Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge
Abstract
:1. Introduction
2. Materials and Methods
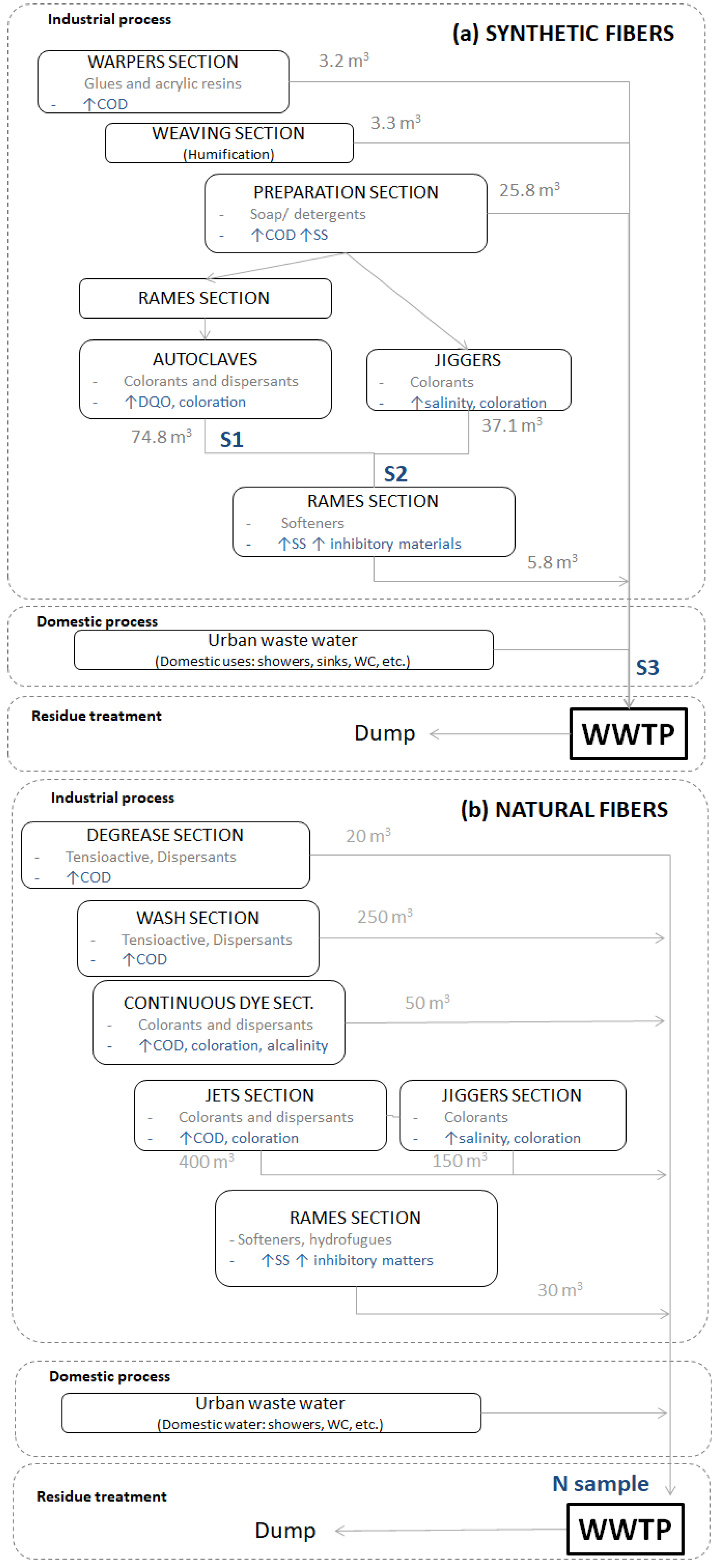
2.1. Liquid Residues Samples
2.2. ASEC Technology
2.3. Sample Collection and Analyses
3. Results and Discussion
3.1. Mass Balance
3.2. Results of the Characterization of the Fluids
3.3. Composition of the Crystallized Solids
3.4. Revalorization
3.5. European Critical Raw Materials and European Green Deals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.; Malik, A. Environmental and Health Effects of Textile Industry Wastewater. In Environmental Deterioration and Human Health; Malik, A., Grohmann, E., Akhtar, R., Eds.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Vicaire, Y. The Greenpeace Detox Campaign. 2017. Available online: https://echa.europa.eu/documents/10162/22979590/230217_substitution_webinar_greenpeace_detox_en.pdf/fa71a525-12df-6978-dadf-2e3ed392d746 (accessed on 3 August 2023).
