis, Astrakhan State Technical University, Astrakhan, Russia, 2019. (In Russian). [Google Scholar]
- Korotaev, V.N.; Babich, D.B.; Chalov, R.S. Atlas of Channel Morphodynamics of the Lower Volga; Izd. Moskovskogo Universiteta: Moskow, Russia, 2009. (In Russian) [Google Scholar]
- Gorelits, O.V.; Zemlyanov, I.V.; Sapozhnikova, A.A. Multiyear and seasonal variability of basic parameters of hydrological regime of the Lower Volga in Volgograd. In Proceedings of the SPNA in the Lower Volga as the Most Important Mechanism of Biodiversity Conservation: Results, Problems and Prospects, Volgograd, Russia, 2010; pp. 186–198. (In Russian). [Google Scholar]
- Polonsky, V.F.; Ostroumova, L.P. Study of hydrological regime of the Volga delta. In Proceedings of the Volga Water Resources: Present and Future, Management Problems, Astrakhan State University, Astrakhan, Russia, 3–5 October 2007; pp. 212–224. (In Russian). [Google Scholar]
- Dubinina, V.G.; Kozlitina, S.V. Water resources management of the southern rivers of Russia with reference to fisheries requirements. Fish. Manag. Ecol. 2004, 7, 157–165. [Google Scholar] [CrossRef]
- Raspopov, V.M. Ecological Bases for the Reproduction of Sturgeons in the Conditions of the Modern Runoff of the River Volga. Ph.D. Thesis, All Russian Institute of Fisheries and Oceanography (VNIRO), Moscow, Russia, 2001. (In Russian). [Google Scholar]
- Avakyan, A.B.; Poddubny, A.G. Reservoirs of the Volga-Kama cascade of hydropower plants and ways to improve their ecological condition. Izv. Akad. Nauk. SSSR Ser. Geograf. 1994, 3, 23–28. (In Russian) [Google Scholar]
- Budkov, S.T. Hydropower of the Volga region and its impact on the nature of the region. Geog. Nat. Resour. 1995, 4, 43–46. (In Russian) [Google Scholar]
- Danilov-Danilyan, V.I. Water Resources of the World and Prospects of Water Complex of Russia; Levko Printing House: Moscow, Russia, 2009; p. 88. (In Russian) [Google Scholar]
- Loganov, V.V.; Gelashvili, D.B. Harm to aquatic biological resources in the reservoirs of the Volga-Kama cascade caused by hydroelectric power plants. Princ. Èkol. 2016, 4, 4–25. (In Russian) [Google Scholar]
- Usova, T.V. Survival of naturally spawned stellate sturgeon during downstream migration in the Volga River. Russ. J. Ecol. 2009, 40, 374–376. [Google Scholar] [CrossRef]
- Lushvin, P.V.; Karpinsky, M.G. Causes of sharp reductions in zoobenthos biomass and their consequences. Rybn. Hozâjstvo 2009, 5, 65–69. (In Russian) [Google Scholar]
- Ruban, G.I.; Khodorevskaya, R.P.; Shatunovskii, M.I. Anthropogenic and environmental factors of natural reproduction decline in Beluga (Huso huso), Russian sturgeon (Acipenser gueldenstaedtii) and stellate sturgeon (A. stellatus) at the Volga-Caspian region. Vopr. Rybolov. 2017, 18, 7–20. (In Russian) [Google Scholar]
- Vlasenko, A.D.; Bulgakova, T.I.; Lepilina, I.N.; Konopleva, I.V.; Safaraliev, I.A. History and status of sturgeon stocks (Acipenseridae) in the Caspian basin. Bull. Mosc. State Tech. Univ. 2020, 23, 105–114. (In Russian) [Google Scholar] [CrossRef]
- Caspian Environment Programme. Available online: www.unep.org/explore-topics/oceans-seas/what-we-do/working-regional-seas/regional-seas-programmes/caspian-sea (accessed on 19 June 2023).
