Abstract
Suckermouth armored catfish (Pterygoplichthys disjunctivus) is one of the most widespread invasive species in Vietnam. However, it is relatively unknown how the species underwent its divergent adaptations to varying local conditions and habitat types, an understanding of which is essential for managing its invasion in Vietnam. We addressed this by analyzing a large number of fish (662 samples) collected in lotic (Dinh River) and lentic (Suoi Trau Reservoir) habitats in southern Vietnam during one year. The allometric growth patterns estimated by von Bertalanffy growth functions were in Dinh River and in Suoi Trau Reservoir. The estimated fish ages were 2.9 to 4.2 years old with an average total length from 206.10 ± 2.09 mm in Suoi Trau Reservoir to 319.22 ± 3.29 mm in Dinh River. The percentage of fish with matured ovaries peaked in August (100%) and was lowest in February (<10%), indicating that these fish breed nearly all year round. The main reproductive season is April–October, with a peak in July–August, as indicated by the gonado-somatic index and monthly changes in the percentage of matured fish. The lengths at 50% maturity were 234.3 and 179.7 mm for females from lotic and lentic habitats, respectively. Lotic fish had a fecundity (4812 ± 383 oocytes/ind.) which was five times greater than that of lentic fish (841 ± 91 oocytes/ind.); however, the relative fecundity of the fish was not statistically different between the two habitats (≈13 oocytes/g). This result was consistent with the larger oocytes: 2.95 ± 0.04 and 2.58 ± 0.01 mm for fish from Dinh River and Suoi Trau Reservoir, respectively. The faster growth and bigger fish with higher fecundity in the lotic habitats suggest that their population growth can accelerate more quickly, potentially affecting local communities more than those in lentic habitats. Our study sheds light on substantial phenotypic divergences in the reproduction and growth of the suckermouth armored catfish between lotic and limnetic habitats in Vietnam.
1. Introduction
Invasive species have recently been identified as one of five important drivers pushing ~1 million species to the brink of extinction [1]. Thousands of species have been moved from their native habitats, as both the accidental and intended results of human activities, and become invasive species in other regions [2]. Invasive species have had serious consequences for the environment such as altering the abundance and diversity of local communities and the function and ecological services of invaded habitats [3,4,5]. Recently, the invasions have represented one of the most serious threats to freshwater biodiversity [6,7]. In freshwater systems, armored catfishes (Pterygoplichthys), originating from South America, have invaded 55 water bodies in 21 countries in Asia, Europe, Africa and North America [8,9]. For example, species invasions by P. pardalis have caused huge damage to freshwater species diversity in India and the Philippines [8,10]. Pterygoplichthys spp. have long digestive tracks (up to 6 m with Lt = 310 mm, mean = 2.7 m with Lt = 250 mm) [11], which are typical for phytophagous and detritophagous fish [12,13], a broad food spectrum, a fast growth rate and high tolerance to environmental conditions. They outcompete native species for food and habitats [11,14,15,16], and they change the composition, structure, reproduction and function of local freshwater communities [15,17,18,19,20]. Pterygoplichthys are a severe threat to freshwater fisheries in Eurasia, the United States of America [21,22] and Mexico [14,23].
In Vietnam, Pterygoplichthys first appeared in the Dong Nai River system and Tri An Reservoir in 2000 [20] after they escaped from local aquariums. These species spread quickly to other freshwater bodies in Vietnam. Currently, Pterygoplichthys are present in all the main river basins and reservoirs in southern Vietnam [15,17,19,20]. Given that these species are thriving in freshwater habitats in Vietnam, it is supposed that they have undergone locally divergent adaptations by changes in morphology, diet, growth, development and reproduction, the key characteristics for successful invasions [11,20]. A few studies have documented some useful but very limited information on the reproduction of this species in Vietnam. The reproductive biology of Pterygoplichthys spp. was first examined in Dinh River from 2010 to 2012 with only 34 individuals (5 females) in October-December 2010, and 10 individuals (6 females) in January 2012 [24]. The catfishes spawned from October to January and their gonads included different stage oocytes (1–3 mm in diameter). These results did not allow us to overview the reproductive cycle of Pterygoplichthys and how they had acquired different growth and reproductive strategies in various habitats. The data obtained through morphological and molecular-genetic analyses, as well as the assessment of the color patterns of fish, have shown that the Vietnamese reservoirs and rivers are inhabited by armored catfishes of two species: Pterygoplichthys pardalis (Castelnau, 1855) and P. disjunctivus (Weber, 1991). The frequency of P. disjunctivus was about three times greater than that of P. pardalis [20], further challenging the efforts for the conservation and management of freshwater habitats in Vietnam.
In this study, we investigated the growth and reproductive biology of P. disjunctivus in lotic and in lentic habitats in southern Vietnam for one year. We determined the length–weight relationship and growth parameters, male–female distinction, sex ratio, gonadal development stages, spawning season, size at the first sexual maturity, fecundity, and oocyte diameter. By comparing these characteristics of fish in different habitats we highlighted phenotypic divergences of P. disjunctivus. Our results provide an important scientific basis for the management and further control of the spread of this invasive species in Vietnam.
2. Materials and Methods
2.1. Sampling Populations
From March 2019 to February 2020, 4 multi-mesh gillnets (2a = 20 mm × 20 mm) were used to collect P. disjunctivus from one locale each in Dinh River (lotic habitat) and Suoi Trau Reservoir (lentic habitat) in southern Vietnam. These are two typical types of water in which suckermouth armored catfish (P. disjunctivus) have appeared [17,19,20] (Figure 1). Fish were caught from 4 pm to 5 am in the following morning. In total, 325 female and 337 male P. disjunctivus were collected from the two study sites. The location, hydrological measurements and some water quality parameters of the sampling stations are presented in Figure 1 and Table 1. We used Hanna HI 9829 (Hanna, Nușfalău, Romania) and Global Water (Emin, Taiwan) to measure water parameters, and total phosphorus and total nitrogen content were analyzed at the Institute of Oceanography (Nha Trang, Vietnam) by the Kjeldahl method.
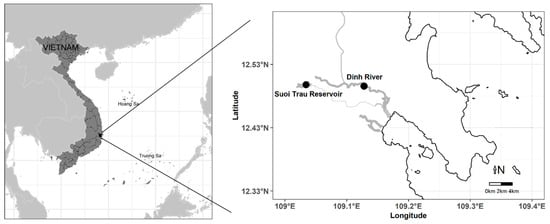
Figure 1.
Map of the two study sites in southern Vietnam (dark spots were sampling locations).

Table 1.
Geographical and physicochemical parameters of the two study sites in southern Vietnam.
Temperature and rainfall data were collected from a meteorology station in Ninh Hoa, a district-level town of Khanh Hoa province in the southern Central Coast region of Vietnam, from January 2019 to January 2020 (Figure 2). Briefly, the temperature varied between 25.2 and 31.4 °C (higher from April to October) and the monthly average rainfall was 0–271.7 mm (higher from September to November).
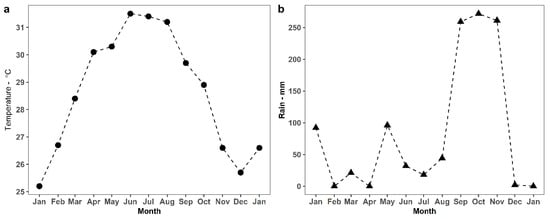
Figure 2.