- Tummino, M.L.; Varesano, A.; Copani, G.; Vineis, C. A glance at novel materials from the textile world to environmental remediation. J. Polym. Environ. 2023, 31, 2826–2854. [Google Scholar] [CrossRef]
- Pacini, H. Seizing the Opportunities of a Circular Economy in Textiles. UNCTAD (United Nations Conference on Trade and Development), 28 June 2021. Available online: https://unctad.org/news/seizing-opportunities-circular-economy-textiles (accessed on 24 April 2023).
- Gupta, R.; Kushwaha, A.; Dave, D.; Mahanta, N.R. Chapter 10—Waste Management in Fashion and Textile Industry: Recent Advances and Trends, Life-Cycle Assessment, and Circular Economy. In Emerging Trends to Approaching Zero Waste, Environmental and Social Perspectives; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–242. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 7185, 137317. [Google Scholar] [CrossRef]
- EPA (United States Environmental Protection Agency). Facts and Figures about Materials, Waste and Recycling: Textiles: Material-Specific Data. 2021. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/textiles-material-specific-data (accessed on 17 May 2021).
- Pandey, K. Fashion Industry May Use Quarter of World’s Carbon Budget by 2050. Down to Earth, 2018. Available online: https://www.downtoearth.org.in/news/environment/fashion-industry-may-use-quarter-of-world-s-carbon-budget-by-2050-61183 (accessed on 24 April 2023).
- Singa, K.; Pandit, P.; Maity, S.; Sharma, S.R. Chapter 11-Harmful environmental effects for textile chemical dyeing practice. In Green Chemistry for Sustainable Textiles Modern Design and Approaches; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2021; pp. 153–164. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Clothes and Textiles. Chemicals in Our Life. Available online: https://chemicalsinourlife.echa.europa.eu/clothes-and-textiles (accessed on 7 August 2023).
- Gaonkar, O. An Overview of Toxic Chemicals in Textiles; Technical Report: December; Toxics Link: New Delhi, India, 2021. [Google Scholar]
- Hassani, A.; Eghbali, P.; Ekicibil, A.; Metin, O. Monodisperse cobalt ferrite nanoparticles assembled on mesoporous graphitic carbon nitride (CoFe2O4/mpg-C3N4): A magnetically recoverable nanocomposite for the photocatalytic degradation of organic dyes. J. Mang. Magn. Mater. 2018, 456, 400–4212. [Google Scholar] [CrossRef]
- Khataee, A.; Eghbali, P.; Irani-Nezhad, M.H.; Hassani, A. Sonochemical synthesis of WS2 nanosheets and its application in sonocatalytic removal of organic dyes from water solution. Ultrason. Sonochem. 2018, 48, 329–339. [Google Scholar] [CrossRef]
- Chen, H.L.; Burns, L.D. Environmental Analysis of Textile Products. Cloth. Text. Res. J. 2006, 24, 546–550. [Google Scholar] [CrossRef]
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Environ. Sci. Pollut. Res. 2018, 25, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- S.I. No. 323/2020-European Union (Waste Directive) Regulations 2020. Available online: https://www.irishstatutebook.ie/eli/2020/si/323/made/en/print?q=waste+directive+ (accessed on 9 August 2023).
- European Commission. The European Green Deal. Brussels, 11.12.2019 COM (2019) 640 final. 2019. Available online: https://scholar.google.com/scholar_lookup? (accessed on 7 August 2023).
- Sasakova, N.; Gregova, G.; Takacova, D.; Mojzisova, J.; Papajova, I.; Venglovsky, J.; Szaboova, T.; Kovacova, S. Pollution of surface and ground water by sources related to agricultural activities. Sec. Waste Manag. Agroecosyst. 2018, 2, 42. [Google Scholar] [CrossRef]
- EU. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control). Available online: http://data.europa.eu/eli/dir/2010/75/oj (accessed on 14 August 2023).
