Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Location and Geological Background
2.2. The Occurrence and Hydrochemistry Characteristics of the Mineral Water
2.3. Sampling and Analytical Methods
2.3.1. Sampling
2.3.2. Nanoparticle Tracking Analysis
2.3.3. Characteristics of the Nanoparticle Analysis
3. Results
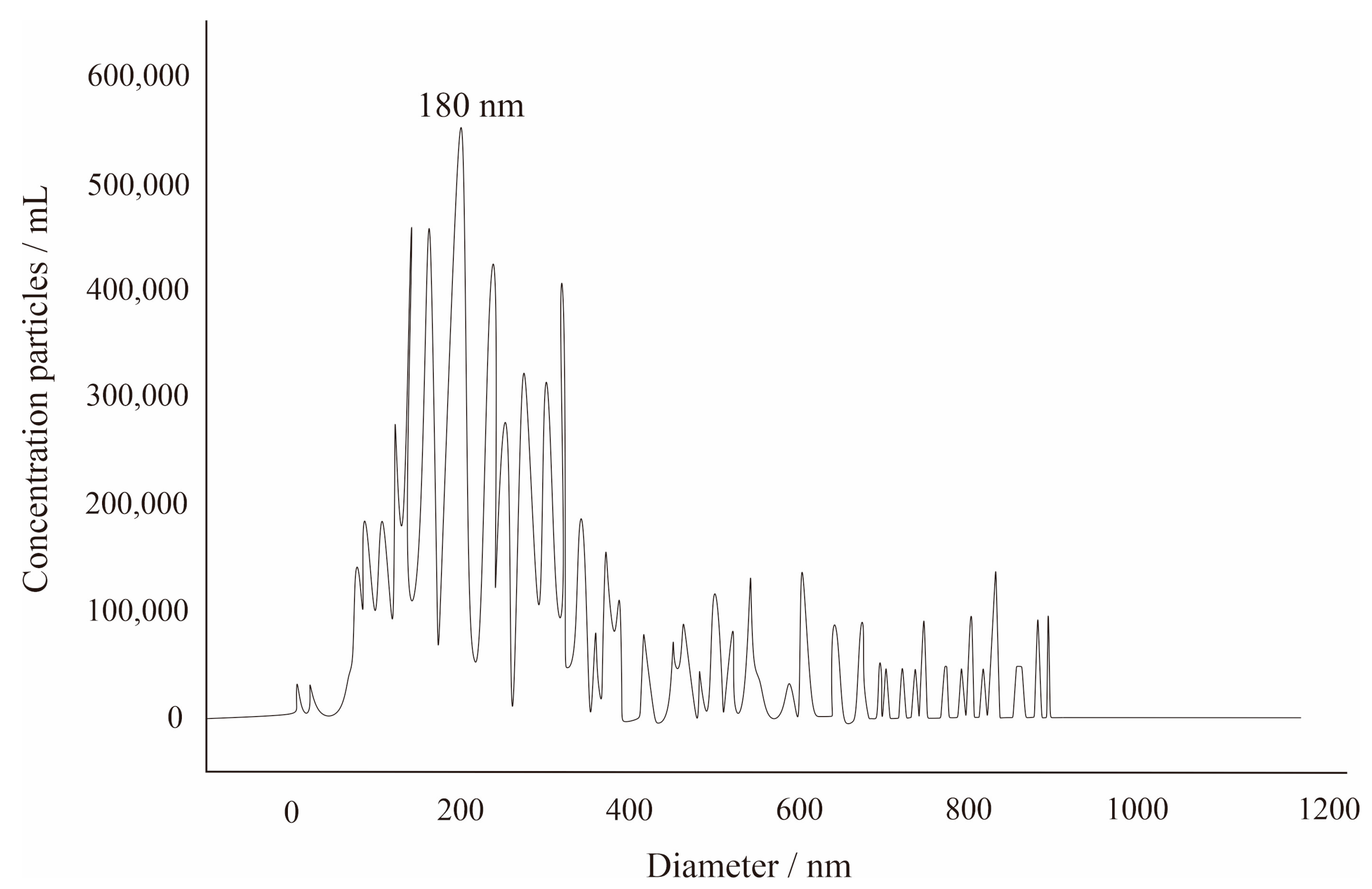
3.1. The Number and Size Distribution of the Environmental Nanoparticles in the Well YY17 Mineral Water
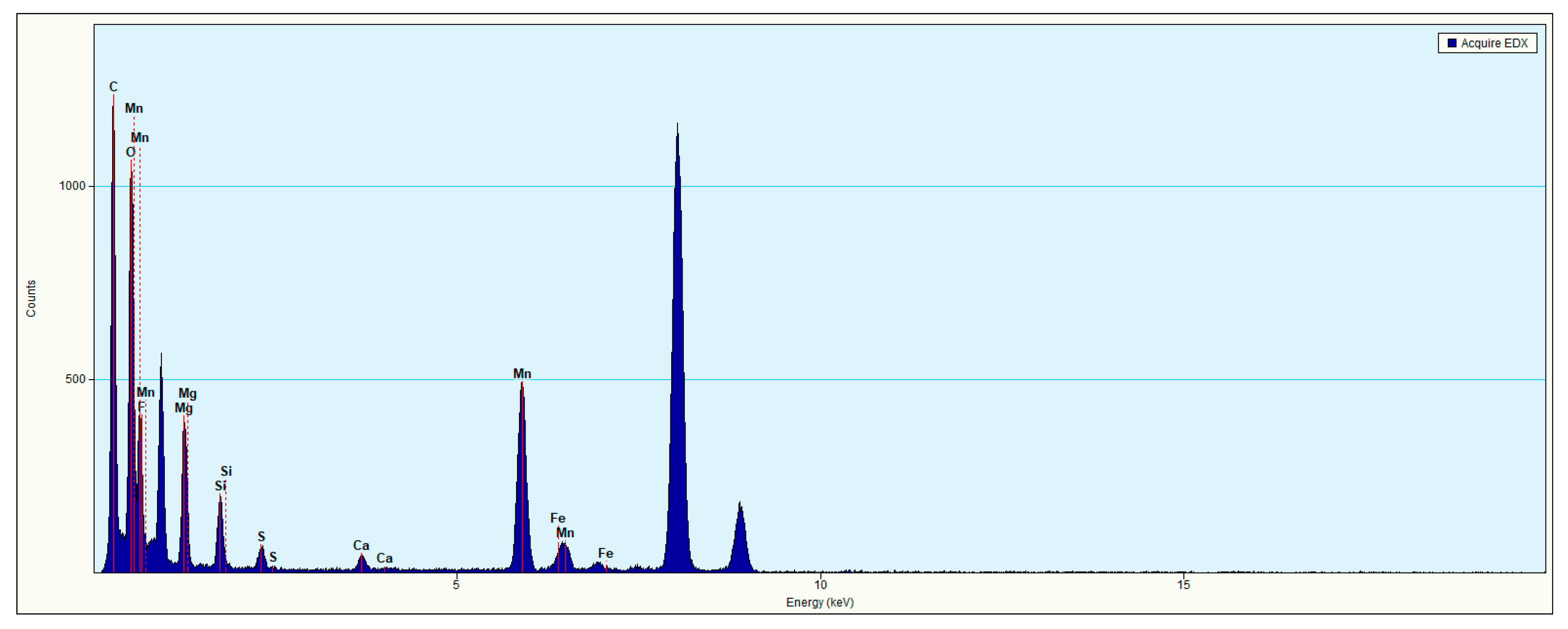
3.2. Characteristics of the Environmental Nanoparticles in the Well YY17 Mineral Water
3.2.1. Ca-Bearing Nanoparticles
3.2.2. Attapulgite Nanorod
3.2.3. MnO2 Nanosheets
3.2.4. TiO2 Nanoparticle
4. Discussion
4.1. A New Form of Elements in Mineral Water
4.1.1. Natural CaCO3 Nanoparticles in Mineral Water
4.1.2. Natural Ca3(PO4)2 Nanoparticles in Mineral Water
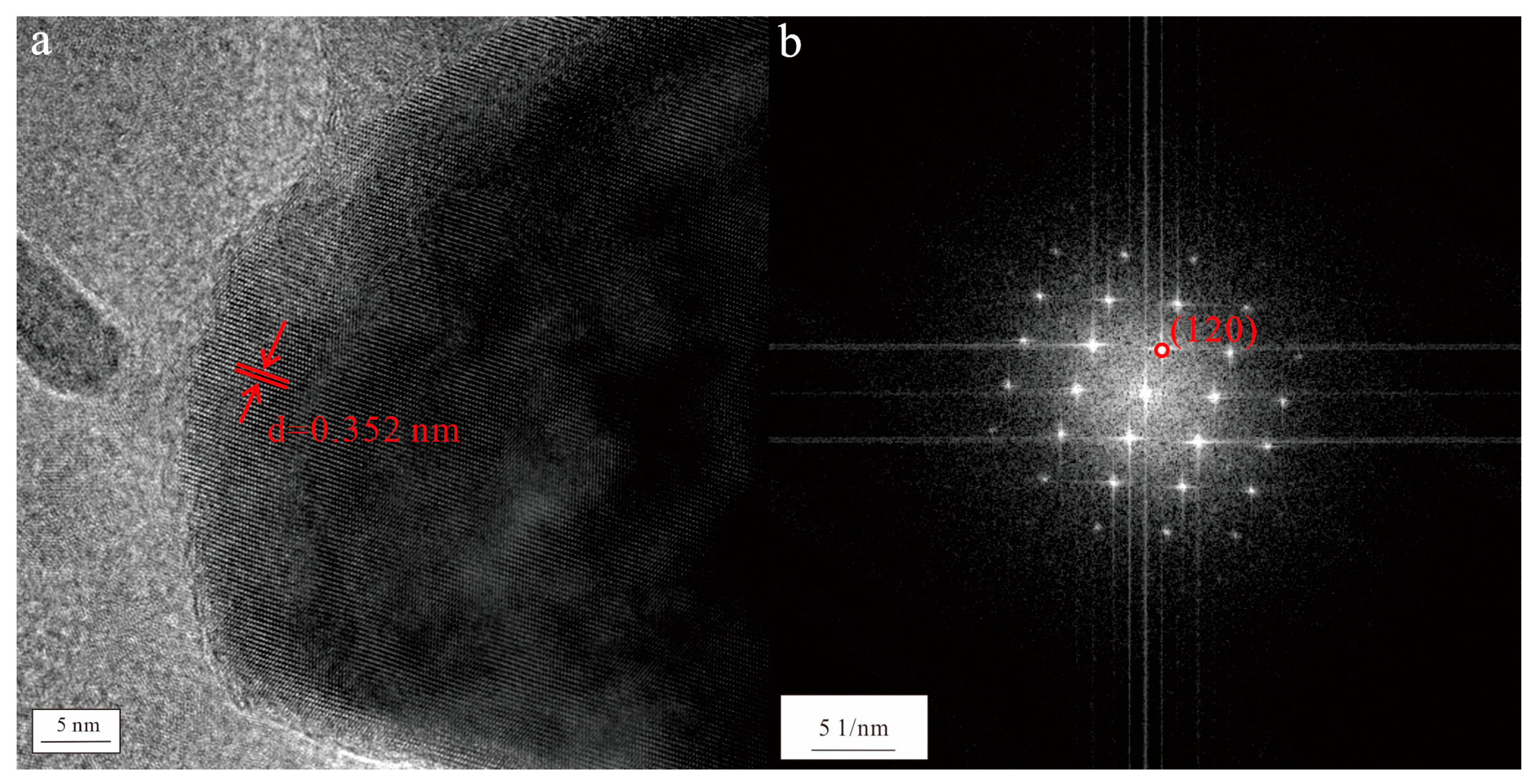
4.1.3. Natural Attapulgite Nanorods in Mineral Water
4.1.4. MnO2 Nanosheets in the Mineral Water
4.1.5. TiO2 Nanoparticles in the Mineral Water
4.2. The Origins of Environmental Nanoparticles in Mineral Water
4.3. Perspectives on the Advancement of Environmental Nanoparticles in Mineral Water
4.3.1. The Opportunity to Understand the Long-Term Stability and Transport Mechanisms of Nanoparticles
4.3.2. Environmental Nanoparticles in Drinking Water Systems
4.3.3. Environmental Nanoparticles Science in Drinking Water Systems
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, N.N.; Zuo, W.S.; Liu, Z.F.; Wang, Y.; Huang, S.; Xu, H.; Li, W. Characteristics of the mineral water resources in Guangdong and suggestions for their exploitation and utilization. Trop. Geogr. 2012, 32, 501–507. [Google Scholar]
- Xu, F. Development, utilization and management of mineral water in the Soviet Union. J. Environ. Health 1988, 3, 62–64. [Google Scholar]
- Wigginton, N.S.; Haus, K.L.; Hochella, M.F., Jr. Aquatic environmental nanoparticles. J. Environ. Monit. 2007, 9, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Geldreich, E.E.; Nash, H.D.; Reasoner, D.J.; Taylor, R.H. The necessity of controlling bacterial populations in potable waters-bottled water and emergency water supplies. J. Am. Water Work. Assoc. 1975, 67, 117–124. [Google Scholar] [CrossRef]
