The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and theDegradation of Aniline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Goethite Synthesis
2.3. Effects of Different Concentrations of AQS on the Fe (III) Bio-Reduction
2.4. Effects of Different Aniline Concentrations on AQS-Mediated Fe (III) Bio-reduction
2.5. Chemical Analysis
2.6. Solid Mineral Characterization
2.7. Kinetic Analyses
2.8. Statistical Analysis
3. Results
3.1. Characterizations of Goethite
3.2. Bio-reductive Transformation of Fe (III)
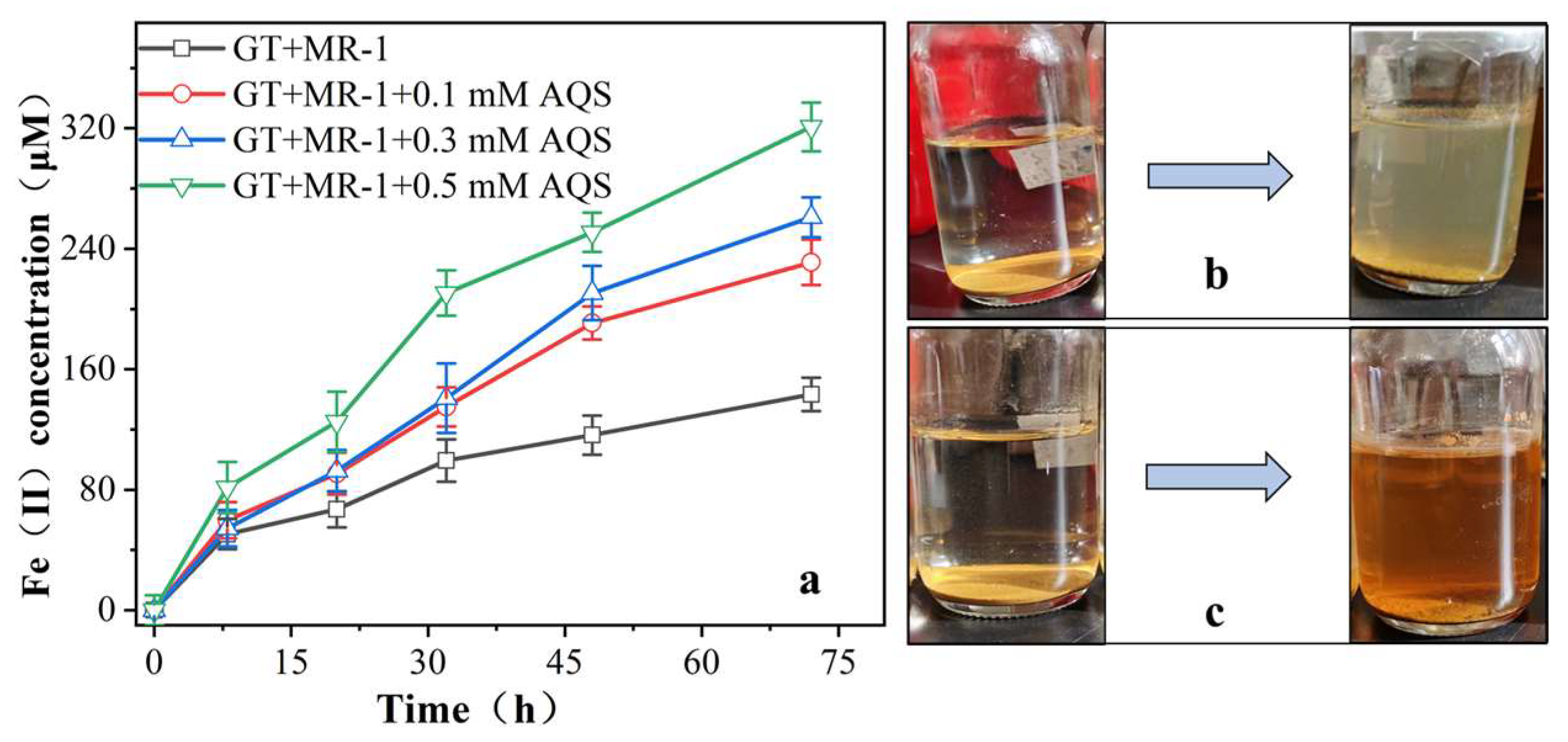
3.2.1. Effect of AQS on the Reduction of Fe (III) by MR-1
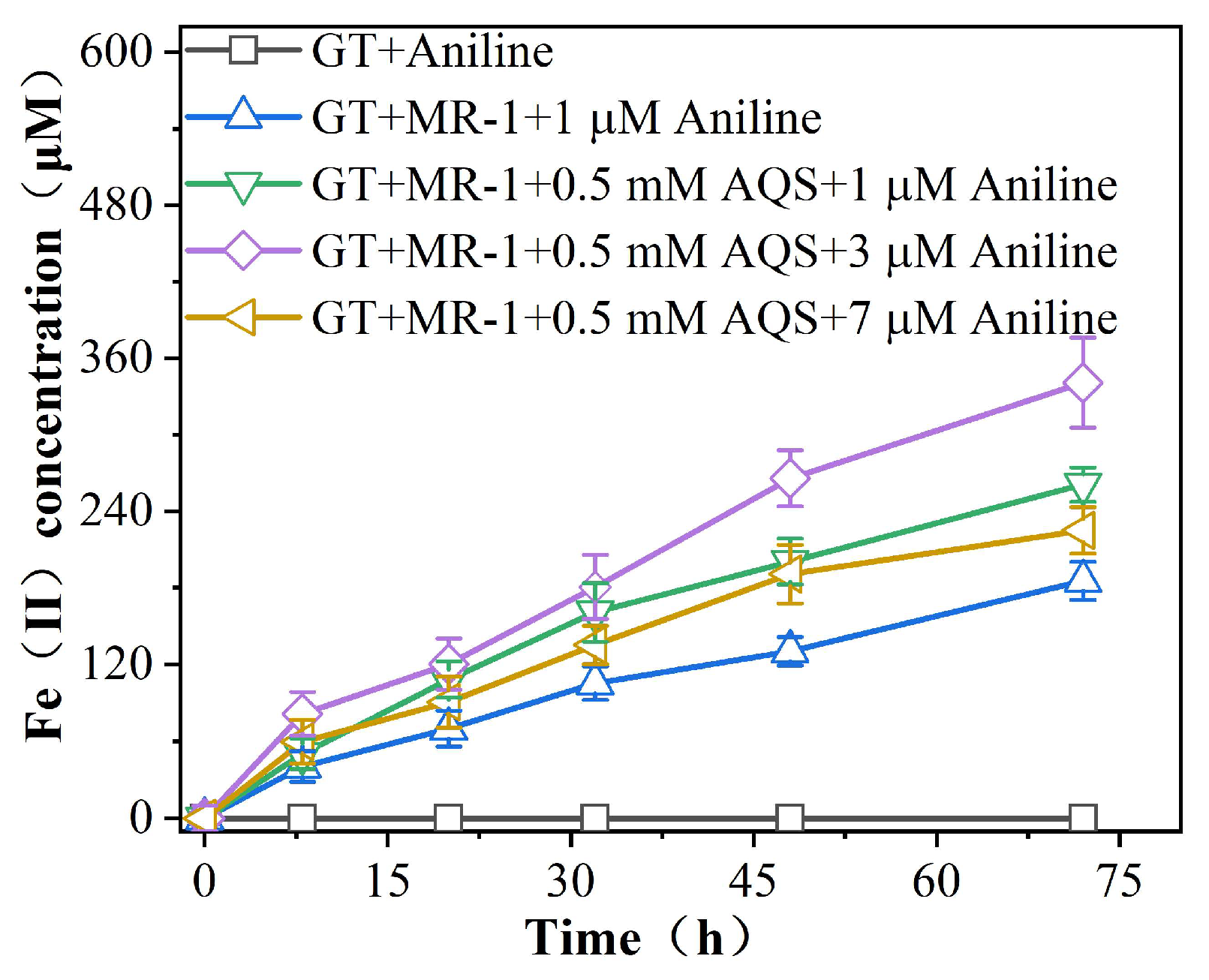
3.2.2. Effect of Aniline on AQS-Mediated Reduction of Fe (III) by MR-1
3.2.3. Consumption and Kinetic Analysis of Sodium Lactate
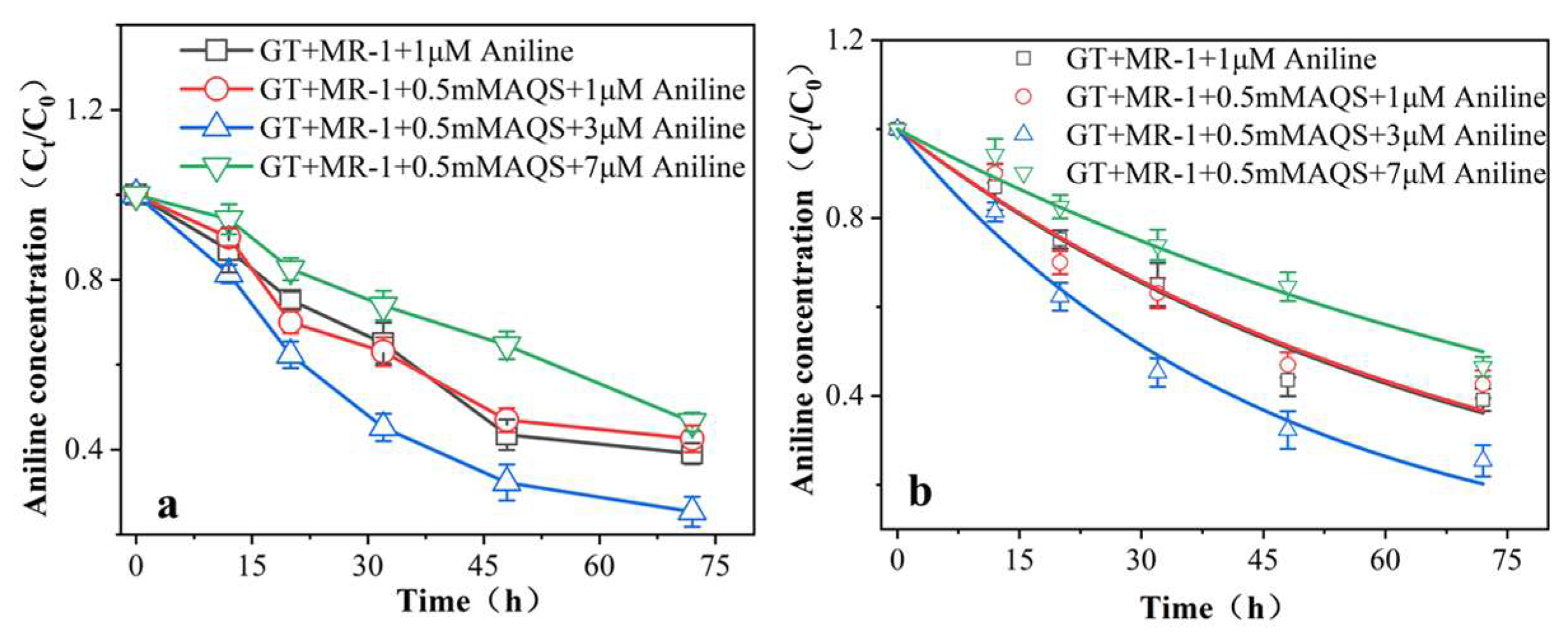
3.2.4. Degradation and Kinetic Analysis of Aniline
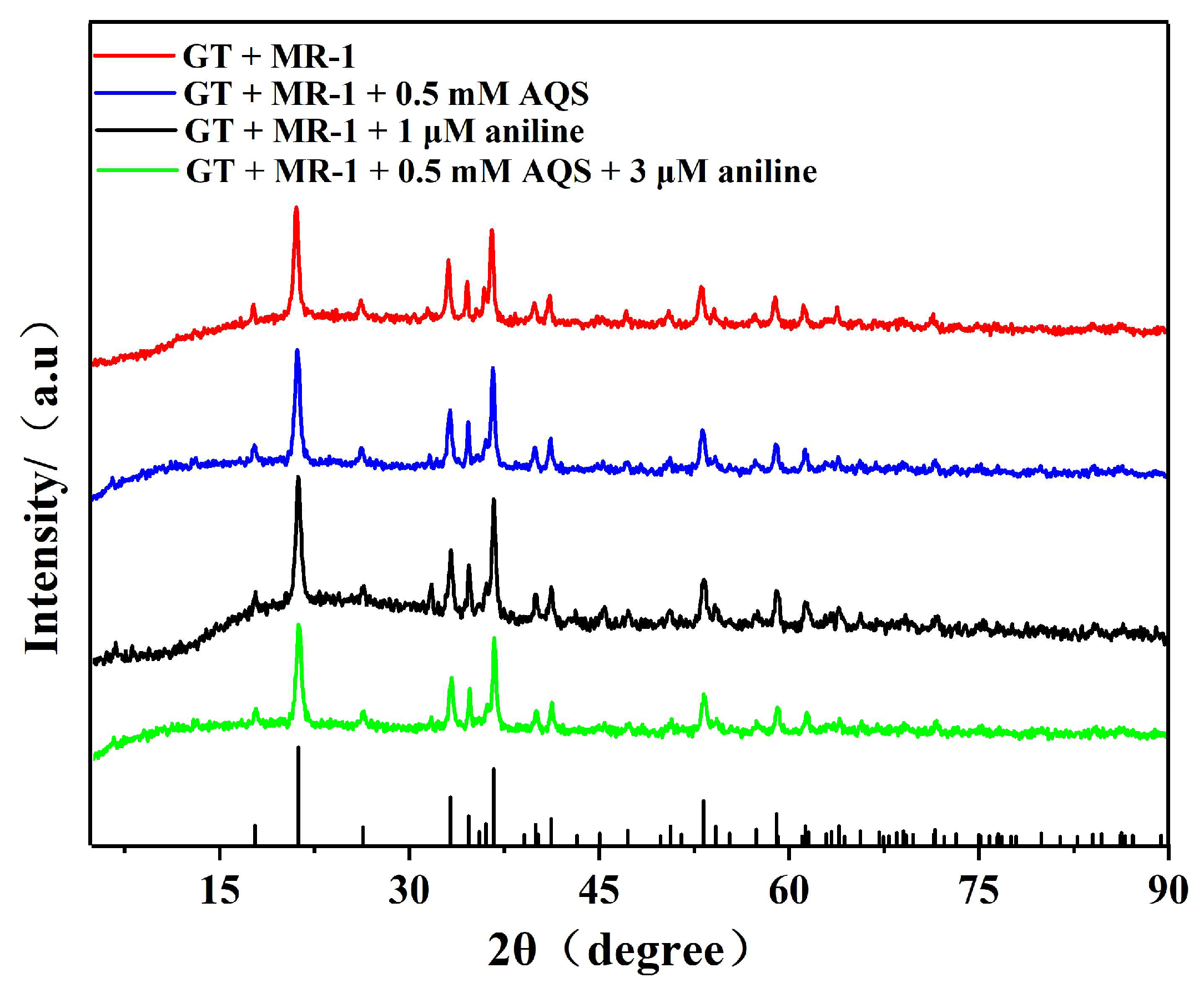
3.3. Solid Mineral Characterisation
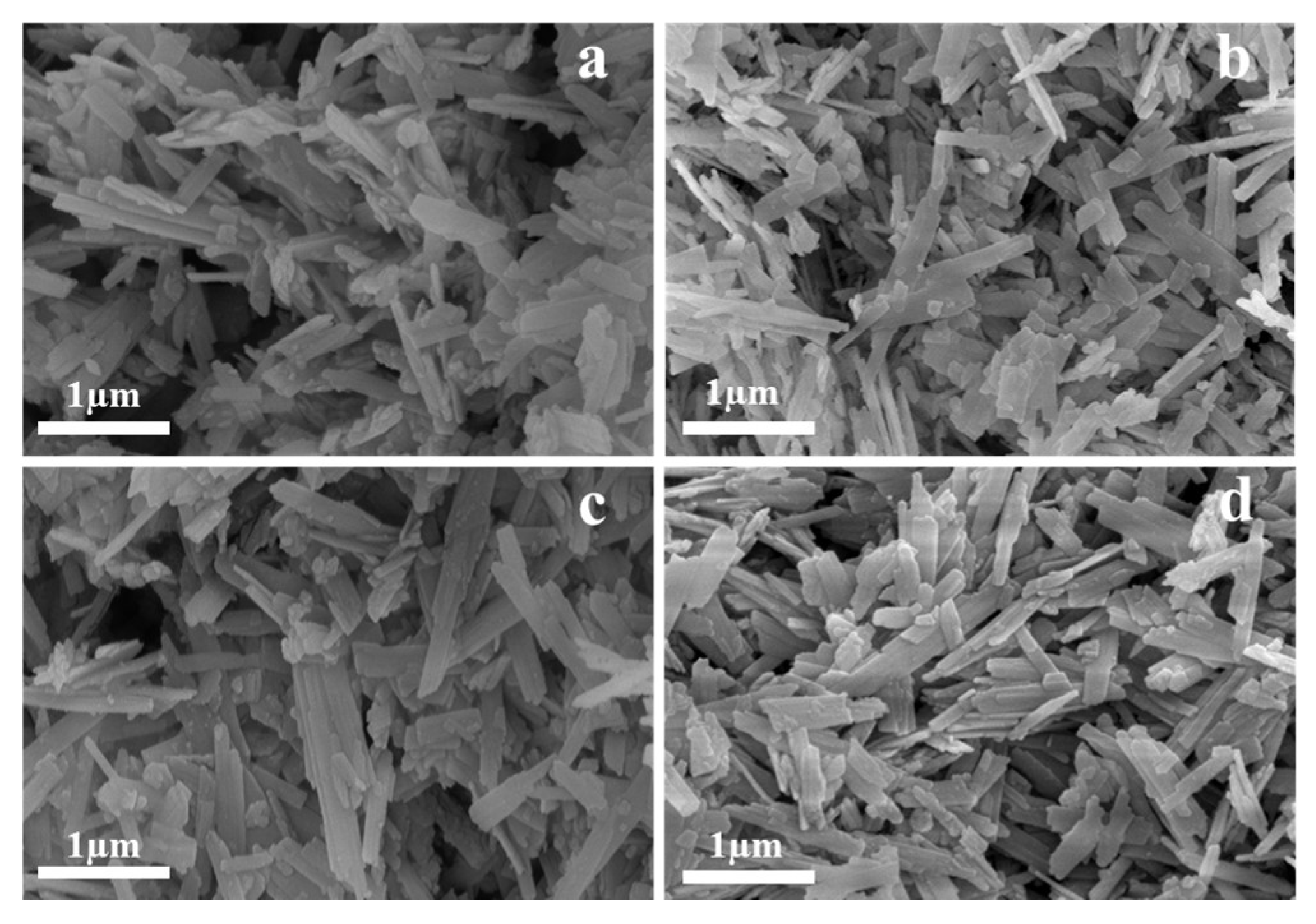
3.3.1. Scanning Electron Microscopy Analysis
3.3.2. X-ray Photoelectron Spectral Analysis
3.3.3. X-ray Diffraction Analysis
4. Discussion
4.1. AQS Enhance the Fe (III) Reduction by Shewanella Oneidensi MR-1
4.2. Aniline Promotes AQS-Mediated Reduction of Fe (III) Oxides by MR-1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.J.; Peng, J.J. Recent advances in studies on dissimilatory Fe(III)-reducing microorganisms. Acta Ecol. Sin. 2012, 32, 1633–1642. [Google Scholar]
- Chen, L.; Zhang, H.X.; Li, Y.; Zheng, S.L.; Liu, F.H. The role of microorganisms in the geochemical iron cycle. Sci. Sin. 2016, 46, 1069–1078. [Google Scholar]
- Zhang, Y.X.; Wang, Y.P.; Wang, H.P. Research Advances in the Mechanisms of Fe (III) Dissimilatory Reduction and the Reduction Products; The Chemical Industry and Engerring Soceiety of China: Beijing, China, 2015; p. 5. [Google Scholar]
- Schuth, S.; Hurraß, J.; Münker, C.; Mansfeldt, T. Redox-dependent fractionation of iron isotopes in suspensions of a groundwater-influenced soil. Chem. Geol. 2015, 392, 74–86. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Strong, J.; Wang, H. Are the biogeochemical cycles of carbon, nitrogen, sulfur, and phosphorus driven by the "FeIII - FeII redox wheel" in dynamic redox environments? J. Soils Sediments 2012, 12, 683–693. [Google Scholar] [CrossRef]
