The Bogs in a Forest–Steppe Region of Western Siberia: Plant Biomass and Net Primary Production (NPP)
Abstract
:1. Introduction
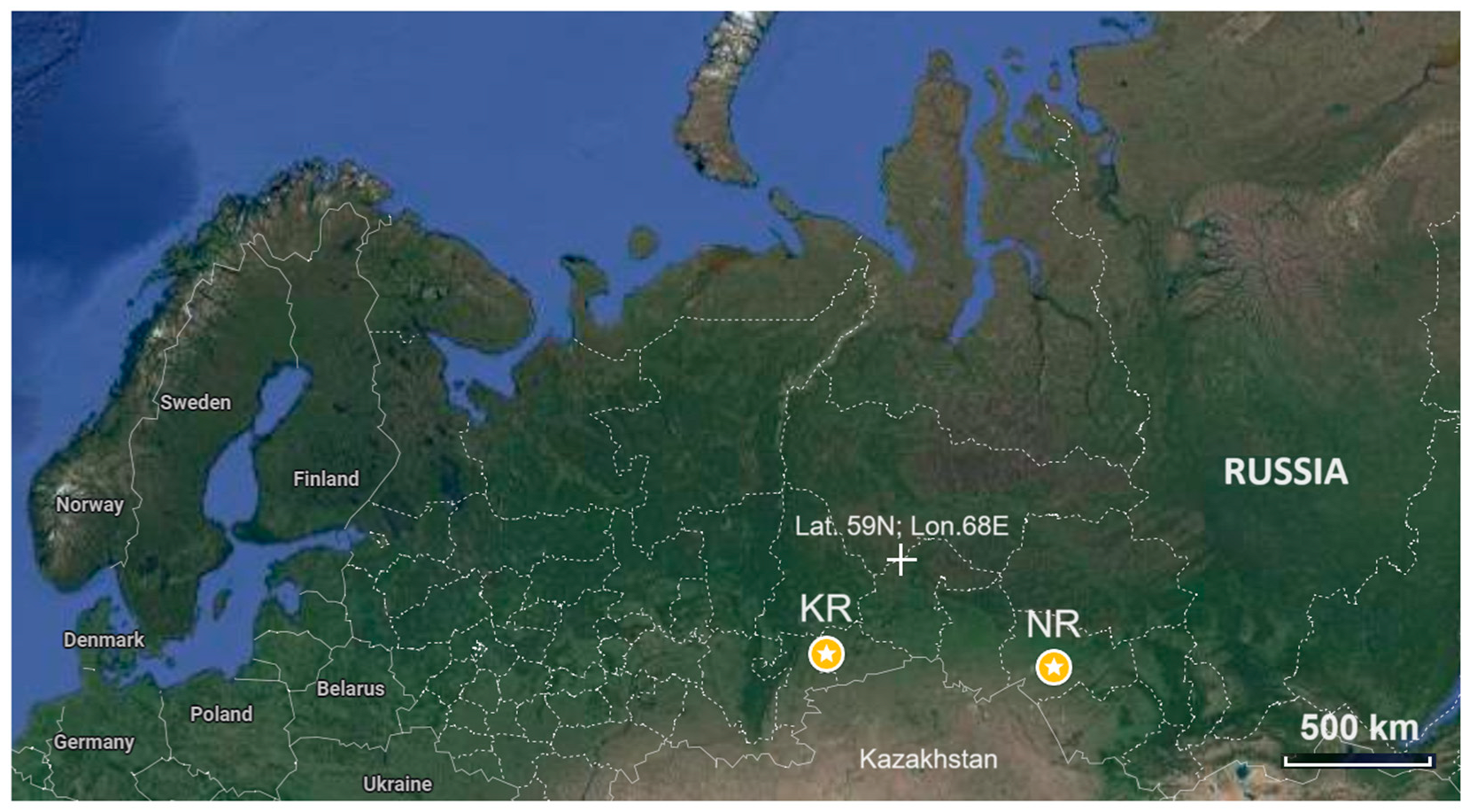
2. Materials and Methods
3. Results
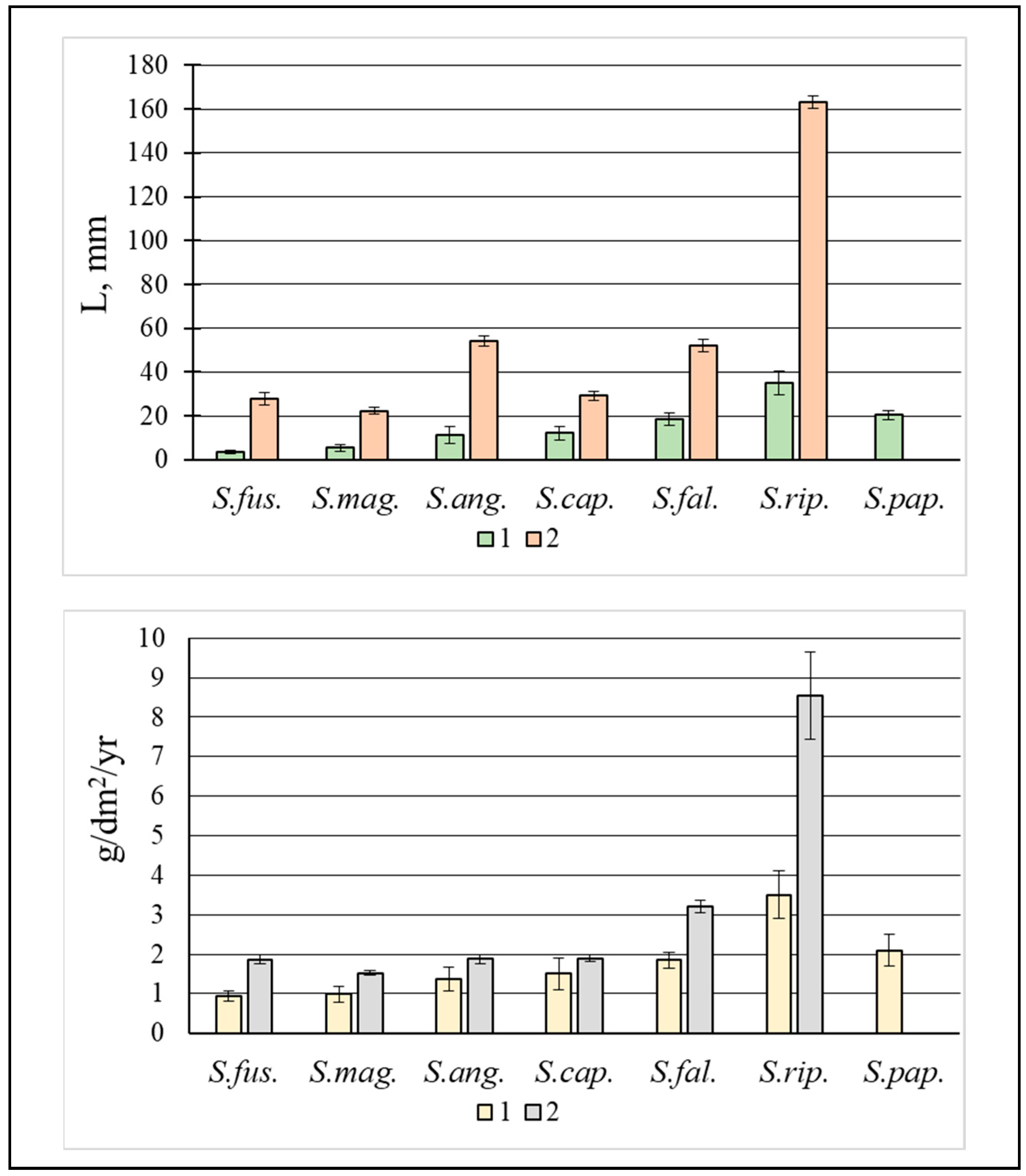
3.1. Dead Biomass (Mortmass)
3.2. Live Biomass
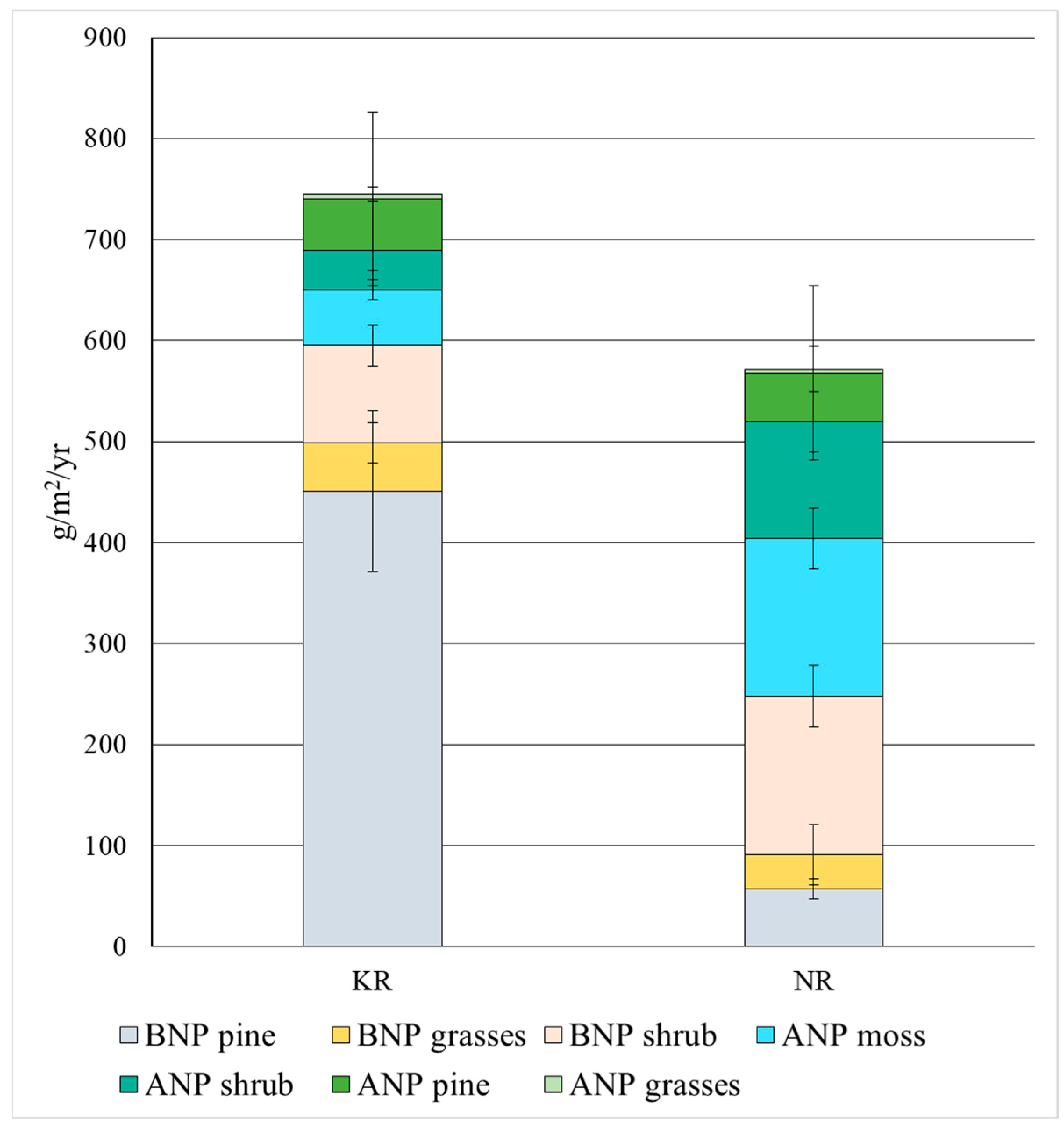
3.3. Net Primary Production (NPP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gorham, E. Northern peatlands: Role in carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Vompersky, S.E.; Ivanova, A.I.; Tsyganova, O.P.; Valiaeva, N.A.; Glukhova, T.V.; Dubinin, F.I.; Markelova, L.G. Wet soils and mires in Russia and their carbon pool. Pochvovedenie 1994, 12, 17–25. (In Russian) [Google Scholar]
- Yu, Z.; Joos, F.; Bauska, T.K.; Stocker, B.D.; Fischer, H.; Loisel, J.; Brovkin, V.; Hugelius, G.; Nehrbass-Ahles, C.; Kleinen, T.; et al. No support for carbon storage of >1000 GtC in northern peatlands. Earth arXiv 2019, reprints. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Kirpotin, S.N.; Antoshkina, O.A.; Berezin, A.E.; Elshehawi, S.; Feurdean, A.; Lapshina, E.D.; Pokrovsky, O.S.; Peregon, A.; Semenova, N.M.; Tanneberger, F.; et al. Great Vasyugan Mire: How the World’s largest peatland helps addressing the World’s largest problems. Ambio 2021, 50, 2038–2049. [Google Scholar] [CrossRef]
- Botch, M.S.; Kobak, K.I.; Vinson, T.S.; Kolchugina, T.P. Carbon pools and accumulation in peatlands of the former Soviet Union. Global Biogeochem. Cycles 1995, 9, 37–46. [Google Scholar] [CrossRef]
- Lapshina, E. Spatial structure of the vegetation cover in the Vasyugan Mire. In Mires from Siberia to Tierra del Fuego; Steiner, G.M., Ed.; Stapfia; OÖ Landes-Kultur GmbH: Linz, Austria, 2005; Volume 85, pp. 296–304. [Google Scholar]
- Lapshina, E.D.; Pologova, N.N.; Muldiyarov, Y.Y. Mires of watershed plains in the south of the boreal region in Western Siberia. Krylovia 2000, 2, 38–43. (In Russian) [Google Scholar]