- Warburton, D.W.; Peterkin, P.I.; Weiss, K.F.; Johnston, M.A. Microbiological quality of bottled water sold in Canada. Can. J. Microbiol. 1986, 32, 891–893. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Gutiérrez, C.; Grande, T. Bacterial flora in bottled uncarbonated mineral drinking water. Can. J. Microbiol. 1987, 33, 1120–1125. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, 6434. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.; Cesar, I.; Grätzel, M. New benchmark for water photooxidation by nanostructured α-Fe2O3 films. J. Am. Chem. Soc. 2006, 128, 15714–15721. [Google Scholar] [CrossRef]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective coating of superparamagnetic iron oxide nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Miser, D.E.; Shin, E.-J.; Hajaligol, M.R.; Rasouli, F. HRTEM characterization of phase changes and the occurrence of maghemite during catalysis by an iron oxide. Appl. Catal. A Gen. 2004, 258, 7–16. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Guan, Q. Research on the origin and water quality evaluation of natural mineral water in Yiyuan county, Shandong province. Groundwater 2021, 43, 25–28. [Google Scholar] [CrossRef]
- Gardiner, C.; Ferreira, Y.J.; Dragovic, R.A.; Redman, C.W.G.; Sargent, I.L. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J. Extracell. Vesicles 2013, 2, 19671. [Google Scholar] [CrossRef]
- Li, Y.; Cao, J.; Hopke, P.K.; Holub, R.F.; Jiang, T. The discovery of the metallic particles of groundwater from the Dongshengmiao polymetallic deposit, Inner Mongolia, and their prospecting significance. J. Geochem. Explor. 2016, 161, 49–61. [Google Scholar] [CrossRef]
- Ramasamy, V.; Anand, P.; Suresh, G. Synthesis and characterization of polymer-mediated CaCO3 nanoparticles using limestone: A novel approach. Adv. Powder Technol. 2018, 29, 818–834. [Google Scholar] [CrossRef]