- Kappler, A.; Haderlein, S.B. Natural organic matter as reductant for chlorinated aliphatic pollutants Environ. Sci. Technol. 2003, 37, 2714–2719. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xi, B.D.; Li, R.; Li, M.X.; Xu, Z.; Yang, Y.N.; Gao, S.B. Advances in Fe (III) bioreduction and its application prospect for groundwater remediation: A review. Front. Environ. Sci. Eng. 2019, 13, 89. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Swanner, E.D.; Behrens, S.; Behrens, S.; Schmidt, C.; Kappler, A. The interplay of microbially mediated and abiotic reactions in the biogeochemical Fe cycle. Nat. Rev. Microbiol. 2014, 12, 797–808. [Google Scholar] [CrossRef]
- Tobler, N.B.; Hofstetter, T.B.; Straub, K.L.; Fontana, D.; Schwarzenbach, R.P. Iron-Mediated Microbial Oxidation and Abiotic Reduction of Organic Contaminants under Anoxic Conditions. Environ. Sci. Technol. 2007, 41, 7765–7772. [Google Scholar] [CrossRef]
- Straub, K.L.; Benz, M.; Schink, B. Iron metabolism in anoxic environments at near neutral pH. FEMS Microbiol. Ecol. 2001, 34, 181–186. [Google Scholar] [CrossRef]
- Thomsen, U.; Thamdrup, B.; Stahl, D.A.; Canfield, D.E. Pathways of organic carbon oxidation in a deep lacustrine sediment, Lake Michigan. Limnol. Oceanogr. 2004, 49, 2046–2057. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Wang, X.-L.; Ma, X.-Y.; Lin, K.-F.; Liu, Y.-D.; Gu, J.-D.; Lu, S.-G.; Shi, L.; Lu, Q.; et al. Biodegradation of benzene homologues in contaminated sediment of the East China Sea. Bioresour. Technol. 2012, 124, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, J.; Han, Y.; Zhu, J.Y.; Zhou, L.X.; Lan, Y.Q. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline. Chemosphere 2018, 200, 373–379. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. OPPT Chemical Fact Sheets, Aniline Fact Sheet, Support Document (CAS No. 62-53-3); United States Environmental Protection Agency: Washington, DC, USA, 1994.
- Li, J.; Jin, Z. Effect of hypersaline aniline-containing pharmaceutical wastewater on the structure of activated sludge-derived bacterial community. J. Hazard. Mater. 2009, 172, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Phillips, E.J.; Lonergan, D.J. Lonergan. Enzymic versus nonenzymic mechanisms for iron (III) reduction in aquatic sediments. Environ. Sci. Technol. 1991, 25, 1062–1067. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Yuan, Y.X.; Song, C.C.; Jiang, M. Advance in Dissimilatory Iron Reduction in Wetland Soils and Sediments. Wetl. Sci. 2020, 18, 122–128. [Google Scholar]