- Bonnail, E.; Vera, S.; Blasco, J.; Conradi, M.; DelValls, T.Á. Metal Pollution and Mining in the Iberian Pyrite Belt: New Remediation Technologies to Improve the Ecosystem Services of the River Basins. Water 2023, 15, 1302. [Google Scholar] [CrossRef]
- Switch2Green. The EU SWITCH to Green Flagship Initiative. A Gateway to Inclusive Green Economy. Available online: https://www.switchtogreen.eu/the-eu-green-deal-promoting-a-green-notable-circular-economy/#:~:text=The%20European%20Green%20Deal%20aims,biodiversity%20loss%20and%20cut%20pollution (accessed on 23 August 2023).
- Portaspecs.com. Why Light Elements are Difficult to Measure with Portable XRF. Portable Spectral Services 2023. Available online: https://www.portaspecs.com/why-light-elements-are-difficult-to-measure-with-portable-xrf/ (accessed on 23 August 2023).
- Partal, R.; Basturk, I.; Hocaoglu, S.M.; Baban, A.; Yilmaz, E. Recovery of water and reusable salt solution from reverse osmosis brine in textile industry: A case study. Water Res. Indus. 2022, 27, 100174. [Google Scholar] [CrossRef]
- Wang, Y. Fiber and Textile Waste Utilization. Waste Biomass Valor. 2010, 1, 135–143. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Islam, M.M. Materials and manufacturing environmental sustainability evaluation of apparel product: Knitted T-shirt case study. Textiles Cloth. Sustain. 2015, 1, 8. [Google Scholar] [CrossRef]
- Vajnhandl, S.; Volmajer Valh, J. The status of water reuse in European textile sector. J. Environ. Manag. 2014, 141, 29–35. [Google Scholar] [CrossRef]
- USGS (U.S. Geological Survey). Mineral Commodity Summaries; Report 210; U.S. Geological Survey: Reston, VA, USA, 2023. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020. [Google Scholar]
- León, R.; Macías, F.; Cánovas, C.R.; Pérez-López, R.; Ayora, C.; Nieto, J.M.; Olías, M. Mine waters as a secondary source of rare earth elements worldwide: The case of the Iberian Pyrite Belt. J. Geochem. Explor. 2021, 224, 106742. [Google Scholar] [CrossRef]
- Chemanalyst. 2023. Available online: https://www.chemanalyst.com/Pricing-data/sulphur-39 (accessed on 14 October 2023).
- Kanagamani, K.; Geethamani, P.; Narmatha, M. Hazardous Waste Management. In Environmental Issues and Sustainable Development; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Elanthendral, G.; Inbathamizh, L.; Sudha, S. Engineered Nanomaterials for Water Treatment Applications. In Modern Nanotechnology; Malik, J.A., Sadiq Mohamed, M.J., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Ravelo-Nieto, E.; Ovalle-Serrano, S.A.; Gutiérrez-Pineda, E.A.; Blanco-Tirado, C.; Combariza, M.Y. Textile wastewater depuration using a green cellulose based Fe3O4 bionanocomposite. J. Environ. Chem. Engine. 2023, 11, 109516. [Google Scholar] [CrossRef]