- Peregon, A.; Maksyutov, S.; Kosykh, N.; Mironysheva-Tokareva, N. Map based inventory of the wetland biomass and NPP in western Siberia. J. Geophys. Res. 2008, 113, G01007. [Google Scholar] [CrossRef]
- Bronzov, A.Y. Raised bogs of the Narymski Krai (Vasyugan Basin). Proc. Peat Inst. 1930, 3, 1–99. (In Russian) [Google Scholar]
- Romanova, E.T. Bog of Western Siberia; Gidrometeoisdat: Leningrad, Russia, 1976; pp. 25–33. (In Russian) [Google Scholar]
- Romanova, E.A. Mire Vegetation. The Land-Cover of the West Siberian Plain; Nauka: Novosibirsk, Russia, 1985; pp. 138–161. (In Russian) [Google Scholar]
- Liss, O.L.; Abramova, L.I.; Avertov, N.A.; Berezina, N.A.; Inisheva, L.I.; Kournikova, T.V.; Sluka, Z.A.; Tolpysheva, T.Y.; Shvedchikova, N.K. Bog Systems of Western Siberia and Their Environmental Significance; Grif and K°.: Tula, Russia, 2001; 584p. [Google Scholar]
- Leifeld, J.; Wüst-Galley, C.; Page, S. Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nat. Clim. Chang. 2019, 9, 945–947. [Google Scholar] [CrossRef]
- Mikhalchuk, A.; Kharanzhevskaya, Y.; Burnashova, E.; Nekhoda, E.; Gammerschmidt, I.; Akerman, E.; Kirpotin, S.; Nikitkin, V.; Khovalyg, A.; Vorobyev, S. Soil Water Regime, Air Temperature, and Precipitation as the Main Drivers of the Future Greenhouse Gas Emissions from West Siberian Peatlands. Water 2023, 15, 3056. [Google Scholar] [CrossRef]
- Valutsky, V.I. Vegetation of forest-steppe ryams in East Baraba. Turczaninowia 2011, 14, 109–119. [Google Scholar]
- Bazilevich, N.I. Biological Productivity of Ecosystems of Northern Eurasia; Nauka: Moscow, Russia, 1993; 293p. (In Russian) [Google Scholar]
- Naumov, A.V.; Kosykh, N.P.; Mironycheva-Tokareva, N.P. Network environment analysis of a model of carbon flows in a peat bog and fen. Mires and Peat 2020, 26, 1–18. [Google Scholar]