- Lagutov, V.; Lagutov, V. Establishment of the International Ural Sturgeon Park to secure sturgeon conservation and to facilitate sustainable integrated water management. In Rescue of Sturgeon Species in the Ural River Basin; Lagutov, V., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 301–323. [Google Scholar]
- Rosenthal, H.; Pourkazemi, M.; Bruch, R. The 5th International Symposium on Sturgeons: A conference with major emphasis on conservation, environmental mitigation and sustainable use of the sturgeon resources. J. Appl. Ichthyol. 2006, 22, 1–4. [Google Scholar] [CrossRef]
- Chibilev, A.A. The Ural Basin: History, Geography, Ecology; Ural Branch of the Russian Academy of Sciences: Ekaterinburg, Russia, 2008. (In Russian) [Google Scholar]
- Kiseleva, R.A. Biological Productivity of the Caspian Sea; Nauka: Moscow, Soviet Union, 1974. (In Russian) [Google Scholar]
- Padalko, Y.A. Socio-economic vulnerability of the population and economy of the regions of the Russian part of the Ural River basin from floods. Succ. Mod. Nat. Sci. 2016, 12, 439–443. (In Russian) [Google Scholar]
- Magritsky, D.V.; Evstigneev, V.M.; Yumina, N.M.; Toropov, P.A.; Kenzhebayeva, A.; Ermakova, G. Changes of runoff in the Ural River basin. Mosc. Univ. Bull. Ser. 5 Geog. 2018, 1, 90–101. (In Russian) [Google Scholar]
- Kim, A.I.; Murzashev, T.K.; Antipova, N.V. Hydrological and hydrochemical characteristics of the Ural River in the Western Kazakhstan region. Fish. Issues 2018, 34, 259–267. (In Russian) [Google Scholar]
- Tulemisova, G.B.; Abdinov, R.S.; Kabdrakhimova, G.Z.; Janetov, T.B. Ecological state of the river Ural. Chem. Bull. Kazakh Nat. Univ. 2017, 85, 18–24. [Google Scholar] [CrossRef]
- Burlibaev, M.Z.; Amirgaliev, N.A.; Shenberger, I.V. Modern hydroecological and toxicological problems of the transboundary runoff of the rivers of the Zhaiyk (Ural) basin and the nature of the transformation of their parameters. Hydrometeorol. Ecol. 2013, 2, 76–107. (In Russian) [Google Scholar]
- Veschev, P.V. Influence of principal factors on the efficiency of natural reproduction of the Volga stellate sturgeon Acipenser stellatus. Russ. J. Ecol. 1998, 29, 310–315. (In Russian) [Google Scholar]
- Ermolin, I.; Svolkinas, L. Assessment of the sturgeon catches and seal bycatches in an IUU fishery in the Caspian Sea. Mar. Policy 2018, 87, 284–290. [Google Scholar] [CrossRef]
- Shalgimbaeva, G.M.; Bokova, E.B.; Popov, N.N.; Mikodina, E.V.; Mugue, N.S.; Asylbekova, S.Z.; Isbekov, K.B. Current state of the stellate sturgeon Acipenser stellatus (Pallas, 1771) of the Ural River. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2016, 4, 32–41. (In Russian) [Google Scholar]
- Bokova, E.B.; Shalgimbaeva, G.M.; Dzhunusova, G.G. The state of reproduction of sturgeon fish from the natural spawning grounds of the Zhaiyk River in 2016. In Proceedings of the International Conference “Freshwater Aquaculture: Mobilization of Resource Potential”, Moscow, Russia, 2017; pp. 241–246. (In Russian). [Google Scholar]
- A Fisherman in Uralsk Recorded a Shoal of Sterlet in the Ural River on His Underwater Camera. Available online: www.uralskweek.kz/2023/03/10/kosyak-sterlyadej-snyal-na-podvodnuyu-kameru-rybak-v-uralske (accessed on 10 March 2023).
- Mikodina, E.V.; Shalgimbaeva, G.M.; Volkov, A.A. Landscape and climate role in the formation of sturgeon reproduction biotopes in the Ural River (Zhaiyk). E3S Web Conf. 2021, 265, 01011. [Google Scholar] [CrossRef]
- Sisengalieva, G.Z.; Bokova, E.B.; Dzhunusova, G.; Tlepov, Z.; Idrisov, S. Atlas of Sturgeon Spawning Grounds of the Ural River; Tip. Atyrauskogo Gos. Univ. Im. Kz. Dosmukhamedova: Atyrau, Kazakhstan, 2004. [Google Scholar]
- Shalgimbaeva, G.M.; Mugue, N.S.; Isbekov, K.B.; Volkov, A.A.; Mikodina, E.V. Ural (Zhaiyk) River spawning grounds of the sturgeon (Acipenseridae) in the Republic of Kazakhstan: Modern situation. J. Ichthyol. 2022, 62, 1439–1453. [Google Scholar] [CrossRef]
- Sivokhip, Z.T.; Pavleichik, V.M.; Chibilev, A.A.; Padalko, Y.A. Problems of dependable water use in the transboundary Ural River basin. Water Res. 2017, 44, 673–684. [Google Scholar] [CrossRef]