- Solano, A.M.S.; de Araújo, C.K.C.; de Melo, J.V.; Peralta-Hernandez, J.M.; da Silva, D.R.; Martínez-Huitle, C.A. Decontamination of real textile industrial effluent by strong oxidant species electrogenerated on diamond electrode: Viability and disadvantages of this electrochemical technology. Appl. Catalysis B Environ. 2013, 130–131, 112–120. [Google Scholar] [CrossRef]
- Amare, E.; Kebed, F.; Berihu, T.; Mulat, W. Field based investigation on phytoremediation potentials of Lemna minor and Azolla filiculoides in tropical, semi-arid regions: Case of Ethiopia. Int. J. Phytoremed. 2017, 20, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Hocini, I.; Khellaf, N.; Benabbas, K.; Djelal, H.; Amrane, A. Identification of the mechanism involved in the removal potential of textile pollutants by the aquatic plant Lemna gibba L. J. Chem. Health Risks 2020, 10, 145–153. [Google Scholar] [CrossRef]
- Khellaf, N.; Djelal, H.; Amrane, A. An Overview of the Valorization of Aquatic Plants in Effluent Depuration through Phytoremediation Processes. Appl. Microbiol. 2022, 2, 309–318. [Google Scholar] [CrossRef]
- Chandanshive, V.; Kadam, S.K.; Khandare, R.V.; Kurade, M.B.; Jeon, B.-H.; Jadhav, J.P.; Govindwar, S.P. In situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere 2018, 210, 968–976. [Google Scholar] [CrossRef]
- López-Rodríguez, D.; Micó-Vicent, B.; Jordán-Núñez, J.; Bonet-Aracil, M.; Bou-Belda, E. Uses of Nanoclays and Adsorbents for Dye Recovery: A Textile Industry Review. Appl. Sci. 2021, 11, 11422. [Google Scholar] [CrossRef]
- Sierra-Solache, R.E.; Muro, C.; Maciel, A.; Illescas, J.; Díaz, M.C.; Carbajal-Franco, G.; Hernández, O.A. Water recovery from textile wastewater treatment by encapsulated cells of Phanerochaete chrysosporium and ultrafiltration system. Biologia 2020, 75, 1717–1729. [Google Scholar] [CrossRef]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Skalar, T.; Dolcet, L.P.; Hurley, R.; van Gestel, C.A. Exploring the impacts of plastics in soil–The effects of polyester textile fibers on soil invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef] [PubMed]
- Grilli, M.L.; Bellezze, T.; Gamsjäger, E.; Rinaldi, A.; Novak, P.; Balos, S.; Piticescu, R.R.; Ruello, M.L. Solutions for Critical Raw Materials under Extreme Conditions: A Review. Materials 2017, 10, 285. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials and the Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar] [CrossRef]
- COMISIÓN EUROPEA. Comunicación de la Comisión al Parlamento Europeo, al Consejo, al Comité Económico y Social Europeo y al Comité de las Regiones. Resiliencia de las Materias Primas Fundamentales: Trazando el Camino Hacia un Mayor Grado de Seguridad y Sostenibilidad. Brussels, 3.9.2020. COM 2020, 474 Final. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:52020DC0474&from=NL (accessed on 15 June 2023).