- Karimi, M.A.; Ranjbar, M. Hydrothermal synthesis and characterization ofhydrothermal synthesis and characterization of CaCO3 nanostructure. Synth. React. Inorg. Met. Nano-Met. Chem. 2016, 46, 635–637. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mishra, S. Nano-calcium carbonate (CaCO3)/polystyrene (PS)core-shell nanoparticle: It’s effect on physical and mechanical properties ofhigh impact polystyrene (HIPS). Polym. Res. 2013, 20, 1–12. [Google Scholar] [CrossRef]
- Barhoum, A.; Van, L.; Lokeren, H.; Rahier, A.G. Van Dufresne, Assche, Roles ofin situ surface modification in controlling the growth and crystallization of CaCO3 nanoparticles, and their dispersion in polymeric materials. J. Mater.Sci. 2015, 50, 7908–7918. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.; Palanisamy, K.; Subramani, K.; Subramanian, V. Polymorphic and Morphological Transformations of CaCO3 under CO2 Atmosphere and under the Influence of EDTA at 60 °C. Int. Lett. Chem. Phys. Astron. 2015, 53, 173–179. [Google Scholar] [CrossRef]
- Lowenstam, H.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium Phosphate and Hydroxyapatite: Crystallographic and Chemical Relations between Octacalcium Phosphate and Hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. Appl. Biomater 2010, 77B, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Mok, Z.H.; Mylonas, P.; Austin, R.; Proctor, G.; Pitts, N.; Thanou, M. Calcium phosphate nanoparticles for potential application as enamel remineralising agent tested on hydroxyapatite discs. Nanoscale 2021, 13, 20002–20012. [Google Scholar] [CrossRef]
- Fang, T.H.; Wang, C.W. Dissolved and particulate phosphorus species partitioning and distribution in the Danshuei River Estuary, Northern Taiwan. Mar. Pollut. Bull. 2020, 151, 110839. [Google Scholar] [CrossRef]
- Belton, D.; Stupp, S.I. Adsorption of ionizable polymers on ionic surfaces: Poly (acrylicacid). Macromolecules 1983, 16, 1143–1150. [Google Scholar] [CrossRef]
- Shakkthivel, P.; Vasudevan, T. Acrylic acid-diphenylamine sul-phonic acid copolymer threshold inhibitor for sulfate and carbonatescales in cooling water systems. Desalination 2006, 197, 179–189. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, J.; Chen, Z.; Mao, J.; Zhang, Z.; Li, X.; Ren, S. Preparation, Characterization, Application and Structure Evolution of Attapulgite: From Nanorods to Nanosheets. Appl. Surf. Sci. 2021, 565, 150398. [Google Scholar] [CrossRef]