- Sivokhip, Z.T.; Pavleichik, V.M.; Chibilev, A.A. Changes in river runoff during winter low water periods in the basin of the Ural River. Dokl. Earth Sci. 2021, 499, 703–707. [Google Scholar] [CrossRef]
- Sivokhip, Z.T.; Pavleichik, V.M. Water resources of transboundary rivers in the steppe zone under modern hydroclimatic conditions. In Advances in Natural, Human-Made, and Coupled Human-Natural Systems Research; Maximova, S.G., Raikin, R.I., Chibilev, A.A., Silantyeva, M.M., Eds.; Springer: Cham, Switzerland, 2023; Volume 2, pp. 3–16. [Google Scholar]
- Kamelov, A.K.; Kadimov, E.L.; Asylbekova, S.Z.; Isbekov, K.B.; Kulikov, E.V. Modern state of natural reproduction of sturgeon fish (Acipenseridae) in the Ural River. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2018, 2, 81–88. [Google Scholar] [CrossRef]
- Ivkina, N.I. Changes in the inflow of water into the Caspian Sea as a result of anthropogenic impact and climate change on the example of the river Zhaiyk (Urals). Hydrometeorol. Ecol. 2016, 3, 50–55. (In Russian) [Google Scholar]
- Padalko, Y.A. An assessment and prognosis of water resources use in regions of the transboundary basin of the Ural River in the medium term. IOP Conf. Ser. Earth Environ. Sci. 2021, 817, 012080. [Google Scholar] [CrossRef]
- Lagutov, V. The Ural River basin: Hydrology, characteristics and water use. In Rescue of Sturgeon Species in the Ural River Basin; Lagutov, V., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 129–161. [Google Scholar]
- Yessenamanova, M.S.; Kulzhanova, G.; Tlepbergenova, A.E.; Yessenamanova, Z.S.; Batyrbayeva, G. Environmental monitoring of water quality in the interstate Ural River. J. Phys. Conf. Ser. 2021, 1889, 032007. [Google Scholar] [CrossRef]
- Lineitseva, A.V. Runoff inflow to the Republic of Kazakhstan along the Ural River for the future until 2035. Hydrometeorol. Ecol. 2011, 2, 64–68. (In Russian) [Google Scholar]
- Kozlova, M.A.; Sivokhip, Z.T. Assessment of water quality dynamics in the transboundary basin of the Ural River. Water Sect. Russ. Prob., Technol. Manag. 2022, 6, 107–119. [Google Scholar] [CrossRef]
- Magritsky, D.V.; Efimova, L.E.; Goncharov, A.V.; Kenzhebaeva, A.Z. Features of modern water use in the lower reaches of the Ural River, its problems and hydroecological consequences. Prob. Steppe Sci. 2022, 1, 28–49. (In Russian) [Google Scholar] [CrossRef]
- Tulemisova, G.; Amangosova, A.; Abdinov, R.; Kabdrakhimova, G.; Orynbasarova, A. Hydrochemical conditions of water bodies of the Ural-Caspian Basin. Eur. J. Nat. Hist. 2018, 1, 7–12. [Google Scholar]
- Assylbekova, S.; Isbekov, K.; Zharkenov, D.; Kulikov, Y. Evaluation of the habitat state of the Zhaiyk River ichthyofauna in modern conditions and its influence on the impacts of anthropogenic factors. EurAsian J. BioSci. 2020, 14, 467–473. [Google Scholar]
- Akhmedenov, K.M.; Chibilev, A.A. Modern ecological problems of restoration and conservation of the sturgeon population in the Ural River basin. South Russ. Ecol. Dev. 2023, 18, 6–16. [Google Scholar] [CrossRef]
- Lattuada, M.; Albrecht, C.; Wilke, T. Differential impact of anthropogenic pressures on Caspian Sea ecoregions. Mar. Pollut. Bull. 2019, 142, 274–281. [Google Scholar] [CrossRef]
- de Mora, S.; Sheikholeslami, M.R.; Wyse, E.; Azemard, S.; Cassi, R. Assessment of metal contamination in coastal sediments of the Caspian Sea. Mar. Pollut. Bull. 2004, 48, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Ruban, G.I.; Khodorevskaya, R.P.; Shatunovskiy, M.I. Long-term dynamics of sturgeon distribution in the northern part of the Caspian Sea (Review). Inland Water Biol. 2019, 12, 443–451. [Google Scholar] [CrossRef]
- Geraskin, P.P.; Metallov, G.F.; Zhuravleva, G.F. The level of physiological well-being of Caspian sturgeon in the marine period of life under conditions of increased impact of anthropogenic factors. In Fisheries Research on the Caspian Sea; Caspian Scientific Research Institute of Fisheries: Astrakhan, Russia, 2002; pp. 423–436. (In Russian) [Google Scholar]
