Characterization of a Moderately Halotolerant Antimony-Removing Desulfovibrio sp. Strain Isolated from Landfill Leachate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Media
2.2. Enrichment of Sb-Removing Bacteria from Landfill Leachate
2.3. Isolation of Antimony-Removing Bacteria
2.4. Sb Removal Tests
2.5. Phylogenetic Characterization of Bacteria
2.6. Chemical Analysis
3. Results
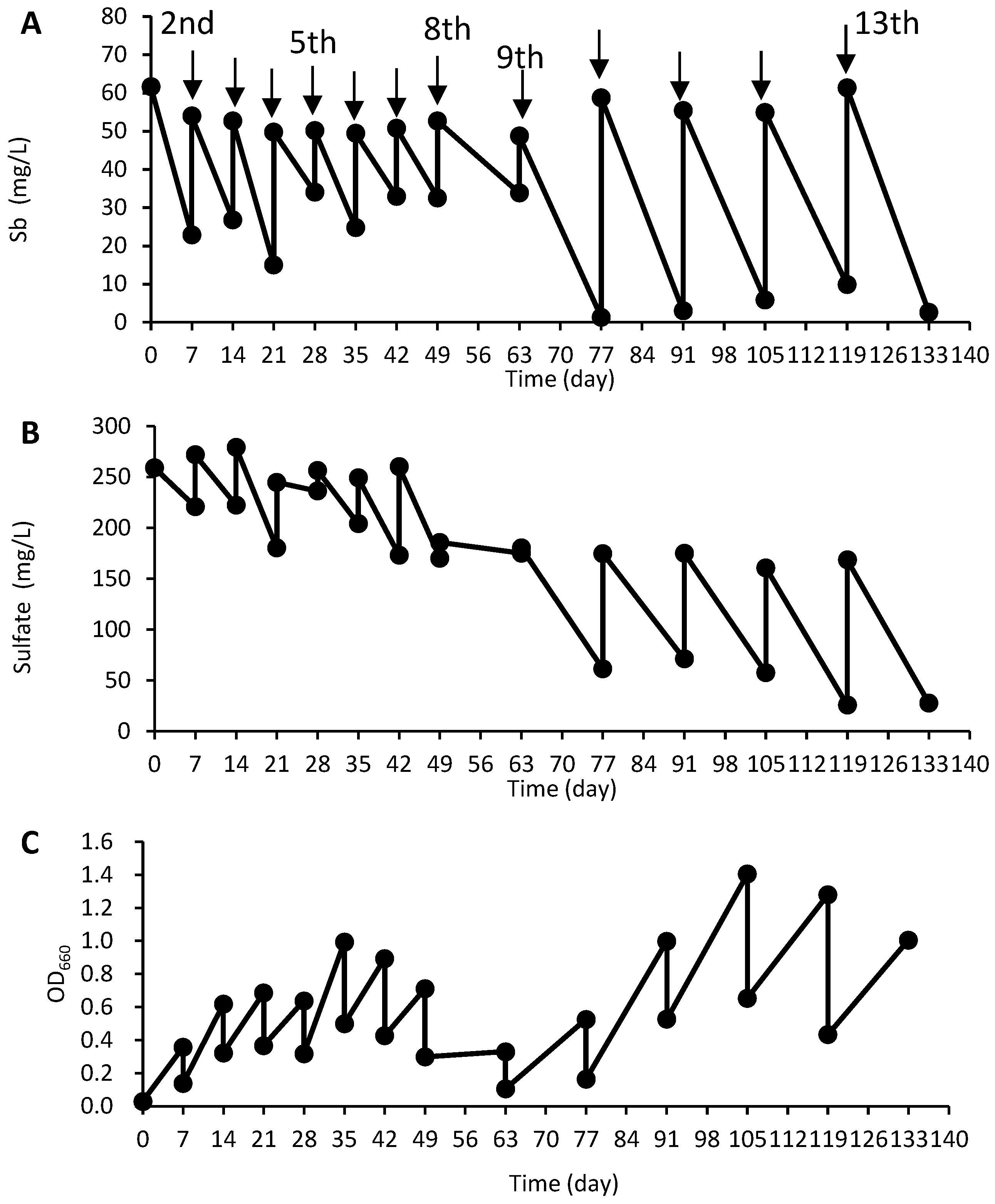
3.1. Enrichment of Sb-Removing Bacteria from Landfill Leachate
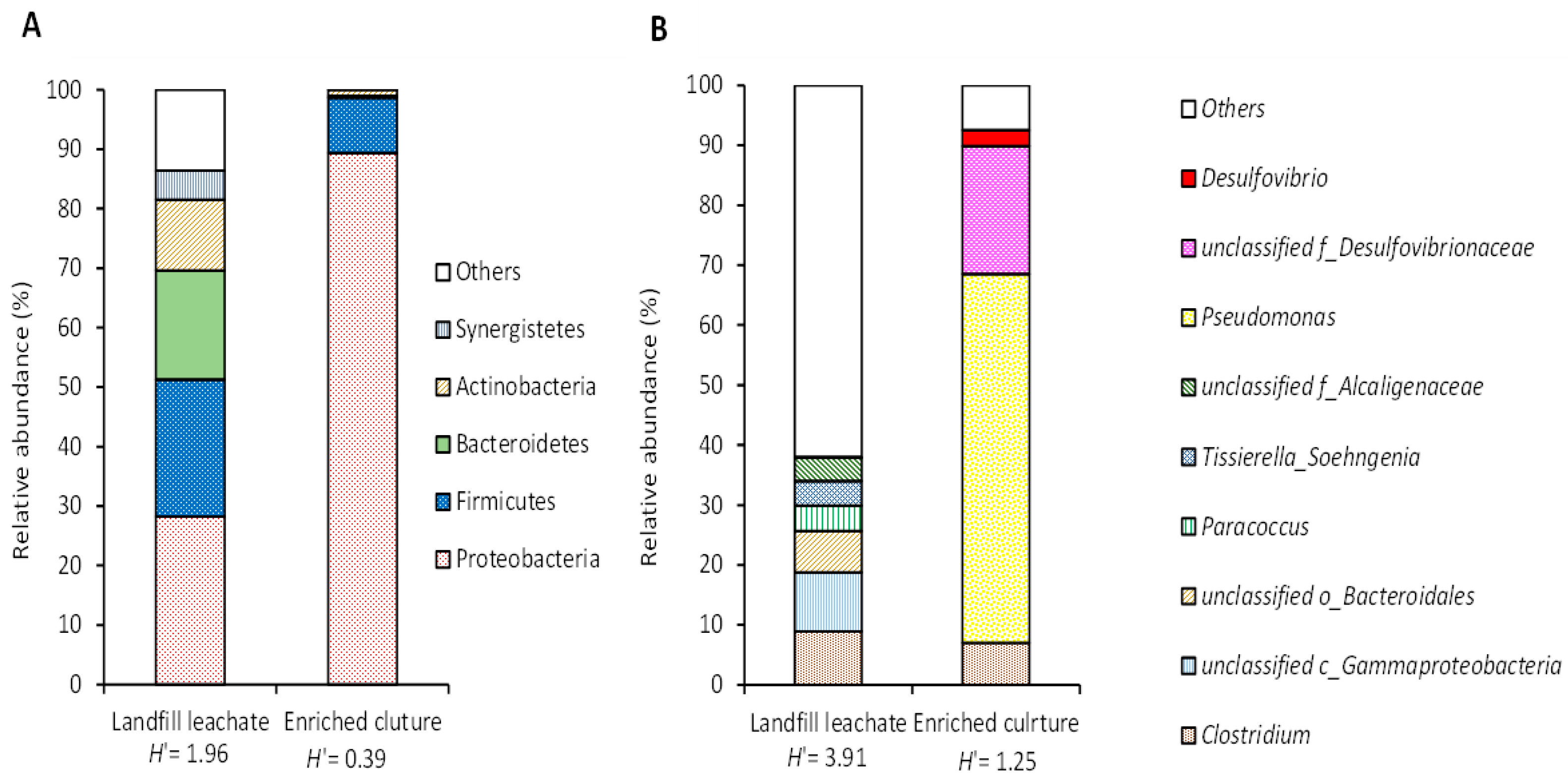
3.2. Microbial Community Composition in Enrichment Culture
3.3. Isolation of an Sb-Removing Bacterium from Enrichment Culture