- Bae, S.; Lee, W. Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim. Cosmochim. Acta 2013, 114, 144–155. [Google Scholar] [CrossRef]
- Orsetti, S.; Laskov, C.; Haderlein, S.B. Electron Transfer between Iron Minerals and Quinones: Estimating the Reduction Potential of the Fe(II)-Goethite Surface from AQDS Speciation. Environ. Sci. Technol. 2013, 47, 14161–14168. [Google Scholar] [CrossRef]
- Roh, Y.; Liu, S.V.; Li, G.; Huang, H.; Phelps, T.J.; Zhou, J. Isolation and characterization of metal-reducing Thermoanaerobacter strains from deep subsurface environments of the Piceance Basin, Colorado. Appl. Environ. Microbiol. 2002, 68, 6013–6020. [Google Scholar] [CrossRef]
- Yang, L.; Steefel, C.I.; Marcus, M.A.; Bargar, J.R. Kinetics of Fe (II)-Catalyzed Transformation of 6-line Ferrihydrite under Anaerobic Flow Conditions. Environ. Sci. Technol. 2010, 44, 5469–5475. [Google Scholar] [CrossRef]
- Till, J.L.; Guyodo, Y.; Lagroix, F.; Ona-Nguema, G.; Brest, J. Magnetic comparison of abiogenic and biogenic alteration products of lepidocrocite. Earth Planet. Sci. Lett. 2014, 395, 149–158. [Google Scholar] [CrossRef]
- Usman, M.; Abdelmoula, M.; Faure, P.; Ruby, C.; Hanna, K. Transformation of various kinds of goethite into magnetite: Effect of chemical and surface properties. Geoderma 2013, 197–198, 9–16. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, I.; Roh, Y. Biomineralization of a poorly crystalline Fe(III) oxide, akaganeite, by an anaerobic Fe(III)-reducing bacterium (Shewanella alga) isolated from marine environment. Geosci. J. 2003, 7, 217–222. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Liu, T.; Yuan, T.; Zhang, W.; Li, F.; Zhou, S.; Li, Y. Electron transfer capacity dependence of quinone-mediated Fe (III) reduction and current generation by Klebsiella pneumoniae L17. Chemosphere 2013, 92, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Nevin, K.P.; Lovley, D.R. Potential for nonenzymatic reduction of Fe (III) via electron shuttling in subsurface sediments. Environ. Sci. Technol. 2000, 34, 2472–2478. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wu, Y. Electron shuttle-mediated microbial extracellular electron transfer: Mechanisms and geochemical implications. Ecol. Environ. 2021, 30, 213–222. [Google Scholar]