- Reader, R.J.; Stewart, J.M. The relationship between net primary production and accumulation for a peatland in southern-eastern Manitoba. Ecology 1972, 53, 1024–1037. [Google Scholar] [CrossRef]
- Vitt, D.H. Growth and production dynamics of boreal mosses over climatic, chemical and topographic gradients. Bot. J. Linn. Soc. 1990, 104, 35–59. [Google Scholar] [CrossRef]
- Moore, T.R.; Bubier, J.L.; Frolking, S.E.; Lafleur, P.M.; Roulet, N.T. Plant biomass and production and CO2 exchange in an ombrotrophic bog. J. Ecol. 2002, 90, 25–36. [Google Scholar] [CrossRef]
- Thormann, M.N.; Bayley, S.E. Aboveground net primary production along a bog–fen–marsh gradient in southern boreal Alberta, Canada. Ecoscience 1997, 4, 374–384. [Google Scholar] [CrossRef]
- Szumigalski, A.R.; Bayley, S.E. Net aboveground primary production along a peatland gradient in central Alberta in relation to environmental factors. Ecoscience 1997, 4, 385–393. [Google Scholar] [CrossRef]
- Campbell, C.; Vitt, D.H.; Halsey, L.A.; Campbell, I.D.; Thormann, M.N.; Bayley, S.E. Net Primary Production and Standing Biomass in Northern Continental Wetlands; Canadian Forestry Service Information Report NOR-X-369; Canadian Forestry Service: Edmonton, AB, Canada, 2000. [Google Scholar]
- Backéus, I. Production and depth distribution of fine roots in a boreal open bog. Ann. Bot. Fenn. 1990, 27, 261–265. [Google Scholar]
- Kosykh, N.P.; Koronatova, N.G.; Naumova, N.B.; Titlyanova, A.A. Above- and below-ground phytomass and net primary production in boreal mire ecosystems of Western Siberia. Wetl. Ecol. Manag. 2008, 16, 139–153. [Google Scholar] [CrossRef]
- Bazilevich, N.I.; Titlyanova, A.A. Biotic Cycle on Five Continents; SB RAS: Novosibirsk, Russia, 2008; 380p. (In Russian) [Google Scholar]