Air temperature (a) and rainfall (b) in Ninh Hoa province, including Dinh River and Suoi Trau Reservoir, Vietnam.
2.2. Sample Processing and Data Collection
The fish were transported to the laboratory at the Coastal Branch of the Vietnam-Russia Tropical Science and Technology Research Center, Nha Trang City, Khanh Hoa Province, Vietnam. Fish were identified as the P. disjunctivus species based on the descriptions of Armbruster and Page (2006) [25]; P. disjunctivus have blotches and dots on their heads, as well as the majority of the dots on an adult’s abdomen coalescing to produce vermiculations. Total length (Lt) was measured to the nearest 0.1 and 1.0 mm, and total weight (Wt) and weight without internal organs (Wwi) were recorded to the nearest 0.1 g using a precision balance, the SHUN DA Digital Scale SD03-KS (Zhejiang, China). Gonad weight (Wg) was recorded to the nearest 0.01 g using a precision balance, the Electronic Precision Balance (KD-TBED-3000; KENDY, Taiwan), and to the nearest 0.0001 g using an Electronic Balance AX 200, No D 422800034 (Shimadzu, Kyoto, Japan).
Ovary development stages of fishes were determined using methods described by Nikolsky (1963) [26] and King (1995) [27], using a microscope (Olympus, CKX53SF; Tokyo, Japan) at 10× and 4× magnifications. Ovary development stages were recognized macroscopically based on the external morphology of the ovary and microscopically based on the sex cell structure.
The ovary of each gonadal development stage was dissected and fixed in 10% neutral formaldehyde solution for at least 24–48 h for histology. After dehydrating by passing the tissue through a series of alcohol solutions of 70, 85, 95 and 100%, the samples were embedded in paraffin. The histological sections (5–7 µm) were stained with hematoxylin and eosin (H&E) for microscopic examination. The samples were photographically analyzed and documented using a microscope (Olympus, CKX53SF) after the scale was calibrated. Images of histological sections were captured using a microscope (Model CKX53SF) connected to a digital camera (Olympus LC30, Olympus, U-T0.5XC-3, SN 7B02181).
The spawning season was identified as a period with more than 50% of fish having ovaries at stages III, IV and V and a greater GSI being observed [28]. A total of 30 ovaries at stages IV and V were used to estimate fecundity. Ovaries were divided into small portions and weighed (Wsp) and fixed in formalin (~1%). Subsequently, all oocytes in each portion were transferred to a counting chamber and the number of yolked oocytes (Oyolked) and number of all oocytes (Ot) were counted using the naked eye as well as a dissecting microscope Olympus SZR61.
Diameters of these top decile oocytes in the breeding season [29,30] were measured using the Measure Arbitrary Line functions of the LCmicro software (Product Version 2.1, Core Version XV 3.15, Package number 5180, copyright year 2016) after the scale was calibrated. Images of these oocytes were captured at 4× magnification using a microscope (Model CKX53SF) connected to a digital camera (Olympus LC30, Olympus, U-T0.5XC-3, SN 7B02181).
2.3. Data Analyses
Length distribution and length–weight relationship [31]
The relationship between total length and the total weight was calculated for each site using a power regression equation:
where:
a is the intercept of the regression or shape coefficient;
b is the allometric or slope parameter [32].
The optimal regression parameters were obtained by minimizing the residual errors using the ordinary least square method [33]. Student’s t-test was utilized to test whether the slope of regression was significantly different from 3, indicating the growth pattern of fish: isometric (b = 3, no change of density and shape as one fish grows), positive allometric (b > 3, the fish becomes relatively stouter or deeper-bodied as it becomes longer) or negative allometric (b < 3, fish becomes slimmer as it grows) [34]. Generally, the allometric coefficient (b) is within the range from 2 to 4 for most fish species [35], and thus this rule of thumb can be used to corroborate the validation of the length and weight relationships of P. disjunctivus in our study. All statistical analyses were performed using R software version 4.0.4 [36] with a significance level (α) equal to 0.05.
The von Bertalanffy growth model (VBGM) was used to describe the growth of P. disjunctivus [37]:
where is the expected or average L at time (or age) t, is the mean asymptotic L, K is the growth coefficient, expressing the rate (year−1) at which the is approached and is the hypothetical age at which fish L equals zero. While VBGM parameters can be estimated by length (weight)–age approach, the length-based method is the most extensively adopted, especially in tropical and subtropical regions [38]. In this study, length frequency datasets with constant class size (30 mm) were used to obtain the optimal growth parameters (corresponding to the maximum of the goodness of fit index, Rn) using the R software version 3.3.2 (ELEFAN functions in TropFishR package) [39]. The theoretical age () was determined by the empirical equation of [40]:
In order to compare the growth performance of different geographical populations, the growth performance index (GPI) f′ (phi-prime) was calculated using the following formula [41]:
The potential or expected longevity (tmax) of P. disjunctivus was calculated based on [40] empirical equation:
where tmax is the approximate maximum age of P. disjunctivus at each study site, and K is the growth constant in the von Bertalanffy growth function.
The gonado-somatic index (GSI) was calculated using the formula [9]:
GSI (%) = 100 × Wg/Wwi
The absolute first-batch fecundity (Fb) was estimated using the following formula [29,30]:
Fb (oocytes/ind.) = (Wg/Wsp) × Oyolked
The absolute total fecundity (Ft) was estimated using the following formula [42]:
Ft (oocytes/ind.) = (Wg/Wsp) × Ot
Relative first-batch fecundity (RFb) was calculated using the following formula [29,30]:
RFb (oocytes/g) = Fb/Wwi
Relative total fecundity (RFt) was calculated using the following formula [42]:
RFt (oocytes/g) = Ft/Wwi
To describe the reproductive strategy of catfishes, we developed an index of prediction for the number of spawns that was calculated as Fb/Ft (%) = Fb/Ft × 100. If index Fb/Ft = 100%, this shows that the catfish is a single spawner; if index Fb/Ft < 100%, this shows that the catfish is a multiple spawner.
The relationship between total fecundity and the total weight was statically determined using the following formula:
where a and b are constant values obtained from the linear model function in RStudio [43].
Ft = aWt + b
Similarly, the relationship between fecundity and total length is statically determined using the following formula:
where a and b are constant values obtained from the linear model function in RStudio [28].
Log(Ft) = aLog(Lt) + b
The fish were considered mature when ovaries were at stages III to V [27]. The size at first sexual maturity of female sucker-mouth catfish was estimated using a logistic regression of the form that was first fitted to the proportion of mature females to estimate size at maturity [27,44]:
in which y is the probability of an individual being mature at determinate; x (total length, mm), a (intercept) and b (slope) are estimated parameters.
y = 1/(1 + exp − (a + b × x),
Size at the first sexual maturity () was calculated as the average total length at which 50% of females had matured for each of habitats (Dinh River and Suoi Trau Reservoir) as:
Confidence intervals (2.5% and 97.5%) for estimates of size at maturity were then derived via bootstrapping procedures within SizeMat (N iterations = 1000) [44]. All analyses were done using the SizeMat package (version 0.2.0) within R statistical software (v 3.3.2, R Development Core Team, R Foundation for Statistical Computing) [44].