- Chen, F.; Lou, D.; Yang, J.; Zhong, M. Mechanical and thermal properties of attapulgite clay reinforced polymethylmethacrylate nanocomposite. Polym. Adv. Technol. 2011, 22, 1912–1918. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.; Zhang, L.; Ni, Q.; Gong, F. Preparation and properties of polycarbonate nanocomposites using attapulgite grafted poly (methyl methacrylate) as a potential nanofiller. J. Appl. Polym. Sci. 2015, 132, 1079–1083. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Chang, D.; Chen, D.; He, H.; Yuan, P.; Xie, J.; Frost, R.L. Characterization and catalytic performance of FegNis/palygorskite for catalyticcracking of benzene. Appl. Clay Sci. 2013, 74, 135–140. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Recent progress in dispersion of palygorskite crystal bundles for nanocomposites. Appl. Clay Sci. 2016, 119, 18–30. [Google Scholar] [CrossRef]
- Xu, J.; Han, W.; Yin, Q.; Song, J.; Zhong, H. Direct Electron Transfer of Glucose Oxidase and Glucose Biosensor Based on Nano-structural Attapulgite Clay Matrix. Chin. J. Chem. 2009, 27, 2197–2202. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Fernandes, F.M.; Wicklein, B.; Alcântara, A.C.; Aranda, P. Fibrous clays based bionanocomposites. Prog. Polym. Sci. 2013, 38, 1392–1414. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Nanoscale clay minerals for functional ecomaterials: Fabrication, applications, and future trends. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Chryssikos, G.D.; Gionis, V.; Kacandes, G.H.; Stathopoulou, E.T.; Suarez, M.; Garcia-Romero, E.; del Rio, M.S. Octahedral cation distribution in palygorskite. Am. Miner. 2009, 94, 200–203. [Google Scholar] [CrossRef]
- Garia-Romero, E.; Suarez, M. Sepiolite-palygorskite: Textural study and genetic considerations. Appl. Clay Sci. 2013, 86, 129–144. [Google Scholar] [CrossRef]