- Vagina, T.A.; Shatochina, N.G. Productivity of plant communities and the influence of soil and weather conditions. In Structure, Functioning and Evolution of the System of Biogecenoses of Baraba; Nauka SB: Novosibirsk, Russia, 1976; pp. 217–359. (In Russian) [Google Scholar]
- Naumov, A.V.; Kosykh, N.P.; Parshina, E.K.; Artymuk, S.Y. Raised bogs of the forest-steppe zone, their condition and monitoring. Sib. J. Ecol. 2009, 2, 251–259. [Google Scholar]
- Kosykh, N.P. Biological productivity of mire in the forest-steppe zone. Bull. TGPU 2009, 3, 87–91. [Google Scholar]
- Bazilevich, N.I. Productivity and biological circulation in the moss swamps of Southern Vasyugan. Plant Resour. 1967, 3, 567–589. (In Russian) [Google Scholar]
- Valutskiy, V.I.; Khramov, A.A. Structure and net primary production of ryams in the south-eastern Vasyugan region. In Theory and Practice of Forest Swamp Management and Hydroforestry Reclamation; ILiD SO AN USSR: Krasnoyarsk, Russia, 1976; pp. 59–82. (In Russian) [Google Scholar]
- Efremov, S.P.; Efremova, T.T.; Bleuten, V. Biological productivity and carbon pool of phytomass in forested wetlands in Western Siberia. Sib. J. Ecol. 2005, 1, 29–44. (In Russian) [Google Scholar]
- Golovatskaya, E.A. Biological productivity of oligotrophic and eutrophic mires in the Southern Taiga of Western Siberia. J. Sib. Fed. Uni. Biol. 2009, 2, 38–53. (In Russian) [Google Scholar]
- Golovatskaya, E.A. Biomass and productivity of the wooden tier in pine-dwarf shrubs-Sphagnum bogs in south taiga of Western Siberia. Forestry 2017, 2, 102–110. (In Russian) [Google Scholar]
- Inisheva, L.I.; Golovatskaya, E.A. Elements of the carbon balance of oligotrophic bogs at the edges of the Vasyugan mires. Ecology 2002, 4, 242–249. (In Russian) [Google Scholar]
- Piyavchenko, N.I. Biological productivity and flow of matter in paludified forests in Western Siberia. Forestry 1967, 3, 32–43. (In Russian) [Google Scholar]