Sex ratio was determined by the ratio of male and female numbers. Chi-square test (R statistical software) was used to test the difference between the numbers of males and females in the natural population of fish. The Kruskal–Wallis analysis was performed to test the differences in length between males and females and between the fish from the two habitats which were non-normal distribution data. p < 0.05 was considered statistically significant.
3. Results
3.1. Length Frequency Distribution and Age Structure
There was a wide range in the total length of P. disjunctivus in both Dinh River (Lt = 177–451 mm, mean = 310.12 mm, for females; and 183–479, mean = 327.33 mm, for males) and Suoi Trau Reservoir (Lt = 82–313 mm, mean = 200.99 mm, for females; and 88–316 mm, mean = 211.54 mm, for males) (Figure 3 and Table 2).
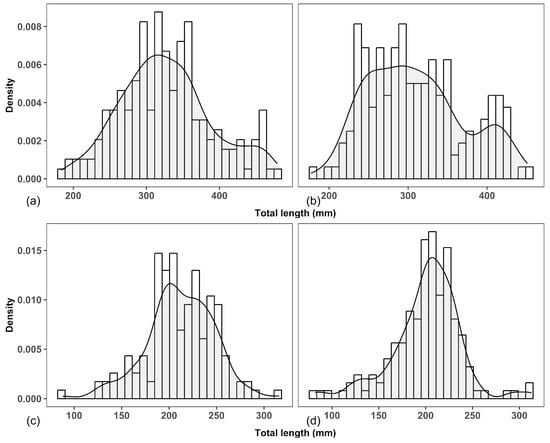
Figure 3.
Total length frequency distribution of males and females of Pterygoplichthys disjunctivus in Dinh River and Suoi Trau Reservoir: (a)—males in Dinh River; (b)—females in Dinh River; (c)—males in Suoi Trau Reservoir; (d)—females in Suoi Trau Reservoir.

Table 2.
Length–weight relationship of the Pterygoplichthys disjunctivus collected throughout the whole year in Vietnam based on the equation (a: intercept and b: slope of the equation). Minimum (min) and maximum (max) of length, weight; CI(b): confidence intervals of b; R2: correlation coefficient of the regression; p is significance of regression with all p being significant at < 0.05; t-test significance was conducted to verify if b is significantly different from consensus b = 3.
There was a statistical difference in the mean total length of females and males and between fish from the two habitats (all p values < 0.01, Kruskal–Wallis). Within each habitat, the difference between the total length of females and males was small; females were about ~5% smaller than males, which was consistent within both habitats. However, the difference in the total length of fish between habitats was pronounced; both females and males from Dinh River were about 1.5 times longer than those from Suoi Trau Reservoir (p < 0.01) (Figure 4 and Table 2). The estimated maximum age of fish was 4.2 and 2.9 years for fish from Dinh River (mean Lt = 319.22 ± 3.29 mm) and Suoi Trau Reservoir (mean Lt = 206.10 ± 2.09 mm), respectively.
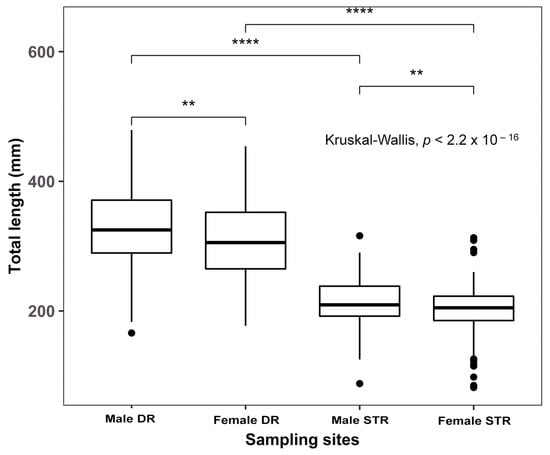
Figure 4.
Boxplot (horizontal line within box: median; boundaries of the box: first and third quartiles; bars: lower and upper inner fences; outliers) from Kruskal–Wallis test for the total length (Lt) of sexual Pterygoplichthys disjunctivus in Dinh River-DR and Suoi Trau Reservoir-STR (**: p < 0.01; ****: p < 0.001).
3.2. The Length–Weight Relationships and Growth Pattern
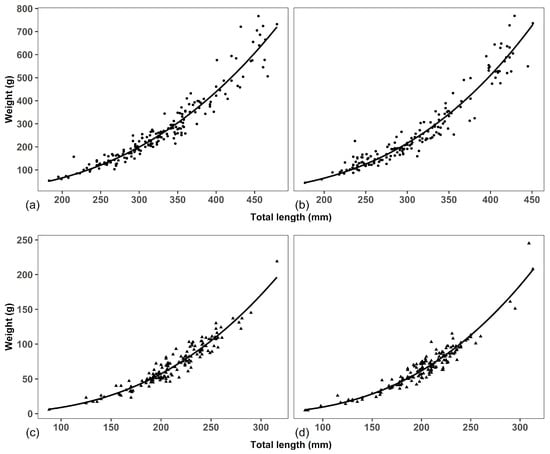
Figure 5.
Total length–weight relationship of males and females of Pterygoplichthys disjunctivus in Dinh River and Suoi Trau Reservoir: (a)—Males in Dinh River; (b)—Females in Dinh River; (c)—Males in Suoi Trau Reservoir; (d)—Females in Suoi Trau Reservoir.
Males in Dinh River: W = 0.00005 L2.66, R2 = 0.94, n = 190, p~0;
Females in Dinh River: W = 0.000023 L2.82, R2 = 0.93, n = 169, p~0;
Males in Suoi Trau Reservoir: W = 0.000035 L2.70, R2 = 0.93, n = 147, p~0;
Females in Suoi Trau Reservoir: W = 0.000039 L2.70, R2 = 0.94, n = 156, p~0;
All males and females had slope coefficients (b values) smaller than 3 regardless of the habitats (in Dinh River: t = −2.99, df = 167, p = 0.003 for females; and t = −6.94, df = 188, p = 6.16 × 10−11 for males; and in Suoi Trau Reservoir: t = −5.54, df = 154, p = 1.93 × 10−7 for females; and t = −4.76, df = 145, p = 4.6 × 10−6 for males), indicating a negative allometric growth pattern for all individuals from the sampling populations (Table 2).
3.3. Growth Parameters
In Dinh River, fish had = 483 mm, K = 0.71, = 0.40, = 5.22 and Rn = 0.366. For those from Suoi Trau Reservoir, they had = 346 mm, K = 1.01, = 0.62, = 5.08 and Rn = 0.598.
3.4. Sex Ratio
Morphology analysis of the gonads of these fish indicated that the sex of small fish (82 mm in length, 4.1 g in weight) could be determined using the naked eye. In both habitats, the ratio of armored catfish males and females was 1:1 (1.12:1 in Dinh River and 0.96:1 in Suoi Trau; p > 0.05). There was no significant difference in the ratio of males and females between the two water bodies (0.53; 0.49; p > 0.05).
3.5. Gonadal Maturation Stages
Six maturity stages, including the resting phase (I), developing phase (II), ripening phase (III), mature (IV), spawning phase (V) and recovering phase (VI), were identified based on the shape and size, the color of the ovaries and histological features (Table 3, Figure 6). Key histological features of the ovaries of armored catfishes in Khanh Hoa province are presented in Figure 6.

Table 3.
Maturity stages of female Pterygoplichthys disjunctivus introduced in Vietnam.
