Integrated Phytobial Remediation of Dissolved Pollutants from Domestic Wastewater through Constructed Wetlands: An Interactive Macrophyte-Microbe-Based Green and Low-Cost Decontamination Technology with Prospective Resource Recovery
Abstract
:1. Introduction
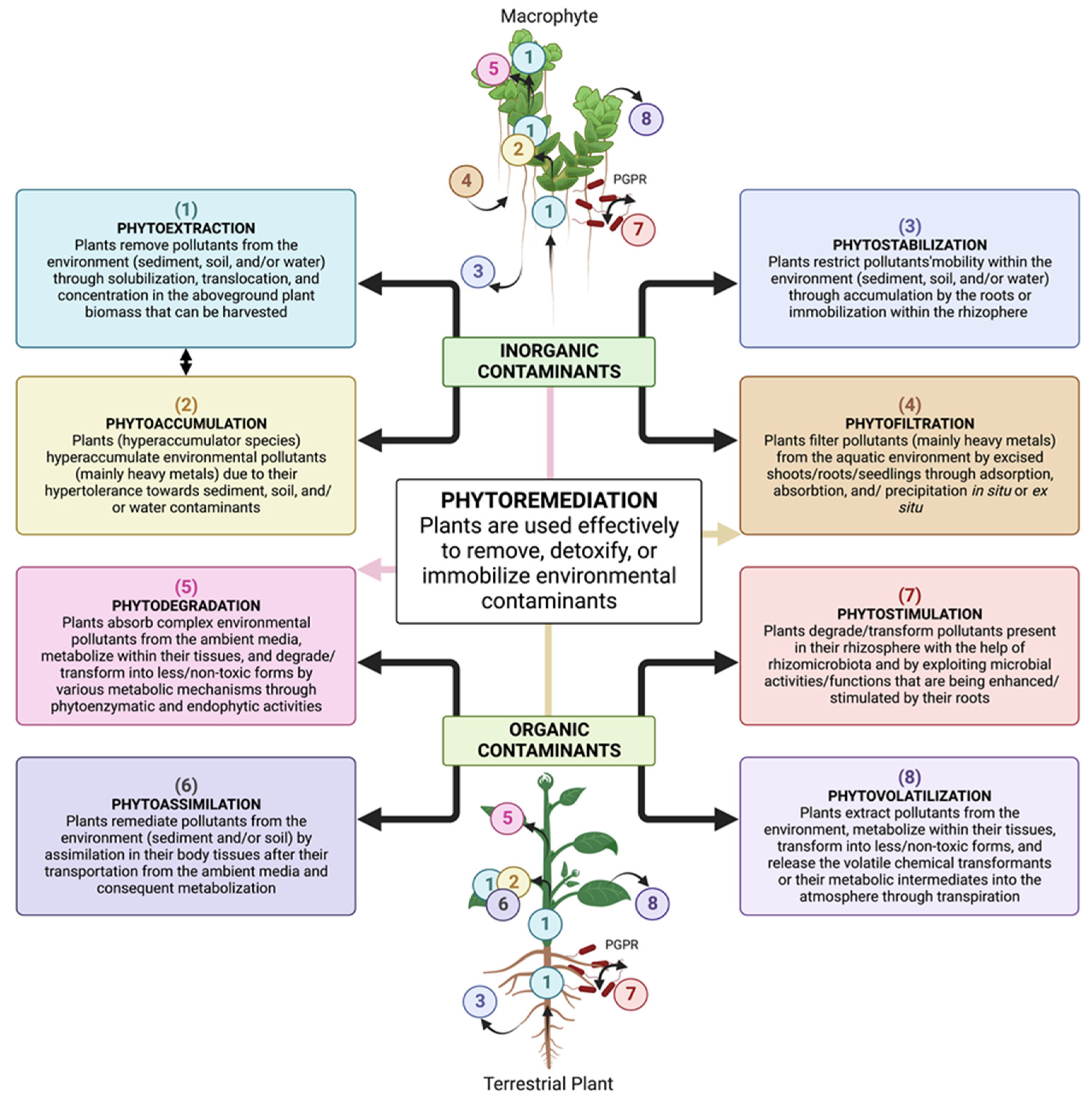
2. Phytoremediation: An In Situ Plant-Based Clean-Up Technology
| Sl. No. | Country/Province Name | Length of the River/Lack (km) | World’s Most Polluted Lakes/River | Source of Pollutant | References |
|---|---|---|---|---|---|
| 1. | BANGLADESH | 27 km | Buri ganga River | Mountains of plastic, sewage-related trash, and residential and industrial waste | [25] |
| 2. | PHILIPPINES | 12.42 km | Marilao River | Open landfills, metal refining, lead-acid batteries, and other harmful metals. | [26] |
| 3. | JORDAN, ISRAEL, and PALESTINE | 251 km | Jordan River | Untreated sewage and Agricultural Runoff. | [27] |
| 4. | NORTHEASTERN AFRICA | 6650 km | Nile River | Intensive load of urban, agricultural, and industrial wastewater | [28] |
| 5. | RUSSIA | 0.75 km | Lake Karachay | Radioactive wastes | [29] |
| 6. | ITALY | 24 km | Sarno River (stream) | Untreated industrial waste and trash from agriculture | [30] |
| 7. | KENYA | 390 km | Nairobi River | Untreated sewage and industrial waste. | [31] |
| 8. | TASMANIA | 13 km | King River | Acid drainage from the old mines. | [32] |