- Liu, G.F.; Zhu, J.Q.; Yu, H.L.; Jin, R.; Wang, J.; Zhou, J. Review on electron-shuttle-mediated microbial reduction of iron oxides minerals. Earth Sci. 2018, 43 (Suppl. S1), 157–170. [Google Scholar]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, L.; Jiang, Y.; Xu, Z.; Gao, S.; Li, M.; Li, R.; Xi, B. Promoting mechanism of electronic shuttle for bioavailability of Fe (III) oxide and its environmental significance. Water Supply 2020, 20, 1157–1166. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, Y.; Zhang, S.; Li, K. Characteristics of Fe (III) reduction by Fe (III)-reducing bacterium Clostridium butyricum LQ25 under the condition of electron shuttles. Acta Microbiol. Sin. 2021, 61, 1496–1506. [Google Scholar]
- Liu, W.; Wu, Y.; Liu, T.; Li, F.; Dong, H.; Jing, M. Influence of incubation temperature on 9,10-anthraquinone-2-sulfonate (AQS)-mediated extracellular electron transfer. Front. Microbiol. 2019, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Sun, B.; Yu, C.; Gu, X.; Ashry, N.; Riahi, Y.; Dai, K.; Huang, Q. Size effect of hematite particles on the Cr (VI) reduction by Shewanella oneidensis MR-1. J. Environ. Chem. Eng. 2021, 9, 105096. [Google Scholar] [CrossRef]
- Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron (II) with 1,10-phenanthroline in the presence of large amounts of iron (III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zachara, J.M.; Shi, L.; Wang, Z.; Moore, D.A.; Kennedy, D.W.; Fredrickson, J.K. Redox Reactions of Reduced Flavin Mononucleotide (FMN), Riboflavin (RBF), and Anthraquinone-2,6-disulfonate (AQDS) with Ferrihydrite and Lepidocrocite. Environ. Sci. Technol. 2012, 46, 11644–11652. [Google Scholar] [CrossRef]
- Liu, C.; Zachara, J.M.; Foster, N.S.; Strickland, J. Kinetics of Reductive Dissolution of Hematite by Bioreduced Anthraquinone-2,6-disulfonate. Environ. Sci. Technol. 2007, 41, 7730–7735. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Tang, Q.-W.; Cao, M.-Y.; Li, J.; Yuan, K.; Wang, P.; Li, W.-W. Enhanced reduction of Fe (III) oxides and methyl orange by Klebsiella oxytoca in presence of anthraquinone-2-disulfonate. Appl. Microbiol. Biotechnol. 2016, 100, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Knackmuss, H.; Stolz, A. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ. Sci. Technol. 2002, 36, 1497–1504. [Google Scholar] [CrossRef]