- Garcia-Romero, E.; Suarez, M. Sepiolite-palygorskite polysomatic series: Oriented aggregation as a crystal growth mechanism in natural environments. Am. Mineral. 2014, 99, 1653–1661. [Google Scholar] [CrossRef]
- Liu, F.; Feng, X.H.; Chen, X.H.; Qiu, G.G.; Tan, W.F.; He, J.Z. Advances in the study of biological genesis of manganese oxide minerals and their characteristics. Earth Sci. Front. 2008, 15, 66–73. [Google Scholar]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287328. [Google Scholar] [CrossRef]
- Lu, A.H.; Lu, X.Y.; Ren, Z.P.; Han, L.R.; Fang, Q.F.; Han, Y. New advances in environmental mineralogy of natural oxides and hydroxides of iron and manganese. Earth Sci. Front. 2000, 7, 473–483. [Google Scholar]
- Sunda, W.G.; Kieber, D.J. Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature 1994, 367, 62–64. [Google Scholar] [CrossRef]
- Chen, Z.; Kim, K.W.; Zhu, Y.G.; McLaren, R.; Liu, F.; He, J.Z. Adsorption (AsIII, V) and oxidation (AsIII) of arsenic by pedogenic Fe–Mn nodules. Geoderma 2006, 136, 566–572. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, X.; Zhou, Z.; Lei, Y.; Ma, M.; Cao, R.; Sun, T.; Xu, J.; Huo, M.; Cao, R.; et al. Prenatal exposure to nanoparticulate titanium dioxide enhances depressive-like behaviors in adult rats. Chemosphere 2014, 96, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cao, J. Metal-containing nanoparticles derived from concealed metal deposits: An important source of toxic nanoparticles in aquatic environments. Chemosphere 2019, 224, 726–733. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for differentregions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Gottschalk, F.; Scholz, R.W.; Nowack, B. Probabilistic material flow modeling for assessing the environmental exposure to compounds: Methodology and an application to engineered nano-TiO2 particles. Environ. Model. Softw. 2010, 25, 320–332. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.Y.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Arvidsson, R.; Molander, S.; Sandén, B.A. Particle flow analysis: Exploring potential use phase emissions of titanium dioxide nanoparticles from sunscreen, paint, and cement. J. Ind. Ecol. 2012, 16, 343–351. [Google Scholar] [CrossRef]