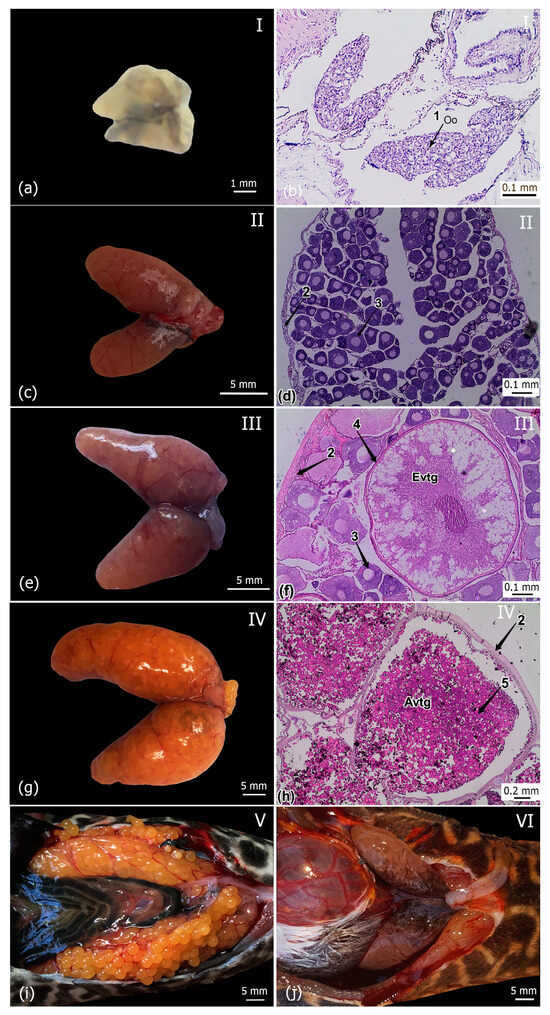
Figure 6.
Macroscopic and microscopic appearance of the ovaries of the female Pterygoplichthys disjunctivus at various maturity stages. Note: (a,b): stage I; (c,d): stage II; (e,f): stage III; (g,h): stage IV; (i): stage V; (j): stage VI; Evtg: early vitellogenic oocyte; Avtg: advanced vitellogenic oocyte; arrow 1—Oo: oogonium; arrow 2—ovarian wall; arrow 3—oocyte during previtellogenesis; arrow 4—oocyte during vitellogenesis; arrow 5—yolk granules in advanced vitellogenic oocyte.
In the resting phase, the ovaries were pale pink to translucent in color, containing mainly primary growth oocytes (PG) with very thin ovarian walls. In the developing phase, no oocyte was visible with the naked eye, and oocytes grew with a nucleus up to 50% of the whole oocyte area. In the ripening phase, oocytes can be seen by the naked eye and oocytes were pale pink in the H&E stained sections. In the mature phase, the ovaries turned to a deep yellow color (straw yellow or red-yellow), and the oocytes were dark purple in the H&E stained sections. In the spawning phase, oocytes were separate and came out with light pressure on the abdomen. In the recovering phase, the ovarian wall was thicker and ovaries were either empty or had a few leftover small eggs.
3.6. Spawning Season
3.6.1. Monthly Changes in the Percentage of Matured Fish
The matured fish presented all year round, but with a higher percentage during April–October compared to November–March. The percentage of fish with matured ovaries peaked in August (100%) and was lowest in February (<10%) (Figure 7a).
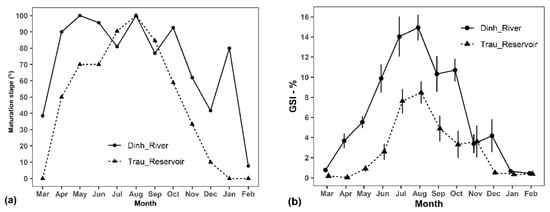
Figure 7.
Monthly percentages of maturation stages (III–V) and monthly changes in the gonado-somatic index (GSI) of the non-native Pterygoplichthys disjunctivus. Note: (a): monthly percentages of maturation stages (III–V); (b): monthly changes in the gonado-somatic index (GSI).
3.6.2. Monthly Changes in the Gonado-Somatic Index (GSI)
The highest GSIs were 16.63 ± 1.25% in fish from Dinh River and 8.48 ± 1.12% in fish from Suoi Trau Reservoir, all in August. The lowest GSI was in February (0.46 ± 0.07%) for fish from Dinh River and in April (0.04 ± 0.03%) for fish from Suoi Trau (Figure 7b).
3.7. Length at First Sexual Maturity (50% Maturity)
The total length at 50% maturity for females was 179.7 mm for fish from Suoi Trau Reservoir and 234.3 mm for fish from Dinh River (Figure 8). The smallest matured fish was observed in Suoi Trau with a Lt of 167 mm and a body weight of 38 g, compared to the smallest in Dinh River with a Lt of 224 mm and a body weight of 84 g.
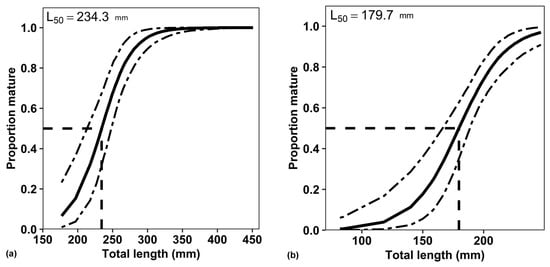
Figure 8.
Length at the first sexual maturity 50% (L50) of the non-native Pterygoplichthys disjunctivus: (a)—Dinh River, (b)—Suoi Trau Reservoir.
3.8. Fecundity
The absolute first-batch fecundity of the P. disjunctivus in Dinh River and Suoi Trau Reservoir was 4812 ± 383 oocytes/ind. (ranged from 103 to 8208 oocytes/ind.) and 841 ± 91 oocytes/ind. (ranged from 150 to 1993 oocytes/ind.), respectively. The relative first-batch fecundity of the P. disjunctivus in Dinh River and Suoi Trau Reservoir was 13.36 ± 0.81 oocytes/g (ranged from 0.69 to 21.32 oocytes/g) and 12.97 ± 1.19 oocytes/g (ranged from 3.26 to 25.23 oocytes/g), respectively. The difference in the absolute first-batch fecundity was no longer present when the fecundity was normalized for the body weight. Specifically, the relative fecundity of the fish was not statistically different between the two habitats (p > 0.05) (Table 4).

Table 4.
Reproductive parameters of Pterygoplichthys disjunctivus from Dinh River (n = 31) and Suoi Trau Reservoir (n = 31).
The absolute total fecundity in Dinh River and Suoi Trau Reservoir was 6000 ± 483 oocytes/ind. (ranged from 1303 to 10,574 oocytes/ind.) and 995 ± 101 oocytes/ind. (ranged from 150 to 2255 oocytes/ind.), respectively. The relative fecundity in Dinh River and Suoi Trau Reservoir was 16.63 ± 0.92 oocytes/g (ranging from 7.05 to 26.00 oocytes/g) and 15.48 ± 1.35 oocytes/g (ranging from 3.26 to 28.55 oocytes/g), respectively. There was no difference in the mean relative fecundity of the fish among the different sampling sites (p > 0.05) (Table 4). The index of Fb/Ft was 79.83 ± 2.73% (ranging from 7.90 to 92.72%) and 84.09 ± 2.00% (ranging from 59.26 to 100%) for fish from Dinh River and Suoi Trau Reservoir, respectively.