- Zhu, W.; Yao, T.; Huang, T. The kinetic characteristics of the microbial dissimilatory reduction of lepidocrocite. Huanjing Kexue Xuebao 2012, 32, 1348–1356. [Google Scholar]
- Okamoto, A.; Hashimoto, K.; Nealson, K.H.; Nakamura, R. Rate enhancement of bacterial extracellular electron transport involves bound flavin semiquinones. Proc. Natl. Acad. Sci. USA 2013, 110, 7856–7861. [Google Scholar] [CrossRef]
- Pinchuk, G.E.; Rodionov, D.A.; Yang, C.; Li, X.; Osterman, A.L.; Dervyn, E.; Geydebrekht, O.V.; Reed, S.B.; Romine, M.F.; Collart, F.R.; et al. Genomic reconstruction of Shewanella oneidensis MR-1 metabolism reveals a previously uncharacterized machinery for lactate utilization. Proc. Natl. Acad. Sci. USA 2009, 106, 2874–2879. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Wang, Y.; Chen, M. Synergy between indigenous bacteria and extracellular electron shuttles enhances transformation and mobilization of Fe (III)/As (V). Sci. Total Environ. 2021, 783, 147002. [Google Scholar] [CrossRef]
- Zheng, Y.; Dai, X.; Wang, L.; Xu, W.; He, Z.; Ma, M. Arsenate reduces copper phytotoxicity in gametophytes of Pteris vittata. J. Plant Physiol. 2008, 165, 1906–1916. [Google Scholar] [CrossRef]
- Rooney-Varga, J.N.; Anderson, R.T.; Fraga, J.L.; Ringelberg, D.; Lovley, D.R. Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. Appl. Environ. Microbiol. 1999, 65, 3056–3063. [Google Scholar] [CrossRef]
- Shuo, L.; Dong, Q. Effects of benzene analogs as sole carbon source on dissimilatory iron reduction. J. Northwest Sci.-Tech. Univ. Agric. For. 2006, 10, 101–106+112. [Google Scholar]
- Anderson, R.T.; Lovley, D.R. Naphthalene and benzene degradation under Fe (III)-reducing conditions in petroleum-contaminated aquifers. Bioremediat. J. 1999, 3, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Woodward, J.C.; Chapelle, F.H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe (III) ligands. Nature 1994, 370, 128–131. [Google Scholar] [CrossRef]