- Piyavchenko, N.I. On the productivity of wetlands in Western Siberia. Plant Resour. 1967, III, 523–533. (In Russian) [Google Scholar]
- Titlyanova, A.A. Net primary production of grasslands and wetland ecosystems. Sib. J. Ecol. 2007, 5, 763–770. (In Russian) [Google Scholar]
- Titlyanova, A.A.; Mironycheva-Tokareva, N.P. Vegetation succession and biological turnover on coal mining spoil. J. Veget. Sci. 1990, 14, 643–652. [Google Scholar] [CrossRef]
- Titlyanova, A.A.; Kosykh, N.P.; Mironycheva-Tokareva, N.P. Dynamics of below-ground plants organs in grasslands. In Root Demographics and Their Efficiencies in Sustainable Agriculture, Grasslands and Forest Ecosystems; Kluwer Acad. Publishers: Dordrecht, The Netherlands, 1998; pp. 247–265. [Google Scholar]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Peregon, A.M.; Parshina, E.K. Net primary production in peatlands of middle taiga region in western Siberia. Russ. J. Ecol. 2008, 39, 466–474. [Google Scholar] [CrossRef]
- Cherepanov, S.K. Vascular Plants of Russia and Neighboring Countries; SPb: St. Petersburg, Russia, 1995; 992p. (In Russian) [Google Scholar]
- Bengtsson, F.; Granath, G.; Rydin, H. Photosynthesis, growth, and decay traits in Sphagnum—A multispecies comparison. Ecol. Evol. 2016, 6, 3325–3341. [Google Scholar] [CrossRef] [PubMed]
- Kosykh, N.P.; Koronatova, N.G.; Granath, G. Effect of Temperature and Precipitation on Linear Increment of Sphagnum fuscum and S. magellanicum in Western Siberia. Russ. J. Ecol. 2017, 48, 173–181. [Google Scholar]
- Bazilevich, N.I.; Grebenshchikov, O.S.; Tishkov, A.A. Geographical Patterns of Structure and Functioning of Ecosystems; Nauka: Moscow, Russia, 1986; 297p. (In Russian) [Google Scholar]
- Wallen, B. Above and below-ground dry mass of the three main vascular plants on hummocks on a subarctic peat bog. Oikos 1986, 46, 51–56. [Google Scholar] [CrossRef]