- European Critical Raw Materials Act. Proposal for a Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/102, COM, 2023. Available online: https://single-market-economy.ec.europa.eu/publications/european-critical-raw-materials-act_en#files (accessed on 15 June 2023).
- Bourg, S.; SCRREEN Project’s Partners. SCRREEN: Solutions for Critical Raw Materials—A European Expert Network. In Extraction 2018; Davis, B., Ed.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Directorate-General for Environment. EU Strategy for Sustainable and Circular Textiles. 2022. Brussels. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:9d2e47d1-b0f3-11ec-83e1-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 3 August 2023).
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular economy and sustainability of the clothing and textile industry. Mater. Circ. Econ. 2021, 3, 12. [Google Scholar] [CrossRef]
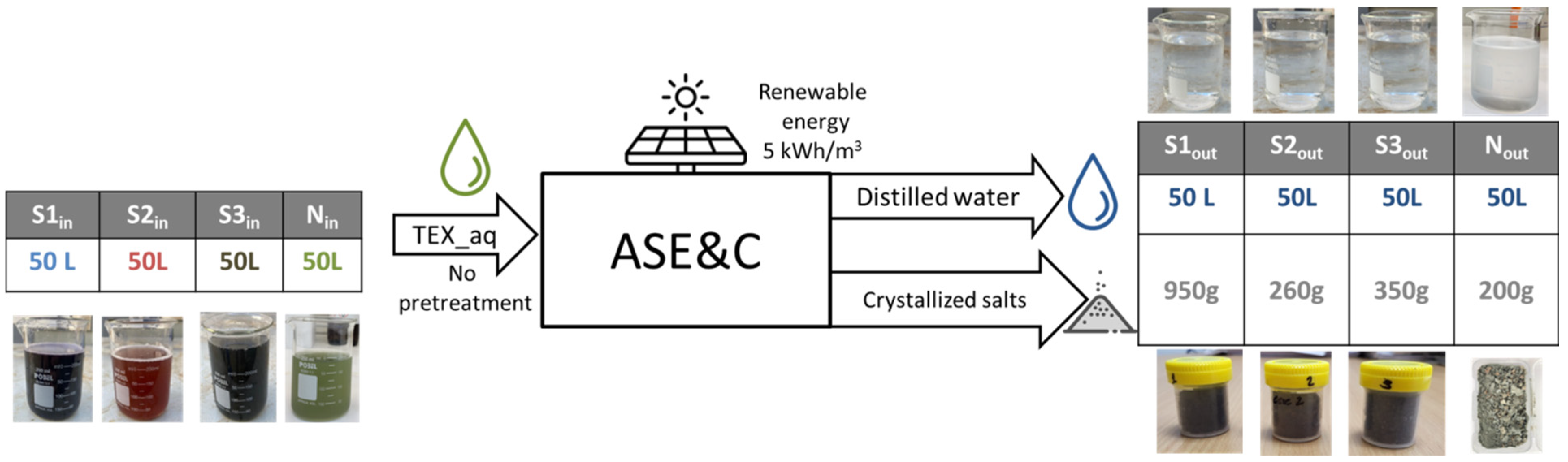

| Input | Output | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Units | S1in | S2in | S3in | Nin | S1out | S2out | S3out | Nout | |
| EC | µS/cm | 11,390 | 2000 | 2660 | 6670 | 16.2 | 16.7 | 16.3 | 38.1 |
| T | °C | 24.3 | 24.4 | 23.9 | 25.4 | 24.7 | 24.4 | 25.1 | 25.3 |
| pH | 6.66 | 8.99 | 7.43 | 7.27 | 6.87 | 6.80 | 7.01 | 6.53 | |
| COD | mg/LO2 | 8950 | 3069 | 3960 | 6900 | <10 | <10 | <10 | <10 |
| TSS | mg/L | 7170 | 2000 | 2660 | 4200 | 10.2 | 10.5 | 10.3 | 9.99 |
| Chlorides | meq/L | 56.13 | 9.86 | 13.11 | 18.31 | 0.08 | 0.08 | 0.08 | 0.06 |
| Sulphates | mg/L | ~8000 | 200 | ~400 | >200 | <25 | <25 | <25 | <25 |
| Elements | |||||||||
| Al | µg/L | 801 | 8063 | 4023 | 117 | n.d. | n.d. | n.d. | n.d. |
| Cd | µg/L | 7.80 | n.d | n.d | 0.2 | n.d. | n.d. | n.d. | n.d. |
| Ca | mg/L | 1999 | 1463 | 1035 | 10.9 | n.d. | n.d. | n.d. | n.d. |
| Co | µg/L | 154 | 14.2 | 169 | 3.2 | n.d. | n.d. | n.d. | n.d. |
| Cu | µg/L | 763 | 241 | 735 | 197 | n.d. | n.d. | n.d. | n.d. |
| Cr | µg/L | 23.4 | 7.00 | 17.2 | 110 | n.d. | n.d. | n.d. | n.d. |
| Mg | µg/L | 4152 | 1562 | 2966 | 40,842 | <5 | n.d. | <5 | <5 |
| Na | mg/L | 4179 | 702 | 4177 | 1951 | <5 | <5 | <5 | <5 |
| Zn | µg/L | 13,833 | 258 | 1242 | 75 | <20 | <20 | <20 | n.d. |
| Li | µg/L | 14.6 | 125 | 192 | 44.4 | n.d. | n.d. | n.d. | n.d. |
| K | mg/L | 12.5 | 11.4 | 7.78 | 92.6 | <0.1 | <0.1 | <0.1 | <0.1 |
| Fe | µg/L | 370 | 1057 | 418 | 88.9 | n.d. | n.d. | n.d. | n.d. |
| Ni | µg/L | 419 | 10.6 | 11.0 | 3.2 | n.d. | n.d. | n.d. | n.d. |
| As | µg/L | 0.75 | 1.54 | 6,33 | 3.60 | n.d. | n.d. | n.d. | n.d. |
| Sr | µg/L | 33.5 | 18.7 | 92.4 | 803 | n.d. | n.d. | n.d. | n.d. |
| Y | µg/L | 14.1 | 37.3 | 138 | 3.00 | n.d. | n.d. | n.d. | n.d. |