- Tor, J.M.; Lovley, D.R. Anaerobic degradation of aromatic compounds coupled to Fe (III) reduction by Ferroglobus placidus. Environ. Microbiol. 2001, 3, 281–287. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Collins, Y.E.; Stotzky, G. Heavy metals alter the electrokinetic properties of bacteria, yeasts, and clay minerals. Appl. Environ. Microbiol. 1992, 58, 1592–1600. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P.; Glaven, R.H.; Lovley, D.R. Microtoming coupled to microarray analysis to evaluate the spatial metabolic status of Geobacter sulfurreducens biofilms. ISME J. 2010, 4, 509–519. [Google Scholar] [CrossRef]
- Gagen, E.J.; Zaugg, J.; Tyson, G.W.; Southam, G. Goethite Reduction by a Neutrophilic Member of the Alphaproteobacterial Genus Telmatospirillum. Front. Microbiol. 2019, 10, 2938. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.S.; Ramsay, J.A. Review: Microbial mechanisms of accessing insoluble Fe (III) as an energy source. World J. Microbiol. Biotechnol. 2003, 19, 215–225. [Google Scholar] [CrossRef]
- Shi, L.; Squier, T.C.; Zachara, J.M.; James, K. Respiration of metal (hydr)oxides by Shewanella and Geobacter: A key role for multihaem c-type cytochromes. Mol. Microbiol. 2010, 65, 12–20. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, E.J. Effects of Electron Transfer Mediators on the Bioreduction of Lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN132. Environ. Sci. Technol. 2008, 42, 6876–6882. [Google Scholar] [CrossRef]
- Lu, M.C. Oxidation of chlorophenols with hydrogen peroxide in the presence of goethite. Chemosphere 2000, 40, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Notini, L.; Byrne, J.M.; Tomaszewski, E.J.; Latta, D.E.; Zhou, Z.; Scherer, M.M.; Kappler, A. Mineral Defects Enhance Bioavailability of Goethite toward Microbial Fe (III) Reduction. Environ. Sci. Technol. 2019, 53, 8883–8891. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, D.; Bu, H.; Deng, L.; Liu, H.; Yuan, P.; Du, P.; Song, H. XRD-based quantitative analysis of clay minerals using reference intensity ratios, mineral intensity factors, Rietveld, and full pattern summation methods: A critical review. Solid Earth Sci. 2018, 3, 16–29. [Google Scholar] [CrossRef]
- Yamamura, S.; Sudo, T.; Watanabe, M.; Tsuboi, S.; Soda, S.; Ike, M.; Amachi, S. Effect of extracellular electron shuttles on arsenic-mobilizing activities in soil microbial communities. J. Hazard. Mater. 2017, 342, 571–578. [Google Scholar] [CrossRef]