- Kajiwara, N.; Ueno, D.; Monirith, I.; Tanabe, S.; Pourkazemi, M.; Aubrey, D.G. Contamination by organochlorine compounds in sturgeons from Caspian Sea during 2001 and 2002. Mar. Pollut. Bull. 2003, 46, 741–747. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.O.; Crossman, J.A.; Williamson, C.; St-Onge, I.; Dion, R.; Manny, B.A.; Gessner, J. If you build it, will they come? Spawning habitat remediation for sturgeon. J. Appl. Ichthyol. 2017, 34, 258–278. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, X.; Zhao, Z.; Tang, W. Conservation and restoration of riverine spawning habitats require fine-scale functional connectivity and functional heterogeneity. Sci. Total Environ. 2023, 857, 159571. [Google Scholar] [CrossRef]
- Baril, A.-M.; Biron, P.M.; Grant, J.W.A. An assessment of an unsuccessful restoration project for lake sturgeon using three-dimensional numerical modelling. N. Am. J. Fish. Manag. 2018, 39, 69–81. [Google Scholar] [CrossRef]
- Doukakis, P.; Erickson, D.; Baimukhanov, M.; Bokova, V.; Erbulekov, A.; Nimatov, A.; Pikitch, E.K. Field and genetic approaches to enhance knowledge of Ural River sturgeon biology. In Rescue of Sturgeon Species in the Ural River Basin; Lagutov, V., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 277–292. [Google Scholar]
- Bouska, K.L.; Healy, B.D.; Moore, M.J.; Dunn, C.G.; Spurgeon, J.J.; Paukert, C.P. Diverse portfolios: Investing in tributaries for restoration of large river fishes in the Anthropocene. Front. Environ. Sci. 2023, 11, 1151315. [Google Scholar] [CrossRef]
- van Uhm, D.P. Black caviar. In The Illegal Wildlife Trade; van Uhm, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 117–160. [Google Scholar]
- Ermolin, I.; Svolkinas, L. Who owns sturgeon in the Caspian? New theoretical model of social responses towards state conservation policy. Biodiv. Conserv. 2016, 25, 2929–2945. [Google Scholar] [CrossRef]
- Ye, Y.; Valbo-Jørgensen, J. Effects of IUU fishing and stock enhancement on the restoration strategies for the stellate sturgeon fishery in the Caspian Sea. Fish. Res. 2012, 131–133, 21–29. [Google Scholar] [CrossRef]
- Raymakers, C. Study on the Social and Economic Aspects of Illegal Fishing in the Caspian Sea; Caspian Environment Programme: Brussels, Belgium, 2002. [Google Scholar]
- Dumont, H. Ecocide in the Caspian Sea. Nature 1995, 377, 673–674. [Google Scholar] [CrossRef]
- De Meulenaer, T.; Raymakers, C. Sturgeons of the Caspian Sea and the International Trade in Caviar; TRAFFIC International: Cambridge, UK; 71p.
- “Bekire-2023”: More than 2 Tons of Illegally Caught Fish Seized. Available online: azh.kz/ru/news/view/91642 (accessed on 1 July 2023).
- Kazakhstan Jails Four Azeris for Illegal Fishing in Caspian. Available online: rferl.org/a/four-azeris-jailed-in-kazakhstan-for-illegal-fishing-in-caspian-sea/2943372 (accessed on 1 July 2023).
- 223 kg of Sturgeon Seized from a Resident of Mangistau. Available online: newsline.kz/article/766105/ (accessed on 2 July 2023).
- A Ton of Redfish Was Taken from Poachers in Atyrau. Available online: tengrinews.kz/kazakhstan_news/tonnu-krasnoy-ryibyi-otobrali-u-brakonerov-v-atyrau-414946/ (accessed on 1 July 2023).
- Fishery Protection Campaign “Bekire-2022” Starts in Kazakhstan. Available online: gov.kz/memleket/entities/ecogeo/press/news/details/349872?lang=ru (accessed on 2 July 2023).
- Poachers’ Cache with Three Tons of Sturgeon Found in Mangistau. Available online: web.archive.org/web/20220524114739/https://tengrinews.kz/other/v-mangistau-nashli-taynik-brakonerov-s-tremya-tonnami-osetra-435860/ (accessed on 24 May 2022).
- Poachers with Half a Ton of Sturgeon Were Running Away from Inspectors in Atyrau. Available online: web.archive.org/web/20220818112702/https://mgorod.kz/nitem/brakonery-s-poltonnoj-osetra-ubegali-ot-inspektorov-v-atyrau/ (accessed on 18 August 2022).
- 300 Kilograms of Sturgeon Seized from Poachers in Aktau. Available online: web.archive.org/web/20221121134929/https://tengrinews.kz/Kazakhstan_news/300-kilogrammov-osetrovyih-izyyli-u-brakonerov-v-aktau-483331/ (accessed on 21 November 2022).