- Shao, G.Y.; Li, C.S.; Liu, R.; Zhang, P.; Zuo, L.; Wang, Y. Silicified Microorganisms and Microorganism-Like Particlesin the Groundwater of an Abandoned Coal Mine. Mine Water Environ. 2023, 42, 489–499. [Google Scholar] [CrossRef]



| Sampling Times | Sr (mg/L) | K+ (mg/L) | Na+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | Cl- (mg/L) | SO42− (mg/L) | HCO3− (mg/L) | NO3− (mg/L) | TDS (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| 25 December 2016 | 2.638 | 1.28 | 5.84 | 68.0 | 35.0 | 4.97 | 54.4 | 309 | 5.74 | 493 |
| 23 May 2017 | 3.01 | 1.19 | 4.93 | 67.6 | 35.9 | 8.75 | 59.3 | 314 | 5.67 | 507 |
| 2.64 | 1.32 | 5.39 | 67.0 | 36.0 | 4.86 | 54.4 | 312 | 5.36 | 496 | |
| 22 September 2017 | 3.01 | 1.25 | 5.16 | 69.4 | 36.4 | 5.25 | 59.6 | 317 | 5.49 | 509 |
| 2.89 | 1.25 | 6.36 | 68.5 | 36.5 | 4.76 | 61.4 | 314 | 5.53 | 499 | |
| 19 December 2017 | 3.00 | 1.14 | 5.07 | 70.8 | 37.8 | 5.60 | 60.6 | 314 | 6.03 | 510 |
| 2.89 | 1.30 | 6.31 | 70.7 | 37.1 | 5.12 | 62.2 | 318 | 4.05 | 511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Zhao, Z.; Lv, M.; Wang, H.; Li, L.; Gang, S.; Zuo, L.; Zhang, P.; Wang, Y.; Li, C.; et al. Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water. Water 2023, 15, 3497. https://doi.org/10.3390/w15193497
Liu R, Zhao Z, Lv M, Wang H, Li L, Gang S, Zuo L, Zhang P, Wang Y, Li C, et al. Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water. Water. 2023; 15(19):3497. https://doi.org/10.3390/w15193497
Chicago/Turabian StyleLiu, Rui, Zhiqiang Zhao, Minghui Lv, Hongwei Wang, Lixia Li, Shenting Gang, Lei Zuo, Peng Zhang, Yaqin Wang, Changsuo Li, and et al. 2023. "Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water" Water 15, no. 19: 3497. https://doi.org/10.3390/w15193497
APA StyleLiu, R., Zhao, Z., Lv, M., Wang, H., Li, L., Gang, S., Zuo, L., Zhang, P., Wang, Y., Li, C., & Lu, Q. (2023). Discovery of Environmental Nanoparticles in a Mineral Water Spring from Yiyuan County, Shandong Province, Eastern China: A New Form of Elements in Mineral Water. Water, 15(19), 3497. https://doi.org/10.3390/w15193497