- Struyk, Z.; Sposito, G. Redox properties of standard humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, C.; Wang, X.; Yan, L.; Zhu, G.; Lu, X. Benzene promotes microbial Fe (III) reduction and flavins secretion. Geochim. Cosmochim. Acta 2019, 264, 92–104. [Google Scholar] [CrossRef]
- Finke, N.; Vandieken, V.; Jørgensen, B.B. Acetate, lactate, propionate, and isobutyrate as electron donors for iron and sulfate reduction in Arctic marine sediments, Svalbard. FEMS Microbiol. Ecol. 2007, 59, 10–22. [Google Scholar] [CrossRef]
- Zhu, W.; Nan, Y.; Huang, T.; Wu, F. The mechanism, thermodynamic and kinetic characteristics of the microbial reduction of goethite mediated by anthraquinone-2-sulfonate. Geomicrobiol. J. 2013, 30, 928–940. [Google Scholar] [CrossRef]
- Lovley, D.R.; Lonergan, D.J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl. Environ. Microbiol. 1990, 56, 1858–1864. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef]
- Lovley, D.R.; Baedecker, M.J.; Lonergan, D.J.; Cozzarelli, I.M.; Elizabeth J., P.; Siegel, D.I. Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 1989, 339, 297–300. [Google Scholar] [CrossRef]
- Wolf, M.; Kappler, A.; Jiang, J.; Meckenstock, R.U. Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ. Sci. Technol. 2009, 43, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; Dijksma, W.; Duong-Dac, T.; Ivanova, A.; Lettinga, G.; Field, J.A. Anaerobic mineralization of toluene by enriched sediments with quinones and humus as terminal electron acceptors. Appl. Environ. Microbiol. 2001, 67, 4471–4478. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Coates, J.D. Anaerobic degradation of monoaromatic hydrocarbons. Appl. Microbiol. Biotechnol. 2004, 64, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R.; Woodward, J.C.; Chapelle, F.H. Rapid anaerobic benzene oxidation with a variety of chelated Fe (III) forms. Appl. Environ. Microbiol. 1996, 62, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Inskeep, W.P.; Harwood, J.A.; Gerlach, R. Impact of ferrihydrite and anthraquinone-2, 6-disulfonate on the reductive transformation of 2, 4, 6-trinitrotoluene by a gram-positive fermenting bacterium. Environ. Sci. Technol. 2005, 39, 7126–7133. [Google Scholar] [CrossRef]