- Birstein, V.J.; Bemis, W.E.; Waldman, J.R. The threatened status of acipenseriform species: A summary. Environ. Biol. Fishes 1997, 48, 427–435. [Google Scholar] [CrossRef]
- Kozhin, N.I.; Gerbilsky, N.L.; Kazansky, B.N. Biotechnics of sturgeon breeding and the principle scheme of a sturgeon hatchery. In Sturgeon Farming in Reservoirs of the USSR; Publishing House of the Academy of Sciences of the USSR: Moscow, Soviet Union, 1963; pp. 29–34. (In Russian) [Google Scholar]
- Vasilyeva, L.M.; Naumov, V.V.; Sudakova, N.V. Features of the current state of artificial reproduction of sturgeons in the Volga-Caspian basin. Nat. Sci. 2015, 4, 90–95. (In Russian) [Google Scholar]
- Vlasenko, A.D.; Zykova, G.F.; Krasikov, E.V. Status of sturgeon stocks in the Caspian basin and ways of their restoration. In Proceedings of the conference dedicated to the 105th anniversary of CaspNIRKH, Astrakhan, Russia, 2002; pp. 58–64. (In Russian). [Google Scholar]
- Burtsev, I.A. Recommendations on improving the efficiency of industrial sturgeon production. Trudy VNIRO 2015, 153, 165–173. (In Russian) [Google Scholar]
- Doukakis, P.; Babcock, E.A.; Pikitch, E.K.; Sharov, A.R.; Baimukhanov, M.; Erbulekov, S.; Bokova, Y.; Nimatov, A. Management and recovery options for Ural River beluga sturgeon. Conserv. Biol. 2010, 24, 769–777. [Google Scholar] [CrossRef]
- Kabdulov, S. Let’s Preserve Fish Wealth. Available online: kazpravda.kz/n/sohranim-rybnye-bogatstva/ (accessed on 17 July 2023). (In Russian).
- Vasilyeva, L.M. Artificial reproduction of sturgeon fish of an enlarged sample. In Proceedings of the International Conference “Reproduction of Natural Populations of Valuable Fish Species,” GosNIORH, 2010; pp. 34–35. (In Russian). [Google Scholar]
- Dosaeva, V.G.; Vasilyeva, T.V.; Astafieva, S.S. Growing large sturgeon fry as one of the ways to preserve the relict fauna of the Caspian Sea during the period of anthropogenic impact. In Proceedings of the international conference “Problems of studying and preserving vertebrate animals of anthropogenic reservoirs”, Saransk, Russia, 2010; pp. 28–31. (In Russian). [Google Scholar]
- Astafieva, S.S.; Vasilyeva, T.V.; Fedoseeva, E.A. The state of artificial reproduction of sturgeons in the West Caspian region and proposals for its development. Act. Prob. Mod. Sci. 2010, 6, 267–271. (In Russian) [Google Scholar]
- Karpyuk, M.I.; Mazhnik, A.Y.; Degtyareva, N.G. Problems of Caspian aquatic bioresources: Today and tomorrow. In Proceedings of the conference dedicated to the 105th anniversary of CaspNIRKH, Astrakhan, Russia, 2002; pp. 132–136. (In Russian). [Google Scholar]
- Burtsev, I.A.; Nikolaev, A.E.; Maltsev, S.A.; Igumnova, L.V. Formation of domesticated broodstock as a guarantee of sustainable hatchery reproduction of sturgeon for sea ranching. J. Appl. Ichthyol. 2002, 18, 4–6. [Google Scholar] [CrossRef]
- Khodorevskaya, R.P.; Nekrasova, S.O. Current state and future of aquatic biological resources reproduction for industrial aquaculture in the Astrakhan region. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2019, 3, 107–116. [Google Scholar] [CrossRef]
- Gorbacheva, L.T.; Gorbenko, E.V.; Savelyeva, E.A.; Chikhacheva, V.P. Results of rearing the Azov beluga to a large mass at industrial exploration, modern problems of the Caspian Sea. In Proceedings of the Conference Dedicated to the 105th Anniversary of CaspNIRKH, Astrakhan, Russia, 2002; pp. 71–77. (In Russian). [Google Scholar]
- Steffensen, K.D.; Powell, L.A.; Koch, J.D. Assessment of hatchery-reared pallid sturgeon survival in the lower Missouri River. N. Amer. J. Fish. Manag. 2010, 30, 671–678. [Google Scholar] [CrossRef]
- Vasilyeva, L.M.; Rabazanov, N.I. Modern problems of artificial reproduction of sturgeon in the Volga-Caspian basin. South Russ. Ecol. Devel. 2022, 17, 6–15. [Google Scholar] [CrossRef]
- Anonymous. On the Activities of the RSE Ural-Atyrau Sturgeon Fish Hatchery; Ural-Atyrau Sturgeon Fish Hatchery: Atyrau, Kazakhstan, 2021. (In Russian) [Google Scholar]
- McClure, M.M.; Utter, F.M.; Baldwin, C.; Carmichael, R.W.; Hassemer, P.F.; Howell, P.J.; Spruell, P.; Cooney, T.D.; Schaller, H.A.; Petrosky, C.E. Evolutionary effects of alternative artificial propagation programs: Implications for viability of endangered anadromous salmonids. Evol. Applic. 2008, 1, 356–375. [Google Scholar] [CrossRef]