| Cs | µg/L | 36.4 | 90.0 | 162 | 0.26 | n.d. | n.d. | n.d. | n.d. |
| Ba | µg/L | 6.84 | 17.8 | 22.4 | 99.5 | n.d. | n.d. | n.d. | n.d. |
| La | µg/L | 41.4 | 47.4 | 51.3 | 0.19 | n.d. | n.d. | n.d. | n.d. |
| Ce | µg/L | 81.7 | 47.2 | 87.1 | n.d | n.d. | n.d. | n.d. | n.d. |
| Pr | µg/L | 13.1 | 12.1 | 15.3 | 0.10 | n.d. | n.d. | n.d. | n.d. |
| Nd | µg/L | 66.6 | 60.2 | 101 | 0.20 | n.d. | n.d. | n.d. | n.d. |
| Sm | µg/L | 8.58 | 14.6 | 5.55 | 0.10 | n.d. | n.d. | n.d. | n.d. |
| Gd | µg/L | 4.73 | 20.0 | 3.41 | 0.56 | n.d. | n.d. | n.d. | n.d. |
| Dy | µg/L | 9.80 | 37.7 | 5.15 | 0.10 | n.d. | n.d. | n.d. | n.d. |
| Er | µg/L | 16.3 | 20.4 | 2.71 | 0.10 | n.d. | n.d. | n.d. | n.d. |
| Yb | µg/L | 14.1 | 30.1 | 23.1 | 0.46 | n.d. | n.d. | n.d. | n.d. |
| Pb | µg/L | 2.14 | 6.44 | 5.75 | 0.15 | n.d. | n.d. | n.d. | n.d. |
| S1 | S2 | S3 | N | ||
|---|---|---|---|---|---|
| S | ton | 304,966 | 66 | 181 | 963 |
| Si | ton | 17.5 | 17.1 | 32.3 | 531 |
| Ca | ton | 5.5 | 3.4 | 10.8 | 416 |
| K | ton | 3.6 | 5.5 | 371 | |
| Mg | ton | 76 | 1080 | ||
| Al | ton | 75 | 251 | ||
| P | ton | 43 | |||
| Sb | ton | 5.5 | |||
| ∑Light elements | ton | 10,194 | |||
| Co | kg | 363 | 149 | 123 | |
| Cu | kg | 638 | 265 | 728 | 4771 |
| Fe | kg | 555 | 497 | 2070 | 3336 |
| Mo | kg | 166 | 69 | 77 | |
| Nb | kg | 93 | 33 | 31 | |
| Ni | kg | 223 | 121 | 77 | |
| Rb | kg | 1048 | 194 | 506 | 506 |
| Se | kg | 62 | 31 | 23 | |
| Sr | kg | 52 | 28 | 73 | 2725 |
| Zn | kg | 8746 | 1007 | 1805 | 1616 |
| Th | kg | 285 | 84 | ||
| Mn | kg | 330 |
| S1 | S2 | S3 | N | |
|---|---|---|---|---|
| S | 76,851 | 16,596 | 45,585 | 242,744 |
| Co | 10,893 | 4477 | 3679 | - |
| Cu | 3828 | 1592 | 4369 | 28,628 |
| Mo | 4316 | 1786 | 1993 | - |
| Nb | 5033 | 1788 | 1653 | - |
| Ni | 2877 | 1559 | 989 | - |
| Se | 1152 | 570 | 425 | - |
| Zn | 17,492 | 2014 | 3610 | 3232 |
| Total | 76,897,041 | 30,380 | 62,303 | 274,604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnail, E.; Vera, S.; Blasco, J.; DelValls, T.Á. Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge. Water 2023, 15, 3781. https://doi.org/10.3390/w15213781
Bonnail E, Vera S, Blasco J, DelValls TÁ. Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge. Water. 2023; 15(21):3781. https://doi.org/10.3390/w15213781
Chicago/Turabian StyleBonnail, Estefanía, Sebastián Vera, Julián Blasco, and Tomás Ángel DelValls. 2023. "Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge" Water 15, no. 21: 3781. https://doi.org/10.3390/w15213781
APA StyleBonnail, E., Vera, S., Blasco, J., & DelValls, T. Á. (2023). Towards a Cleaner Textile Industry: Using ASEC to Decrease the Water Footprint to Zero Liquid Discharge. Water, 15(21), 3781. https://doi.org/10.3390/w15213781