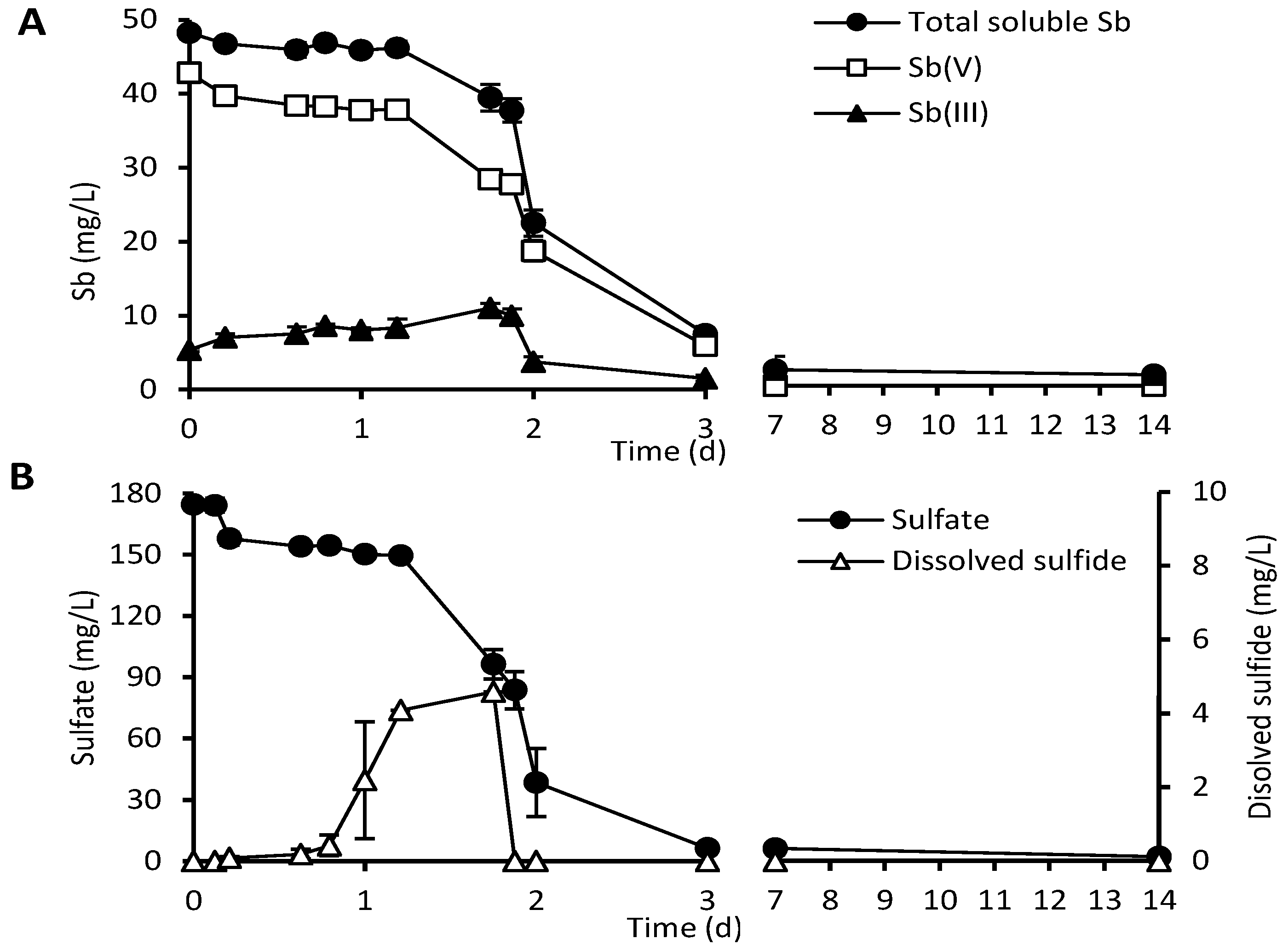
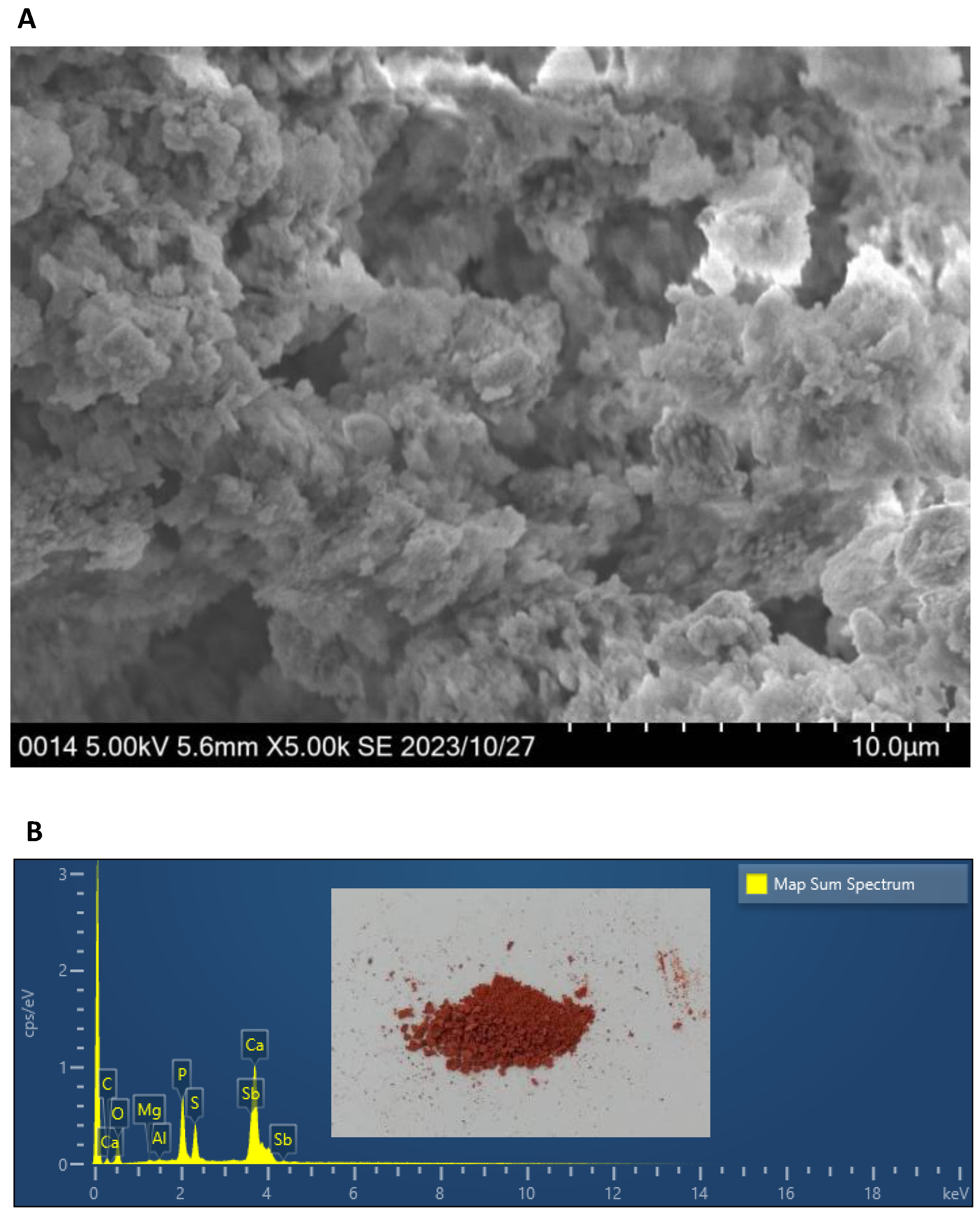
3.4. Removal of Sb and Sulfate by NSLLH1b and the Resulting Precipitates
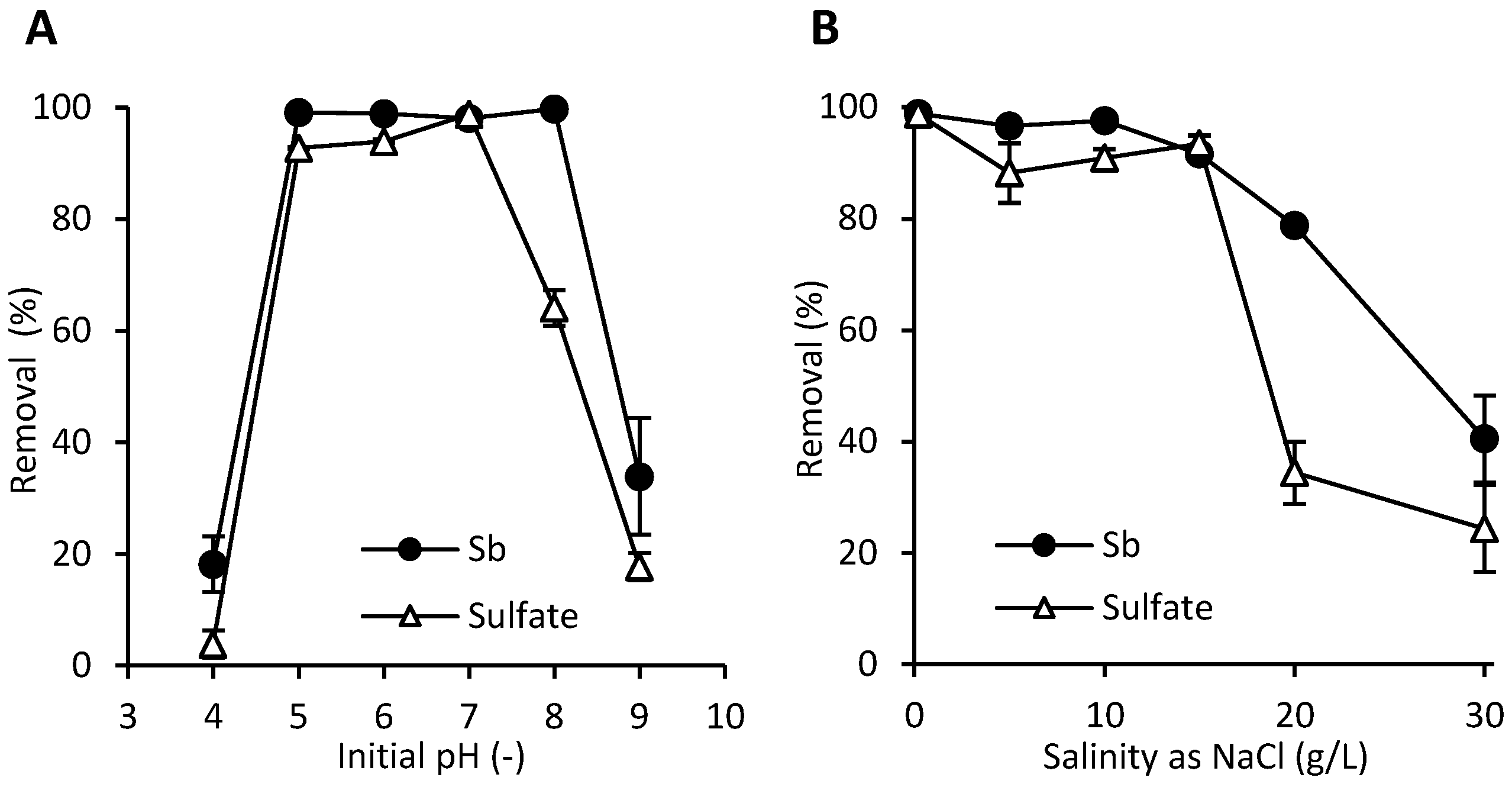
3.5. Factors Affecting Sb(V) Removal by NSLLH1b
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shotyk, W.; Krachler, M.; Chen, B. Antimony: Global environmental contaminant. J. Environ. Monit. 2015, 7, 1135–1136. [Google Scholar] [CrossRef]
- Li, J.; Zheng, B.; He, Y.; Zhou, Y.; Chen, X.; Ruan, S.; Yang, Y.; Dai, C.; Tang, L. Antimony contamination, consequences, and removal techniques: A review. Ecotoxicol. Environ. Saf. 2018, 156, 125–134. [Google Scholar] [CrossRef]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem.-Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef]
- USEPA. National Primary Drinking Water Regulations; EPA 816-F-09-004; United States Environmental Protection Agency: Washington, DC, USA, 2009. Available online: http://water.epa.gov/drink/contaminants/upload/mcl-2.pdf (accessed on 30 October 2023).