- Osborne, M.J.; Dowling, T.E.; Scribner, K.T.; Turner, T.F. Wild at heart: Programs to diminish negative ecological and evolutionary effects of conservation hatcheries. Biol. Conserv. 2020, 251, 108768. [Google Scholar] [CrossRef]
- Assylbekova, S.Z.; Mikodina, E.V.; Isbekov, K.B.; Shalgimbayeva, G.M. Experience, principles and parameters in the sturgeon quality assessment by anomalies in early ontogenesis (a review). Biology 2022, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Bemis, W.E.; Kynard, B. Sturgeon rivers: An introduction to acipenseriform biogeography and life history. Environ. Biol. Fishes 1997, 48, 167–183. [Google Scholar] [CrossRef]
- Nelson, T.C.; Doukakis, P.; Lindley, S.T.; Schreier, A.D.; Hightower, J.E.; Hildebrand, L.R.; Whitlock, R.E.; Webb, M.A.H. Research tools to investigate movements, migrations, and life history of sturgeons (Acipenseridae), with an emphasis on marine-oriented populations. PLoS ONE 2013, 8, e71552. [Google Scholar] [CrossRef]
- Sergeev, A.A.; Barmintseva, A.E.; Chavychalova, N.I.; Tolochkova, M.E. Genetic monitoring of natural sturgeon spawning in the lower Volga for the period 2017–2018. In Proceedings of the XVI International Congress of Ichthyology, Lausanne, Switzerland, 2–6 September 2019. [Google Scholar]
- Allendorf, F.W.; Leary, R.F.; Spruell, P.; Wenburg, J.K. The problems with hybrids: Setting conservation guidelines. Trends Ecol. Evol. 2001, 16, 613–622. [Google Scholar] [CrossRef]
- Bekbergenova, V. Current biological data on the ship sturgeon Acipenser nudiventris Lovetsky, 1828 (Review). In XV International Scientific Conference “INTERAGROMASH 2022; Beskopylny, A., Shamtsyan, M., Artiukh, V., Eds.; Springer: Cham, Switzerland, 2023; pp. 750–758. [Google Scholar] [CrossRef]
- Levin, A.V.; Kokoza, A.A.; Burykin, M.V. Survival and growth of sturgeon fry at the first stages of the marine life period. In Reproduction of Sturgeon Stocks in the Caspian and Azov-Black Sea Basins; VNIRO: Moscow, Soviet Union, 1987; pp. 65–75. (In Russian) [Google Scholar]
- Chebanov, M.S.; Galich, E.V. Sturgeon Hatchery Manual; UN Food and Agriculture Organization: Ankara, Turkey, 2013. [Google Scholar]
- A Disappearing River: The Fate of the Ural. Available online: www.novostan.org/en/Kazakhstan/disappearing-river-can-the-ural-fate-be-averted/ (accessed on 20 July 2023).
- Valbo-Jørgensen, J.; Marmulla, G.; Welcomme, R.L. Migratory fish stocks in transboundary basins—Implications for governance, management and research. In Rescue of Sturgeon Species in the Ural River Basin; Lagutov, V., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 61–86. [Google Scholar]
- Ozenbayeva, A.; Yerezhepkyzy, R.; Yessetova, S.; Jangabulova, A.; Beissenbayeva, M. Legal regulation of transboundary water resources of the Republic of Kazakhstan. Environ. Devel. 2022, 44, 100781. [Google Scholar] [CrossRef]
- Aliyeva, G.; Laumulin, M. Transboundary environmental cooperation of Kazakhstan and Russia: Problems and prospects. Cent. Asia Cauc. 2021, 22, 140–152. [Google Scholar] [CrossRef]
- Mamatova, Z.; Ibrokhimov, D.; Dewulf, A. The wicked problem of dam governance in Central Asia: Current trade-offs, future challenges, prospects for cooperation. Int. J. Water Gov. 2016, 4, 15. [Google Scholar]
- Graham, N.A.; Pueppke, S.G.; Uderbayev, T. The current status and future of Central Asia’s fish and fish resources: Confronting a wicked problem. Water 2017, 9, 701. [Google Scholar] [CrossRef]
- Pueppke, S.G.; Nurtazin, S.T.; Graham, N.A.; Qi, J. Central Asia’s Ili River ecosystem as a wicked problem: Unraveling complex interrelationships at the interface of water, energy, and food. Water 2018, 10, 541. [Google Scholar] [CrossRef]
- Hopson, R.; Cram, F. Tackling wicked problems in complex evaluation ecologies. In Tackling Wicked Problems in Complex Ecologies: The Role of Evaluation; Hopson, R., Cram, F., Eds.; Stanford University Press: Stanford, CA, USA, 2018; pp. 3–24. [Google Scholar]
- Rittel, H.W.J.; Webber, M.M. Dilemmas in a general theory of planning. Policy Sci. 1973, 4, 155–169. [Google Scholar] [CrossRef]