| 9. | ARGENTINA | 64 km | Matanza River (stream) | Sewage and waste water treatment, farming and fossil fuel power plants | [33] |
| 10. | SOUTH OF CHICAGO | 25.7 km | Grand Calumet River | Industrial waste and municipal effluent. | [34] |
| 11. | ARGENTINA AND URUGUAY | 499 km | Plate River | Urban runoff. | [35] |
| 12. | AUSTRALIA | 2508 km | Murray-Darling River | Tillage, cultivation, fertilizers, and animal droppings. | [36] |
| 13. | BRAZIL | 853 km | Doce River | Agriculture (e.g., fertilizers and pesticides) and inadequate disposal of municipal waste. | [37] |
| 14. | NORTHERN NEW JERSEY | 130 km | Passaic River | Industrialization Watershed. Layers of dioxin, mercury, PCBs, and other harmful chemicals. | [38] |
| 15. | EUROPE | 2857 km | Danube River | Organic material, nutrients, hazardous substances, and plastics. | [39] |
| 16. | INDIA | 1376 km | Yamuna | Untreated wastewater, etc. | [40] |
| 17. | INDIA | 2525 km | Ganges | Sewage dumped industrial waste, agricultural runoff, partially burned or unburned bodies from funeral pyres, and animal carcasses. | [41] |
| 18. | TIBET, INDIA and PAKISTAN | 3180 km | Indus River | Municipal, industrial wastewater discharges and, return-agriculture flows through drainage structures. | [42] |
| 19. | CHINA | 6300 km | Yangtze River | Industrial wastewater, agricultural chemical fertilizer, ship garbage and acid rain | [43] |
| 20. | CHINA | 5464 km | Yellow River | Factory discharges and sewage. | [44] |