The fecundity was positively correlated with body weight and well explained (Ft = 13.676Wt − 290.128; R2 = 0.684; t = 7.93; p < 0.001; n = 31) and directly proportional to body weight for fishes in Dinh River (Figure 9a). Only with the R2 value (0.511) was it possible to explain this correlation in Suoi Trau Reservoir (Ft = 25.015Wt − 918.175; t = 5.406; p < 0.001; n = 30) (Figure 9b).
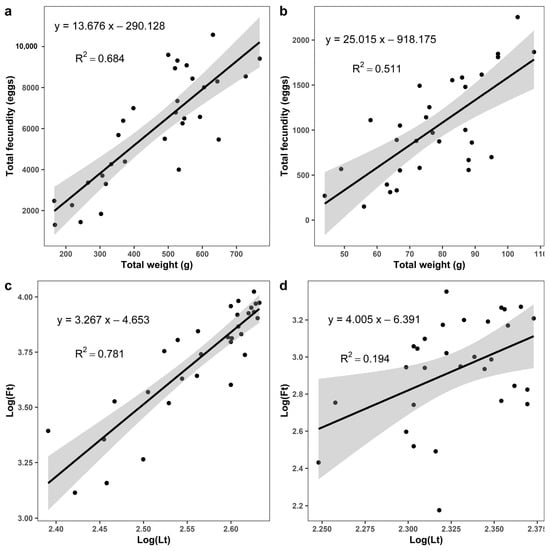
Figure 9.
Correlation between total fecundity (Ft, eggs) and total weight (Wt, g) of the Pterygoplichthys disjunctivus. The grey area indicates a 95% confidence interval: (a)—Dinh River; (b)—Suoi Trau Reservoir. Correlation between total fecundity—Log(Ft) and total length—Log(Lt): (c)—Dinh River; (d)—Suoi Trau Reservoir.
Similarly, the fecundity was positively correlated with total length (Log(Ft) = 3.267Log(Lt) − 4.653; R2 = 0.781; t = 10.181; p < 0.001; n = 31) and directly proportional to total length for fishes in Dinh River (Figure 9c). Only with the R2 value (0.194) could the correlation in Suoi Trau Reservoir be calculated (Log(Ft) = 4.005Log(Lt) − 6.391; t = 2.597; p = 0.015; n = 30) (Figure 9d).
3.9. Oocyte Diameter
The diameter of the top decile oocytes for stage IV–V ovaries of the P. disjunctivus in Dinh River and Suoi Trau Reservoir were 2.95 ± 0.04 mm (ranged from 2.50 to 3.41 mm) and 2.58 ± 0.01 mm (ranged from 2.42 to 2.80 mm), respectively. There was a significant difference in the mean diameter of the top decile oocytes between fish from lotic and those from lentic habitats (Table 4).
4. Discussion
We documented the first evidence of how suckermouth armored catfishes had undergone phenotypic divergences when inhabiting lotic and lentic habitats in Vietnam. Indeed, all the growth and reproductive results indicated that P. disjunctivus in lotic habitats had faster growth, higher fecundity, and greater longevity; these results were consistent in a large number of specimens collected from every month throughout the year. Furthermore, these populations were within the same climate region, suggesting that they may experience the same climatic conditions (Table 1). The substantial phenotypic divergences of the fish in lotic and lentic habitats may directly relate to hydrology, water flow (Table 1), food availability and predators. More details concerning how P. disjunctivus has undergone phenotypic divergences in growth and reproduction that are critical for its invasion of lotic and lentic habitats in Vietnam will be discussed in the following paragraphs.
In this study, both males and females of P. disjunctivus collected from the Dinh River (lotic habitat) and Suoi Trau Reservoir (lentic habitat) had negative allometric growth (b = 2.66–2.82), indicating that these fish were likely to be slimmer as they grew bigger. This finding was consistent with the growth pattern of P. disjunctivus from Adolfo López Mateos El Infiernillo Reservoir in Mexico (b = 2.3–2.7) [42], and of P. disjunctivus from Marikina River in the Philippines (b = 2.74) [45].
Another important line of evidence for the strong phenotypic divergence of P. disjunctivus was that the size, maximal size and age of both males and females from Dinh River (lotic habitat) were about 45–50% greater than those from Suoi Trau Reservoir (lentic habitat). Indeed, the maximum length and lifespan of P. disjunctivus from Dinh River were 483 mm and 4.2 years old, and for those from Suoi Trau they were 346 mm and 2.9 years old. The differences between the two types of water bodies (Table 1) might result in variations in food, dissolved oxygen, territory, or interspecific competition and harvesting as “trashfish”, subsequently affecting the size of the fish. In general, P. disjunctivus have rapid growth (100 mm/year) and a relatively short lifespan (ca. 5 years) [30,46]. The lifespans of P. disjunctivus in both Dinh River and Suoi Trau Reservoir were less than the estimated lifetime of this species in Volusia Blue Spring, Florida, USA (5.25 years) [30,46], although they had a similar length (L∞ = 500–520 mm). The shorter lifetime may relate to the higher average temperatures of Vietnamese climate conditions compared to Florida climate conditions, as lifetime and temperature typically correlate negatively [47].
In the current study, six stages of the gonadal development of P. disjunctivus caught from Dinh River and Suoi Trau Reservoir were described and these were similar to Jumawan and Herrera (2014) [48]. However, the weight of stage I gonads in the P. disjunctivus in this study was 10-fold smaller than that for fish collected in Marikina River in the Philippines [48], which may relate to the difference in size at maturity; or the catfishes collected in Marikina River may have been stage II gonads, because the color of stage I gonads in this study was “pale pink”, but the stage I gonads from Marikina River were “pink”. Furthermore, this fish species can alter its reproductive strategies (either annual single spawner or annually repeated spawner), and its GSI index in adults varies greatly, which may explain the difference. Moreover, the sex ratio in both habitats was practically 1:1. That is advantageous for the non-native species in terms of reproduction because, as the male builds a large nest burrow during the breeding season [49], there will always be at least one female available to mate, thus ensuring the establishment of P. disjunctivus in the sites.
Interestingly, the size at 50% maturity for females of P. disjunctivus in Suoi Trau Reservoir and Dinh River was 179.7 mm Lt and 234.3 mm Lt, respectively, which is much smaller than in their native habitats. In South America, the suckermouth armored catfishes begin to breed at a size of 250 mm, with unknown Ls (standard length) or Lt [14]. In North America, the 50% sexual maturity size of P. disjunctivus was 192 mm Ls in El Infiernillo Reservoir, Mexico [42], and 260 mm Ls in Volusia Blue Spring Florida, USA [29,30]. In invaded habitats in Asia, the 50% sexual maturity size was 240 mm Lt for P. disjunctivus in East Kolkata Wetlands, India [16], and 260 mm Ls for P. disjunctivus in Marikina River in the Philippines [48]. The smaller size at maturity may be a response to the exploitation pressure, removing the majority of large-size, matured animals from the populations [50]. This is likely the case for suckermouth armored catfishes as they are harvested as “trashfish” which can be used for making fertilizers. Furthermore, breeding females with small sizes can indicate early sexual maturation as an attempt to maximize their reproductive success, a typical characteristic of an opportunistic strategy [51]. Additionally, the smaller size at maturation is one of the universal patterns of ectothermic animals in response to elevated temperature [52,53]; the development outpaces the growth when temperature increases until the upper thermal optimum [54]. Corroborating this, the average water temperature in both Dinh River and Suoi Trau Reservoir varies between 25.4–32.9 °C, higher than in Florida, USA, where it is around 23 °C [30]. A smaller size at the first sexual maturity in response to elevated temperature has been observed in aquatic invertebrates (Pseudodiaptomus annandalei) in Vietnam [55], but no one has yet documented the evidence for fish in Vietnam.