- EU Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption. European Council, EU. 1998L0083- EN27.10.2015-003.001. Adopted by the Council. 1998. Available online: https://aei.pitt.edu/10298/1/10298.pdf (accessed on 30 October 2023).
- World Health Organization (WHO). Guidelines for Drinking-Water Quality, in Incorporating the First Addendum, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- QCVN 01:2009/BYT; National Technical Regulation on Drinking Water Quality. Ministry of Health: Hanoi, Vietnam, 2009.
- Intrakamhaeng, V.; Clavier, K.A.; Liu, Y.; Townsend, T.G. Antimony mobility from E-waste plastic in simulated municipal solid waste landfills. Chemosphere 2020, 241, 125042. [Google Scholar] [CrossRef]
- Moody, C.M.; Townsend, T.G. A comparison of landfill leachates based on waste composition. Waste Manag. 2017, 63, 267–274. [Google Scholar] [CrossRef]
- Wang, H.; Chen, F.; Mu, S.; Zhang, D.; Pan, X.; Lee, D.J.; Chang, J.S. Removal of antimony (Sb(V)) from Sb mine drainage: Biological sulfate reduction and sulfide oxidation-precipitation. Bioresour. Technol. 2013, 146, 799–802. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Ren, B. Treatment of antimony mine drainage: Challenges and opportunities with special emphasis on mineral adsorption and sulfate reducing bacteria. Water Sci. Technol. 2016, 73, 2039–2051. [Google Scholar] [CrossRef]
- Cappuyns, V.; Van Campen, A.; Helser, J. Antimony leaching from soils and mine waste from the Mau Due antimony mine, North-Vietnam. J. Geochem. Explor. 2021, 220, 06663. [Google Scholar] [CrossRef]
- Zhang, G.; Ouyang, X.; Li, H.; Fu, Z.; Chen, J. Bioremoval of antimony from contaminated waters by a mixed batch culture of sulfate-reducing bacteria. Int. Biodeterior. Biodegrad. 2016, 115, 148–155. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Liu, S.; Fu, Z.; Chen, J.; Ma, C. Bioremoval of arsenic and antimony from wastewater by a mixed culture of sulfate-reducing bacteria using lactate and ethanol as carbon sources. Int. Biodeterior. Biodegrad. 2018, 126, 152–159. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, M.; Gao, N.; Chu, W.; An, N.; Wang, Q.; Wang, S. Removal of antimonate from wastewater by dissimilatory bacterial reduction: Role of the coexisting sulfate. J. Hazard. Mater. 2018, 341, 36–45. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Ma, C.; Li, D. Antimony removal from wastewater by sulfate-reducing bacteria in a bench-scale upflow anaerobic packed-bed reactor. Acta Geochim. 2020, 39, 203–215. [Google Scholar] [CrossRef]
- Xi, Y.; Lan, S.; Li, X.; Wu, Y.; Yuan, X.; Zhang, C.; Yunguo, L.; Huang, Y.; Quan, B.; Wu, S. Bioremediation of antimony from wastewater by sulfate-reducing bacteria: Effect of the coexisting ferrous ion. Int. Biodeterior. Biodegrad. 2020, 148, 104912. [Google Scholar] [CrossRef]
- Li, H.; Fei, Y.; Xue, S.; Zhang, G.; Bian, Z.; Guo, F.; Wang, L.; Chai, R.; Zhang, S.; Cui, Z.; et al. Removal of antimony in wastewater by antimony tolerant sulfate-reducing bacteria isolated from municipal sludge. Int. J. Mol. Sci. 2022, 23, 1594. [Google Scholar] [CrossRef]
- Yu, H.; Yan, X.; Weng, W.; Xu, S.; Xu, G.; Gu, T.; Guan, X.; Liu, S.; Chen, P.; Wu, Y.; et al. Extracellular proteins of Desulfovibrio vulgaris as adsorbents and redox shuttles promote biomineralization of antimony. J. Hazard. Mater. 2022, 426, 127795. [Google Scholar] [CrossRef]
- Ramírez-Patiño, J.; Pérez-Trevilla, J.; Cervantes, F.J.; Moreno-Andrade, I. Removal of antimony by dissimilatory and sulfate-reducing pathways in anaerobic packed bed bioreactors. J. Chem. Technol. Biotechnol. 2023, 98, 932–939. [Google Scholar] [CrossRef]