| Experimental Conditions | K for Sodium Lactate Consumption (h−1) | R2 for Sodium Lactate Consumption |
|---|---|---|
| GT + MR-1 | 0.0023 ± 0.002 | 0.87 |
| GT + MR-1 + 0.1 mM AQS | 0.0097 ± 0.002 | 0.99 |
| GT + MR-1 + 0.3 mM AQS | 0.0132 ± 0.001 | 0.98 |
| GT + MR-1 + 0.5 mM AQS | 0.0181 ± 0.002 | 0.98 |
| GT + MR-1 + 1 μM Aniline | 0.0052 ± 0.003 | 0.97 |
| GT + MR-1 + 0.3 mM AQS + 1 μM Aniline | 0.0144 ± 0.005 | 0.94 |
| GT + MR-1 + 0.5 mM AQS + 3 μM Aniline | 0.0191 ± 0.001 | 0.96 |
| GT + MR-1 + 0.5 mM AQS + 7 μM Aniline | 0.0125 ± 0.002 | 0.97 |
| Experimental Conditions | K for Aniline Consumption (h−1) | R2 for Aniline Consumption |
|---|---|---|
| GT + MR-1 + 1 μM Aniline | 0.0139 ± 0.001 | 0.97 |
| GT + MR-1 + 0.5 mM AQS + 1 μM Aniline | 0.0143 ± 0.002 | 0.95 |
| GT + MR-1 + 0.5 mM AQS + 1 μM Aniline | 0.0232 ± 0.001 | 0.98 |
| GT + MR-1 + 0.5 mM AQS + 1 μM Aniline | 0.0098 ± 0.001 | 0.99 |
| Fe | GT + MR-1 | GT + MR-1 + 0.5 mM AQS | GT + MR-1 + 1 μM Aniline | GT + MR-1 + 0.5 mM AQS + 3 μM Aniline | ||||
|---|---|---|---|---|---|---|---|---|
| B.E./eV | at% | B.E./eV | at% | B.E./eV | at% | B.E./eV | at% | |
| Fe2+2p3/2 | 709.2 | 32.2 | 709.67 | 40.81 | 709.24 | 37.3 | 709.7 | 41.32 |
| Fe2+2p1/2 | 722.25 | 722.89 | 722.48 | 722.9 | ||||
| Fe3+2p3/2 | 710.75 | 67.8 | 710.92 | 59.19 | 710.7 | 62.27 | 710.85 | 58.68 |
| Fe3+2p1/2 | 724.33 | 724.46 | 724.18 | 724.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Wang, C.; Dong, Z.; Che, Q.; Wang, Z.; Zhu, Y. The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and theDegradation of Aniline. Water 2023, 15, 3686. https://doi.org/10.3390/w15203686
Tang M, Wang C, Dong Z, Che Q, Wang Z, Zhu Y. The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and theDegradation of Aniline. Water. 2023; 15(20):3686. https://doi.org/10.3390/w15203686
Chicago/Turabian StyleTang, Mengmeng, Chaoyong Wang, Zaitian Dong, Qianjin Che, Zetang Wang, and Yuxuan Zhu. 2023. "The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and theDegradation of Aniline" Water 15, no. 20: 3686. https://doi.org/10.3390/w15203686
APA StyleTang, M., Wang, C., Dong, Z., Che, Q., Wang, Z., & Zhu, Y. (2023). The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and theDegradation of Aniline. Water, 15(20), 3686. https://doi.org/10.3390/w15203686