In this study, P. disjunctivus was found to breed all year round, indicated by the monthly changes in GSI and the percentage of fish in matured stages. The main reproductive season would be from April to October, with a peak in July-August (dry season) as indicated by the highest GSI values of fish collected from both Dinh River and Suoi Trau Reservoir being during this period. This finding was consistent with a previous study which reported that Pterygoplichthys spp. in Dinh River was fertile from October to January of the next year (GSI: 6.59% to 15.77%) [24]. These results also agree with the observation that the main breeding season of P. disjunctivus is in the summer regardless of invading habitats worldwide. For example, in Mexico and the United States of America, P. disjunctivus reproduces in summer from May to October or November [29,30,42]. In other geographical areas with only two seasons (rainy and dry seasons), the main reproductive season of P. disjunctivus occurs in the rainy season. For example, in the Philippines, the P. disjunctivus reproduce from June to September [48], and from July to November in India [16]. Frequent reproduction over an extended breeding season may be an opportunistic life-history strategy adopted by non-native oviparous fish and be useful to maintain a continuous input of young (i.e., larvae, alevins and juveniles) into the population. In hydrologically unstable environments such as Dinh River, which is subject to floods, the continuous input of newborn individuals would assure a higher survival rate and therefore the maintenance of a viable population in a stochastic environment [7,56].
The P. disjunctivus can change its reproductive strategy to adapt to the local environment [30]. The reproductive strategies of P. disjunctivus in southern Vietnam were diverse. They can be single spawners with only one time of reproduction per year, as indicated by the index Fb/Ft = 100%, or multiple spawners which can reproduce several times a year, as indicated by Fb/Ft index varying from 7.9% to nearly 100% (Table 4). In India and the Philippines, P. disjunctivus are multiple spawners only [16,48]. In general, multiple spawning increases the survival chances of a species [57] and offers several advantages, such as (i) increasing the number of eggs spawned throughout the reproductive period [26], (ii) exposing the spawn to a variety of environmental conditions so that at least some of the offspring may develop under ideal conditions [58], and (iii) reducing competition between larvae/juveniles and the predation of eggs and larvae [59]. The alteration in the reproductive strategies of P. disjunctivus may be interpreted as an adaptation to variations in environmental, biological or geographical conditions [30], which needs to be clarified in freshwater habitats in southern Vietnam.
One important finding of this study was that the absolute fecundity of P. disjunctivus was five times higher in Dinh River than in Suoi Trau Reservoir, although the distance between the two sampling sites is only 8 km and they have the same weather conditions. The difference in absolute fecundity of P. disjunctivus was associated with the larger body size of fish collected from these two habitats, as confirmed by the positive correlations between these two parameters (Figure 9). Indeed, the difference was no longer present when the fecundity was normalized for the body size, as there was no significant difference in the relative fecundity of P. disjunctivus between lotic and lentic habitats. Furthermore, the types of water bodies or water quality parameters (Table 1), and especially the more intense harvesting of fish in Suoi Trau Reservoir during the drought season, may also contribute to this difference. The relative fecundity of P. disjunctivus in this study was comparable to P. disjunctivus from El Infiernillo Reservoir, Mexico (RFt = 12.5 eggs/g) [42], and from the East Kolkata Wetlands in India (RFt = 8–22 egg/g) [16].
The average diameter of the top size class of eggs in P. disjunctivus from Dinh River was moderately larger (~13%) than that for the fish from Suoi Trau Reservoir, which may relate to their adaptive responses to the different habitat conditions and harvesting intensity. Generally, the smaller egg size in reservoirs may result from unfavorable environmental conditions such as high water temperature variation, greater water level changes, food shortages accompanied by a long dry season, as well as dissolved oxygen and other parameters that also were different in the two water bodies. Indeed, the size of fish from Dinh River was larger than in Suoi Trau Reservoir (Table 3), and fish obtained from Dinh River had a higher abundance of food in the digestive tract (benthic, planktonic, amphibious and terrestrial food items) than those from Suoi Trau Reservoir (only benthic and planktonic) [11]. This leads to the larger diameter of the top size class of eggs in Dinh River compared to those in Suoi Trau Reservoir. In other studies in lotic habitats, for example, in Volusia Blue Spring, Florida, USA (latitude ~28° N), the average diameter of the top decile class size during the period 2005–2007 was 3.44 mm and for the period 2012–2014 it was 3.01 mm [29,30], while the diameter of the top size class of eggs was 3–4 mm for fish from Marikina River in the Philippines (latitude ~14° N) [48], which are larger than the top decile class size in Dinh River, Vietnam (latitude ~12° N). It seems that the size of the egg is smaller in lower latitudes, which needs further investigation.
5. Conclusions
In conclusion, this study provided important knowledge about the reproductive biology and growth of the armored catfish P. disjunctivus in different water bodies in the south of Vietnam. The fish from two different water bodies significantly varied in the length of the fish, size of oocytes, fecundity, the total length at maturity and allometric growth pattern, but there was no difference in the ratio between males and females and relative fecundity. Macroscopic observations and histological examinations of the gonads of these fish indicated that they could breed all year round, with a peak from July to August. Deeper studies on the adaptive behavior of fish in flowing and stagnant water in laboratory conditions need to be conducted in the future.
Author Contributions
All authors contributed to the study conception and design. T.D.D.: ideas, field work, data collection and analysis, first draft preparation and editing, and funding. V.T.H.: ideas, field work, data collection and analysis, and manuscript editing. M.D.: ideas, data analysis and manuscript editing. H.M.S.: ideas, data collection and analysis, and manuscript editing. N.T.D.H.: data analysis and manuscript editing. I.A.S.: ideas, data analysis, manuscript editing and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out within the frameworks of the Joint Vietnam-Russia Tropical Science and Technology Research Center (Ecolan 3.2. Mission number 5) and the State Assignment no. 121051100104-6.