- Xue, G.; Wang, Q.; Qian, Y.; Gao, P.; Su, Y.; Liu, Z.; Chen, H.; Li, X.; Chen, J. Simultaneous removal of aniline, antimony and chromium by ZVI coupled with H2O2: Implication for textile wastewater treatment. J. Hazard. Mater. 2019, 368, 840–848. [Google Scholar] [CrossRef]
- Qi, Y.; Fan, C.; Quan, X.; Xi, F.; Liu, Z.; Cao, Q.; Wu, Z.; Yue, Q.; Gao, B.; Xu, X.; et al. In-situ recycling strategy for co-treatment of antimony-rich sludge char and leachate: Pilot-scale application in an engineering case. Chem. Eng. J. 2022, 446, 137315. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Nakajima, J.; Takaoka, M.; Hang, N.T.A. Heavy metal speciation in landfill leachate and its association with organic matter. IOP Conf. Ser. Earth Environ. Sci. 2019, 266, 012006. [Google Scholar] [CrossRef]
- Hoai, S.T.; Nguyen Lan, H.; Thi Viet, N.T.; Nguyen Hoang, G.; Kawamoto, K. Characterizing seasonal variation in landfill leachate using leachate pollution index (LPI) at Nam Son solid waste landfill in Hanoi, Vietnam. Environments 2021, 8, 17. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Caporaso, J.G. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ 2018, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, S.J.; Saunders, A.M.; Albertsen, M.; Nierychlo, M.; McIlroy, B.; Hansen, A.A.; Karst, S.M.; Nielsen, J.L.; Nielsen, P.H. MiDAS: The field guide to the microbes of activated sludge. Database 2015, 2015, bav062. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- The Japanese Pharmacopoeia Sixteenth Edition. In Microbial attributes of Nonsterile Pharmaceutical Products; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2011.
- Wysocki, R.; Chéry, C.C.; Wawrzycka, D.; Van Hulle, M.; Cornelis, R.; Thevelein, J.M.; Tamás, M.J. The glycerol channel Fps1p mediates the uptake of arsenite and antimonite in Saccharomyces cerevisiae. Mol. Microbiol. 2001, 40, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Roschi, E.; Marison, I.W.; Comminellis, C.; von Stockar, U. Physiologic studies with the sulfate-reducing bacterium Desulfovibrio desulfuricans: Evaluation for use in a biofuel cell. Enzym. Microb. Technol. 1996, 18, 358–365. [Google Scholar] [CrossRef]
- Chen, H.; Yu, X.; Wang, X.; He, Y.; Zhang, C.; Xue, G.; Liu, Z.; Lao, H.; Song, H.; Chen, W.; et al. Dyeing and finishing wastewater treatment in China: State of the art and perspective. J. Clean. Prod. 2021, 326, 129353. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binneman, K. Antimony recovery from end-of-life products and industrial process residues: A Critical review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, C.T.K.; Sawada, K.; Soda, S. Characterization of a Moderately Halotolerant Antimony-Removing Desulfovibrio sp. Strain Isolated from Landfill Leachate. Water 2023, 15, 3872. https://doi.org/10.3390/w15223872
Pham CTK, Sawada K, Soda S. Characterization of a Moderately Halotolerant Antimony-Removing Desulfovibrio sp. Strain Isolated from Landfill Leachate. Water. 2023; 15(22):3872. https://doi.org/10.3390/w15223872
Chicago/Turabian StylePham, Chinh Thi Kieu, Kazuko Sawada, and Satoshi Soda. 2023. "Characterization of a Moderately Halotolerant Antimony-Removing Desulfovibrio sp. Strain Isolated from Landfill Leachate" Water 15, no. 22: 3872. https://doi.org/10.3390/w15223872
APA StylePham, C. T. K., Sawada, K., & Soda, S. (2023). Characterization of a Moderately Halotolerant Antimony-Removing Desulfovibrio sp. Strain Isolated from Landfill Leachate. Water, 15(22), 3872. https://doi.org/10.3390/w15223872