- Batie, S. Wicked problems and applied economics. Am. J. Agric. Econ. 2008, 90, 1176–1191. [Google Scholar] [CrossRef]
- Svolkinas, L.; Holmes, G.; Dmitrieva, L.; Ermolin, I.; Suvorkov, P.; Goodman, S.J. Stakeholder consensus suggests strategies to promote sustainability in an artisanal fishery with high rates of poaching and marine mammal bycatch. People Nat. 2023, 5, 1187–1206. [Google Scholar] [CrossRef]
- Strukova, E.; Guchgeldiyev, O.; Evans, A.; Katunin, D.; Khodorevskaya, R.; Kim, Y.; Akhundov, M.; Mammadli, T.; Shahivar, R.; Muradov, O.; et al. Exploitation of the Caspian Sea bioresources (with focus on economics of bioresources utilization). In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–44. [Google Scholar] [CrossRef]
- Cooke, S.J.; Paukert, C.; Hogan, Z. Endangered river fish: Factors hindering conservation and restoration. Endanger. Species Res. 2012, 17, 179–191. [Google Scholar] [CrossRef]
- Akhmadiyeva, Z.; Abdullaev, I. Water management paradigm shifts in the Caspian Sea region: Review and outlook. J. Hydrol. 2019, 568, 997–1006. [Google Scholar] [CrossRef]
- Sivokhip, Z.T.; Chibilev, A.A. Transboundary river basins: Basic principles for solving the problems of interstate cooperation. Geogr. Nat. Resourc. 2022, 43, 218–227. [Google Scholar] [CrossRef]
- Karatayev, M.; Rivotti, P.; Mourão, Z.S.; Konadu, D.D.; Shah, N.; Clarke, M. The water-energy-food nexus in Kazakhstan: Challenges and opportunities. Energy Procedia 2017, 125, 63–70. [Google Scholar] [CrossRef]
- Warner, J.F.; van Buuren, A. Reframing long-term controversies in transboundary river management. The intermediate role of puzzling and powering in tackling wicked problems. Futures 2016, 76, 18–29. [Google Scholar] [CrossRef]
- Lagutov, V. (Ed.) Rescue of Sturgeon Species in the Ural River Basin; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Parrott, L. The modelling spiral for solving ‘wicked’ environmental problems: Guidance for stakeholder involvement and collaborative model development. Methods Ecol. Evol. 2017, 8, 1005–1011. [Google Scholar] [CrossRef]
- Williot, P.; Arlati, G.; Chebanov, M.; Gulyas, T.; Kasimov, R.; Kirschbaum, F.; Patriche, N.; Pavlovskaya, L.P.; Poliakova, L.; Pourkazemi, M.; et al. Status and management of Eurasian sturgeon: An overview. Int. Rev. Hydrobiol. 2002, 87, 483–506. [Google Scholar] [CrossRef]
- McCall, R.; Burge, J. Untangling wicked problems. Artif. Intell. Eng. Des. Anal. Manuf. 2016, 30, 200–210. [Google Scholar] [CrossRef]
- Lukyanenko, V.I.; Valisev, A.S.; Lukyanenko, V.V.; Khavarov, M.V. On the increasing threat of extermination of the unique Caspian sturgeon populations and the urgent measures required to save them. J. Appl. Ichthyol. 1999, 15, 99–102. [Google Scholar] [CrossRef]
- Anderson, W.G.; Schreier, A.; Crossman, J.A. Conservation aquaculture—A stocking story. Fish Physiol. 2022, 39, 39–109. [Google Scholar] [CrossRef]
- Bakhshalizadeh, S.; Tchaikovsky, A.; Bani, A.; Prohaska, T.; Zitek, A. Using fin ray chemistry to discriminate hatchery reared juvenile ago-0 Persian sturgeons by their origin in the Southern Caspian Sea region using split stream ICP-MS/MC ICP-MS. Fish. Res. 2021, 243, 106093. [Google Scholar] [CrossRef]
- White, S.L.; Kazyak, D.C.; Darden, T.L.; Farrae, D.J.; Lubinski, B.A.; Johnson, R.L.; Eakles, M.S.; Balazik, M.T.; Brundage III, H.M.; Fox, A.G.; et al. Establishment of a microsatellite genetic baseline for North American Atlantic sturgeon (Acipenser o. oxyrhinchus) and range-wide analysis of population genetics. Conserv. Genet. 2021, 22, 977–992. [Google Scholar] [CrossRef]
- Lubchenco, J.; Haugan, P.M. (Eds.) Illegal, unreported and unregulated fishing and associated drivers. In The Blue Compendium; Springer: Cham, Switzerland, 2023; pp. 553–591. [Google Scholar] [CrossRef]
- Zuzanna, K.; Tomasz, U.; Michal, G.; Robert, P. How high-tech solutions support the fight against IUU and ghost fishing: A review of innovative approaches, methods and trends. IEEE Access 2022, 10, 112539–112554. [Google Scholar] [CrossRef]