| 21. | CHINA and MYANMAR | 3289 km | Salween River | Textile factories’ waste. | [45] |
| 22. | INDONESIA | 297 km | Citarum River | Industrial waste and high organic waste from cattle. | [46] |
| 23. | INDONESIA | 320 km | Brantas River | Micro-plastics pollution | [47] |
| 24. | USA | 3034 km | Rio Grande River | High concentrations of salts, microorganisms, and industrial and agricultural contaminants. | [48] |
| 25. | USA | 3766 km | Mississippi River | Agricultural runoff | [49] |
2.1. Different Plant Strategies Used for Phytoremediation
2.1.1. Phytoextraction
2.1.2. Phytodegradation
2.1.3. Phytofiltration
2.1.4. Phytostimulation
2.1.5. Phytovolatilization
2.2. Sources and Types of Pollutants in Domestic Wastewater
2.3. Phytoremediation of Domestic Sewage/Wastewater
3. Constructed Wetlands (CWs) in Wastewater Decontamination
3.1. Types and Modes of Action of Constructed Wetlands (CWs)
3.1.1. Surface-Flow Constructed Wetlands (SFCWs)

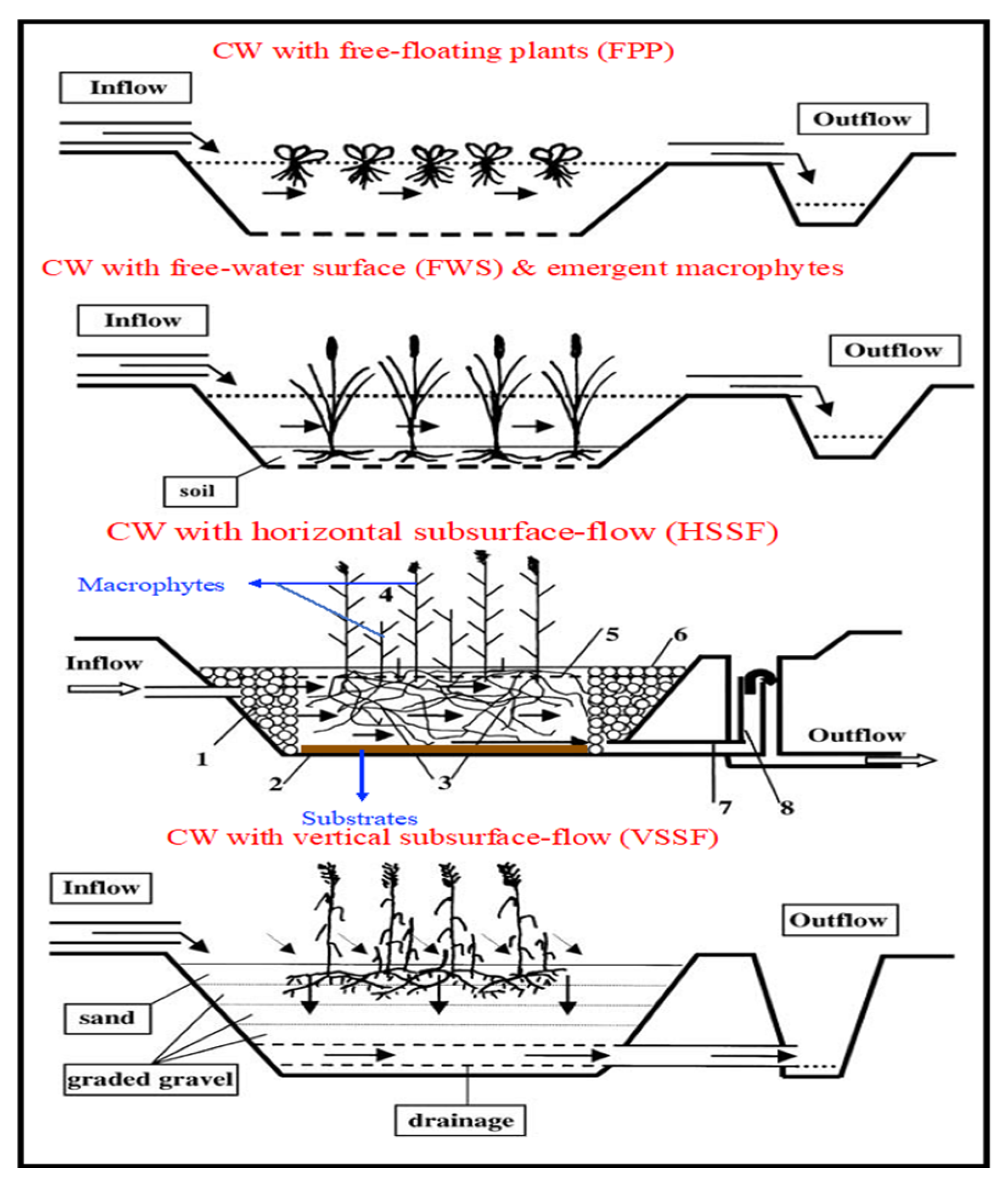
3.1.2. Subsurface-Flow Constructed Wetlands (SSFCWs)
Horizontal Subsurface-Flow Constructed Wetlands (HSSFCWs)
Vertical Subsurface-Flow Constructed Wetlands (VSSFCWs)
3.1.3. Hybrid Constructed Wetlands (HCWs)
3.2. Operation and Maintenance of Constructed Wetland (CW) Systems
4. Contaminant Removal in Different Constructed Wetland (CW) Types
4.1. Role of Macrophytes or Vegetation
- Plants must adapt to local environmental conditions.