Data Availability Statement
The datasets analyzed during this current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors are grateful to Khuong V. Dinh, Pavlov E.D., Gusakov V.A., Le Tran Thuy Duong, Dao Tuan Vu, co-workers in the Coastal Branch of the Joint Vietnam-Russia Tropical Science and Technology Research Center, local fishermen for contributing to sample collection and the Vingroup Joint Stock Company. The authors are also thankful to the Ninh Hoa and Khanh Hoa Hydrometeorological Agency for providing meteorological data for the study area and water bodies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Díaz, S.M.; Settele, J.; Brondízio, E.; Ngo, H.; Guèze, M.; Agard, J.; Arneth, A.; Balvanera, P.; Brauman, K.; Butchart, S. The Global Assessment Report on Biodiversity and Ecosystem Services: Summary for Policy Makers; IPBES Secretariat: Bonn, Germany, 2019; p. 60. [Google Scholar]
- Burgiel, S.W.; Muir, A.A. Invasive Species, Climate Change and Ecosystem-Based Adaptation: Addressing Multiple Drivers of Global Change; Global Invasive Species Programme (GISP), ZA: Washington, DC, USA, 2010; p. 55. ISBN 978-92-9059-287-7. [Google Scholar]
- Torchin, M.E.; Mitchell, C.E. Parasites, pathogens, and invasions by plants and animals. Front. Ecol. Environ. 2004, 2, 183–190. [Google Scholar] [CrossRef]
- Schofield, P.J.; Loftus, W.F. Non-native fishes in Florida freshwaters: A literature review and synthesis. Rev. Fish Biol. Fish. 2015, 25, 117–145. [Google Scholar] [CrossRef]
- Magalhães, A.L.B.; Bezerra, L.A.V.; Daga, V.S.; Pelicice, F.M.; Vitule, J.R.S.; Brito, M.F.G. Biotic differentiation in headwater creeks after the massive introduction of non-native freshwater aquarium fish in the Paraíba do Sul River basin, Brazil. Neotrop. Ichthyol. 2021, 19, e200147. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Magalhães, A.L.B.; Brito, M.F.G.; Silva, L.G.M. The fluorescent introduction has begun in the southern hemisphere: Presence and life-history strategies of the transgenic zebrafish Danio rerio (Cypriniformes: Danionidae) in Brazil. Stud. Neotrop. Fauna Environ. 2022, 1–13. [Google Scholar] [CrossRef]
- Orfinger, A.B.; Goodding, D.D. The global invasion of the suckermouth armored catfish genus Pterygoplichthys (Siluriformes: Loricariidae): Annotated list of species, distributional summary, and assessment of impacts. Zool. Stud. 2018, 57, 7. [Google Scholar] [CrossRef]
- Samat, A.; Yusoff, F.; Arshad, A.; Ghaffar, M.; Nor, S.; Magalhaes, A.; Das, S. Reproductive biology of the introduced sailfin catfish Pterygoplichthys pardalis (Pisces: Loricariidae) in Peninsular Malaysia. Indian J. Fish. 2016, 63, 35–41. [Google Scholar] [CrossRef]
- Bijukumar, A.; Smrithy, R.; Sureshkumar, U.; George, S. Invasion of South American suckermouth armoured catfishes Pterygoplichthys spp. (Loricariidae) in Kerala, India-a case study. J. Threat. Taxa 2015, 7, 6987–6995. [Google Scholar] [CrossRef]
- Stolbunov, I.A.; Gusakov, V.A.; Dien, T.D.; Thanh, N.T.H. Food Spectrum, Trophic and Length-Weight Characteristics of Nonindigenous Suckermouth Armored Catfishes Pterygoplichthys spp. (Loricariidae) in Vietnam. Inland Water Biol. 2021, 14, 597–605. [Google Scholar] [CrossRef]
- Kramer, D.L.; Bryant, M.J. Intestine length in the fishes of a tropical stream: 2. Relationships to diet-the long and short of a convoluted issue. Environ. Biol. Fishes 1995, 42, 129–141. [Google Scholar] [CrossRef]
- Pavlov, D.; Kasumyan, A. Feeding diversity in fishes: Trophic classification of fish. J. Ichthyol. 2002, 42, S137. [Google Scholar]
- Alfaro, R.E.M.; Cudmore, C.; Orr, R.; Fisher, J.P.; Balderas, S.C.; Courtenay, W.R.; Osorio, P.K.; Mandrak, N.; Torres, P.A.; Damián, M.A.; et al. Trinational Risk Assessment Guidelines for Aquatic Alien Invasive Species: Test Cases for the Snakeheads (Channidae) and Armored Catfishes (Loricariidae) in North American Inland Waters; Commission for Environmental Cooperation: Montreal, QC, Canada, 2009; p. 100. [Google Scholar]
- Stolbunov, I.A.; Dien, T.D. Mass alien fish species in the fish fauna of inland waters in Central Vietnam. Inland Water Biol. 2019, 12, 477–480. [Google Scholar] [CrossRef]
- Suresh, V.R.; Ekka, A.; Biswas, D.K.; Sahu, S.K.; Yousuf, A.; Das, S. Vermiculated sailfin catfish, Pterygoplichthys disjunctivus (Actinopterygii: Siluriformes: Loricariidae): Invasion, biology, and initial impacts in east Kolkata Wetlands, India. Acta Ichthyol. Piscat. 2019, 49, 221–233. [Google Scholar] [CrossRef]
- Gusakov, V.A.; Stolbunov, I.A.; Dien, T.D. Modern distribution of armored catfishes (Siluriformes: Loricariidae) in Central Vietnam. Inland Water Biol. 2018, 11, 179–183. [Google Scholar] [CrossRef]
- Hussan, A.; Sundaray, J.; Mandal, R.; Hoque, F.; Das, A.; Chakrabarti, P.; Adhikari, S. Invasion of non-indigenous suckermouth armoured catfish of the genus Pterygoplichthys (Loricariidae) in the East Kolkata Wetlands: Stakeholders’ perception. Indian J. Fish. 2019, 66, 29–42. [Google Scholar] [CrossRef]
- Stolbunov, I.A.; Dien, T.D.; Armbruster, J.W. Suckermouth-armored Catfish (Siluriformes: Loricariidae) of Central and Southern Vietnam. Inland Water Biol. 2020, 13, 627–639. [Google Scholar] [CrossRef]
- Stolbunov, I.A.; Dien, T.D.; Karabanov, D.P. Taxonomic composition and distribution of alien suckermouth armored Catfish (Siluriformes: Loricariidae) in Southern Vietnam. Inland Water Biol. 2021, 14, 263–273. [Google Scholar] [CrossRef]
- Nico, L.G.; Loftus, W.F.; Reid, J.P. Interactions between non-native armored suckermouth catfish (Loricariidae: Pterygoplichthys) and native Florida manatee (Trichechus manatus latirostris) in artesian springs. Aquat. Invasions 2009, 4, 511–519. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Vadas, R.L., Jr.; Ruiz-Carus, R.; Galib, S.M. Amazon sailfin catfish Pterygoplichthys pardalis (Loricariidae) in Bangladesh: A critical review of its invasive threat to native and endemic aquatic species. Fishes 2018, 3, 14. [Google Scholar] [CrossRef]
- Mendoza, R.; Luna, S.; Aguilera, C. Risk assessment of the ornamental fish trade in Mexico: Analysis of freshwater species and effectiveness of the FISK (Fish Invasiveness Screening Kit). Biol. Invasions 2015, 17, 3491–3502. [Google Scholar] [CrossRef]
- Zworykin, D.D.; Budaev, S.V. Non-indigenous armoured catfish in Vietnam: Invasion and systematics. Ichthyol. Res. 2013, 60, 327–333. [Google Scholar] [CrossRef]