- Kopoteva, T.A.; Kosykh, N.P. Comparative characteristics of the structure of phytomass and productivity of mesotrophic shrub-Sphagnum bogs in the boreal region. Sib. J. Ecol. 2011, 2, 301–307. [Google Scholar]
- Wallen, B. Methods for studing below-ground production in mire ecosystems. Suo 1992, 43, 155–162. [Google Scholar]
- Murphy, M.T.; McKinley, A.; Moore, T.R. Variation in above-and below-ground vascular plant biomass and water table on a temperate ombrotrophic peatland. Botany 2009, 87, 845–853. [Google Scholar] [CrossRef]
- Saarinen, T. Vascular Plants as Input of Carbon in Boreal Sedge Fens: Control of Production and Partitioning of Biomass; Publications in Botany, University of Helsinki: Yliopistopaino, Finland, 1999; 66p. [Google Scholar]
| Fractions of Dead Biomass (Mortmass) | KR | NR |
|---|---|---|
| Litter and above-ground residues | 461 ± 168 | 187 ± 29 |
| Wooden residues (pine trees) | 7584 ± 2376 | 0 |
| Vascular plants | 660 ± 272 | 856 ± 468 |
| Sphagnum mosses | 11,373 ± 3156 | 6016 ± 1391 |
| Total | 20,077 ± 3666 | 7059 ± 1881 |
| Fractions of Live Biomass | KR | NR |
|---|---|---|
| Pine above-ground phytomass | 6550 ± 782 | 995 ± 141 |
| Pine roots (0–30 cm) | 1474 ± 142 | 409 ± 95 |
| Moss phytomass | 135 ± 17 | 419 ± 69 |
| Above-ground phytomass of grasses | 5 ± 2 | 4 ± 1 |
| Above-ground phytomass of shrubs | 118 ± 7 | 460 ± 112 |
| Below-ground phytomass of grasses (incl. sedges) | 53 ± 2 | 28 ± 5 |
| Below-ground parts of shrubs | 629 ± 10 | 1386 ± 127 |
| Total (except wooden fractions) | 940 ± 291 | 2297 ± 220 |
| Total (incl. wooden fractions/pine trees) | 8964 ± 195 | 3701 ± 281 |
| Chamaedaphne calyculata | Ledum palustre | Vaccinium vitis-idaea | ||||
|---|---|---|---|---|---|---|
| Study site | Green | Shoots | Green | Shoots | Green | Shoots |
| KR | 9.3 | 29.7 | 7.2 | 24.1 | 19.6 | 21.4 |
| NR | 37.2 | 179.6 | 50.5 | 172.1 | 5.7 | 2.9 |
| Net Primary Production (NPP) | KR | NR |