- Rastorguev, S.M.; Nedoluzhko, A.; Mazur, A.M.; Gruzdeva, N.M.; Volkov, A.A.; Barmintseva, A.E.; Mugue, N.S.; Prokhortchouk, E.B. High-throughput SNP-genotyping analysis of the relationships among Ponto-Caspian sturgeon species. Ecol. Evol. 2013, 3, 2612–2618. [Google Scholar] [CrossRef]
- Sergaliyev, N.H.; Sariev, B.T.; Tumenov, A.N.; Bakiyev, S.S. Efficiency of tagging methods for sturgeon fish producers. Exp. Biol. 2020, 85, 105–113. [Google Scholar] [CrossRef]
- Liss, S.A.; Li, H.; Deng, Z.D. A subdermal tagging technique for juvenile sturgeon using a new self-powered acoustic tag. Anim. Biotelemitry 2022, 10, 7. [Google Scholar] [CrossRef]
- Balint, P.J.; Stewart, R.E.; Desai, A.; Walters, L.C. Wicked Environmental Problems: Managing Uncertainty and Conflict; Island Press: Washington, DC, USA, 2011. [Google Scholar]
- Billard, R.; Lecointe, G. Biology and conservation of sturgeon and paddlefish. Rev. Fish Biol. Fish. 2000, 10, 355–392. [Google Scholar] [CrossRef]
- Jarić, I.; Gessner, J. Analysis of publications on sturgeon research between 1996 and 2010. Scientometrics 2012, 90, 715–735. [Google Scholar] [CrossRef]
- Ostrom, E. Governing the Commons: The Evolution of Institutions for Collective Action; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Weber, E.P.; Steenson, A.P. Collaborative governance, science, and policy outcomes. In New Strategies for Wicked Problems: Science and Solutions in the 21st Century; Weber, E.P., Lach, D., Steel, B.S., Eds.; Oregon State University Press: Corvallis, OR, USA, 2017; pp. 185–206. [Google Scholar]



| Date and Location | Seizure/Action | Reference |
|---|---|---|
| 2018, Qaraqiya | Arrest at the border and seizure of sturgeon | [115] |
| 2018, Mangistau | Sturgeon weighing 223 kg | [116] |
| 2020, Atyrau | 345 sturgeon weighing > 1 ton | [117] |
| 2021, Various | 4.6 tons of sturgeon | [118] |
| 2021, Mangistau | 3 tons of sturgeon in industrial freezers | [119] |
| 2022, Atyrau | 0.5 tons of sturgeon | [120] |
| 2022, Aktau | 76 sturgeon weighing 300 kg | [121] |
| 2023, Atyrau | 93 people convicted of IUU fishing and trade | [114] |
| Wicked Problem Characteristic | Examples | Ural Sturgeon References |
|---|---|---|
| Difficult to define with multiple solutions | Shifting paradigms of fundamental issue; lack of regional cooperation; no consensus on preventing IUU fishing | [28,150,159] |
| Assumptions changing | Centralized Soviet control suddenly relaxed in 1991; climate change emerges as an issue | [88,154] |
| Tradeoffs | Hydroelectric power vs. sturgeon; stocking to support fishing vs. stocking to re-establish natural populations | [100,132] |
| Dynamic with strong economic drivers | Fishing regulations change over time; powerful incentives to produce caviar | [3,41,160] |
| Incomplete and contradictory knowledge | Lack of basin-wide data; knowledge gaps in sturgeon life histories; inability to quantify effects of poaching | [106,150,161] |
| Interconnected with other wicked problems | Water–energy–food; overall management of the Caspian basin; geopolitical conflicts | [162,163,164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pueppke, S.G.; Nurtazin, S.T.; Murzashev, T.K.; Galymzhanov, I.S.; Graham, N.A.; Konysbayev, T. Re-Establishing Naturally Reproducing Sturgeon Populations in the Caspian Basin: A Wicked Problem in the Ural River. Water 2023, 15, 3399. https://doi.org/10.3390/w15193399
Pueppke SG, Nurtazin ST, Murzashev TK, Galymzhanov IS, Graham NA, Konysbayev T. Re-Establishing Naturally Reproducing Sturgeon Populations in the Caspian Basin: A Wicked Problem in the Ural River. Water. 2023; 15(19):3399. https://doi.org/10.3390/w15193399
Chicago/Turabian StylePueppke, Steven G., Sabir T. Nurtazin, Turesh K. Murzashev, Islam S. Galymzhanov, Norman A. Graham, and Talgarbay Konysbayev. 2023. "Re-Establishing Naturally Reproducing Sturgeon Populations in the Caspian Basin: A Wicked Problem in the Ural River" Water 15, no. 19: 3399. https://doi.org/10.3390/w15193399
APA StylePueppke, S. G., Nurtazin, S. T., Murzashev, T. K., Galymzhanov, I. S., Graham, N. A., & Konysbayev, T. (2023). Re-Establishing Naturally Reproducing Sturgeon Populations in the Caspian Basin: A Wicked Problem in the Ural River. Water, 15(19), 3399. https://doi.org/10.3390/w15193399