- Armbruster, J.W.; Page, L.M. Redescription of Pterygoplichthys punctatus and description of a new species of Pterygoplichthys (Siluriformes: Loricariidae). Neotrop. Ichthyol. 2006, 4, 401–410. [Google Scholar] [CrossRef]
- Nikolsky, G. The Ecology of Fishes; Academic Press: London, UK, 1963. [Google Scholar]
- King, M. Fisheries Biology Assessment and Management; Fishing News Books: Oxford, UK, 1995. [Google Scholar]
- Sang, H.M.; Lam, H.S. Reproductive biology of blue tang fish (Paracanthurus hepatus Linnaeus, 1776) in Khanh Hoa seawater, Viet Nam. Indian J. Geo-Mar. Sci. 2018, 47, 839–845. [Google Scholar]
- Gibbs, M.; Shields, J.; Lock, D.; Talmadge, K.; Farrell, T. Reproduction in an invasive exotic catfish Pterygoplichthys disjunctivus in Volusia Blue Spring, Florida, USA. J. Fish Biol. 2008, 73, 1562–1572. [Google Scholar] [CrossRef]
- Gibbs, M.; Watson, P.; Johnson-Sapp, K.; Lind, C. Reproduction revisited—A decade of changes in the reproductive strategies of an invasive catfish, Pterygoplichthys disjunctivus (Weber, 1991), in Volusia Blue Spring, Florida. Aquat. Invasions 2017, 12, 225–239. [Google Scholar] [CrossRef]
- Dong, X.; Xiang, T.; Ju, T.; Li, R.; Ye, S.; Lek, S.; Liu, J.; Grenouillet, G. Age, growth, mortality and recruitment of thin sharpbelly Toxabramis swinhonis Günther, 1873 in three shallow lakes along the middle and lower reaches of the Yangtze River basin, China. PeerJ 2019, 7, e6772. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations. Fish. Res. Board Can. Bull. 1975, 191, 1–382. [Google Scholar]
- O’Brien, C.M. Modelling and quantitative methods in fisheries, second edition by Malcolm Haddon. Int. Stat. Rev. 2012, 80, 201–202. [Google Scholar] [CrossRef]
- Ye, S.; Li, Z.; Feng, G.; Cao, W. Length-weight relationships for thirty fish species in Lake Niushan, a Shallow Macrophytic Yangtze Lake in Chin. Asian Fish. Sci. 2007, 20, 217–226. [Google Scholar] [CrossRef]
- Koutrakis, E.; Tsikliras, A. Length–weight relationships of fishes from three northern Aegean estuarine systems (Greece). J. Appl. Ichthyol. 2003, 19, 258–260. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 3 February 2022).
- Von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Sparre, P. Introduction to Tropical Fish Stock Assessment. Part 1. Manual. FAO Fish. Tech. Paper 1998, 306, 407. [Google Scholar]
- Gayanilo, F.C.; Sparre, P. FAO-ICLARM Stock Assessment Tools II: User’s Guide; Food & Agriculture Org.: Rome, Italy, 2005. [Google Scholar]
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; Food & Agriculture Org.: Rome, Italy, 1983. [Google Scholar]
- Pauly, D.; Munro, J. Once more on the comparison of growth in fish and invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Rueda-Jasso, R.A.; Campos-Mendoza, A.; Arreguín-Sánchez, F.; Díaz-Pardo, E.; Martínez-Palacios, C.A. The biological and reproductive parameters of the invasive armored catfish Pterygoplichthys disjunctivus from Adolfo López Mateos El Infiernillo Reservoir, Michoacán-Guerrero, Mexico. Rev. Mex. Biodivers. 2013, 84, 318–326. [Google Scholar] [CrossRef]
- Hasan, M.; Hosen, M.H.A.; Miah, M.I.; Ahmed, Z.F.; Chhanda, M.S.; Shahriar, S.I.M. Fecundity, length at maturity and gonadal development indices of river catfish (Clupisoma garua) of the old Brahmaputra River in Bangladesh. Egypt. J. Aquat. Res. 2020, 46, 259–263. [Google Scholar] [CrossRef]
- McLeay, L.J.; Doubell, M.J.; Linnane, A.J. Spatial and temporal variations in female size at maturity of a Southern Rock Lobster (Jasus edwardsii) population: A likely response to climate change. PLoS ONE 2019, 14, e0225144. [Google Scholar] [CrossRef]
- Jumawan, J.C.; Herrera, A.A.; Jumawan, J.H.; Benjamin, V., Jr. Size structure and reproductive phenology of the suckermouth sailfin catfish Pterygoplichthys disjunctivus (Weber, 1991) from Marikina River, Philippines. ARPN J. Agric. Biol. Sci. 2016, 11, 18–23. [Google Scholar]
- Gibbs, M.A.; Kurth, B.N.; Bridges, C.D. Age and growth of the loricariid catfish Pterygoplichthys disjunctivus in Volusia Blue Spring, Florida. Aquat. Invasions 2013, 8, 207–218. [Google Scholar] [CrossRef]
- Truong, K.N.; Vu, N.-A.; Doan, N.X.; Le, M.-H.; Vu, M.T.; Dinh, K.V. Predator cues increase negative effects of a simulated marine heatwave on tropical zooplankton. J. Exp. Mar. Biol. Ecol. 2020, 530, 151415. [Google Scholar] [CrossRef]
- Jumawan, J.; Herrera, A. Ovary morphology and reproductive features of the female suckermouth sailfin catfish, Pterygoplichthys disjunctivus (Weber 1991) from Marikina River, Philippines. Asian Fish. Sci. 2014, 27, 75–89. [Google Scholar] [CrossRef]
- Nico, L.; Jelks, H.; Tuten, T. Non-Native suckermouth armored catfishes in Florida: Description of Nest Borrows and Burrow Colonies with assessment of Shoreline Conditions. Aquat. Nuis. Species Res. Program Bull. 2009, 9, 1–30. [Google Scholar]
- De Roos, A.M.; Boukal, D.S.; Persson, L. Evolutionary regime shifts in age and size at maturation of exploited fish stocks. Proc. R. Soc. B Biol. Sci. 2006, 273, 1873–1880. [Google Scholar] [CrossRef]
- Winemiller, K.O. Life-history strategies and the effectiveness of sexual selection. Oikos 1992, 63, 318–327. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W.; Sarmiento, J.L.; Dunne, J.; Frölicher, T.L.; Lam, V.W.; Deng Palomares, M.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Chang. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Forster, J.; Hirst, A.G.; Woodward, G. Growth and development rates have different thermal responses. Am. Nat. 2011, 178, 668–678. [Google Scholar] [CrossRef]
- Doan, N.X.; Vu, M.T.; Pham, H.Q.; Wisz, M.S.; Nielsen, T.G.; Dinh, K.V. Extreme temperature impairs growth and productivity in a common tropical marine copepod. Sci. Rep. 2019, 9, 4550. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.L.B.; Jacobi, C.M. Asian aquarium fishes in a Neotropical biodiversity hotspot: Impeding establishment, spread and impacts. Biol. Invasions 2013, 15, 2157–2163. [Google Scholar] [CrossRef]
- Hunter, J.; Lo, N.C.; Leong, R.J. Batch fecundity in multiple spawning fishes. NOAA Tech. Rep. NMFS 1985, 36, 67–77. [Google Scholar]
- Growns, I. A numerical classification of reproductive guilds of the freshwater fishes of south-eastern Australia and their application to river management. Fish. Manag. Ecol. 2004, 11, 369–377. [Google Scholar] [CrossRef]
- McEvoy, L.; McEvoy, J. Multiple spawning in several commercial fish species and its consequences for fisheries management, cultivation and experimentation. J. Fish Biol. 1992, 41, 125–136. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).