|---|---|---|
| ANP 1 of pine trees | 51 ± 3 | 48 ± 5 |
| ANP of grasses | 5 ± 1 | 4 ± 1 |
| ANP of shrubs | 39 ± 7 | 116 ± 22 |
| ANP of Sphagnum mosses | 55 ± 8 | 156 ± 30 |
| BNP 2 of grasses | 48 ± 9 | 34 ± 7 |
| BNP of shrubs | 96 ± 20 | 157 ± 30 |
| BNP of pine trees | 451 ± 80 | 57 ± 10 |
| Total ANP (except wooden fractions) | 99 ± 5 | 276 ± 43 |
| Total BNP (except wooden fractions) | 144 ± 42 | 191 ± 40 |
| Total NPP (ANP + BNP, except wooden fractions) | 243 ± 57 | 467 ± 59 |
| Total ANP | 150 ± 26 | 324 ± 43 |
| Total BNP | 595 ± 98 | 248 ± 85 |
| Total NPP (ANP + BNP) | 745 ± 152 | 572 ± 111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosykh, N.P.; Koronatova, N.G.; Mironycheva-Tokareva, N.P.; Vishnyakova, E.K.; Ivchenko, T.G.; Kurbatskaya, S.S.; Peregon, A.M. The Bogs in a Forest–Steppe Region of Western Siberia: Plant Biomass and Net Primary Production (NPP). Water 2023, 15, 3526. https://doi.org/10.3390/w15203526
Kosykh NP, Koronatova NG, Mironycheva-Tokareva NP, Vishnyakova EK, Ivchenko TG, Kurbatskaya SS, Peregon AM. The Bogs in a Forest–Steppe Region of Western Siberia: Plant Biomass and Net Primary Production (NPP). Water. 2023; 15(20):3526. https://doi.org/10.3390/w15203526
Chicago/Turabian StyleKosykh, Natalia P., Natalia G. Koronatova, Nina P. Mironycheva-Tokareva, Evgenia K. Vishnyakova, Tatiana G. Ivchenko, Svetlana S. Kurbatskaya, and Anna M. Peregon. 2023. "The Bogs in a Forest–Steppe Region of Western Siberia: Plant Biomass and Net Primary Production (NPP)" Water 15, no. 20: 3526. https://doi.org/10.3390/w15203526
APA StyleKosykh, N. P., Koronatova, N. G., Mironycheva-Tokareva, N. P., Vishnyakova, E. K., Ivchenko, T. G., Kurbatskaya, S. S., & Peregon, A. M. (2023). The Bogs in a Forest–Steppe Region of Western Siberia: Plant Biomass and Net Primary Production (NPP). Water, 15(20), 3526. https://doi.org/10.3390/w15203526