- Plants must be practicable under local climatic conditions and may tolerate/resist potential pests, insects, and diseases.
- Plants should tolerate various contaminants (e.g., N, OM, P, etc.) in the wastewater.
- Plants should be easily adjusted in local CW environments to show relatively fast growth and spreading.
- Plants should have a high pollutant elimination capacity.
4.2. Role of Substrate Materials
- The media supports the growth of planted vegetation.
- It stabilizes the bed (contact effect with the roots of developed plants).
- It provides a media filtration effect.
- It ensures high permeability and reduces possible clogging problems.
- It provides an attractive attachment area for many microbes (biofilm formation) that are involved in pollutant removal processes.
- It supports many transformation and elimination processes.
4.3. Role of (Plant Root-Associated) Microbes
4.4. Role of Influent-Feeding Mode
4.5. Role of Constructed Wetland (CW) as a Catalyst in Phytoremediation
4.5.1. Removal of Total Dissolved Solids (TDS)
4.5.2. Removal of Biological Oxygen Demand (BOD)
4.5.3. Removal of Total Nitrogen (TN)
4.5.4. Removal of Nitrates (NO3−)
Biological Removal Mechanism(s)
4.5.5. Removal of Phosphate (PO42−)
Physical Removal Mechanism(s)
Chemical Removal Mechanism(s)
Biological Removal Mechanism(s)
4.5.6. Removal of Heavy or Trace Metals
4.5.7. Removal of Pathogens
5. Design of a Representative Constructed Wetland (CW) System
6. Prospective Resource Recovery in Constructed Wetland (CW) Systems for Circular Bioeconomy (CBE)
7. Performance Assessment of Constructed Wetlands (CWs) and Macrophytes for Domestic Sewage Treatment
8. Pros and Cons of Phytoremediation through Constructed Wetland (CW) Systems
9. Recommendations and Prospects
- The role of different types of CWs and environmental conditions needs to be explored, especially in rural areas with no centralized wastewater treatment system.
- When studying the interaction between the ecological environments of plants and local substrates in a CW, the role of the microbial community must be examined.
- Dissolved nutrients and other contaminants assimilated by the wetland vegetation have been reported to be released into the water when plants wither, die, and decay during the winter, potentially resulting in poor removal performances of CWs. As a result, research and development (R&D) on optimal plant-harvesting techniques and the conservation and recycling of plant-based resources in CW systems are critical.
- A few limitations associated with this technology have raised grave concerns about its widespread practice, like climate adaptability, less efficient plant morphological traits, phytodisposal, seasonal dependencies, and time consumption. To overcome such barriers, plant geneticists have recently emphasized the application of plant biotechnology in developing new species of macrophytes with increased capabilities of dissolved nutrient/pollutant extraction through breeding techniques, protoplast fusion, and mutagenesis.
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of contaminated waters: An eco-friendly technology based on aquatic macrophytes application. Egypt. J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Khan, F.A.; Ansari, A.A. Eutrophication: An ecological vision. Bot. Rev. 2005, 71, 449–482. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Anderson, T.A.; Schwab, A.P.; Hsu, F.C. Phytoremediation of soils contaminated with organic pollutants. Adv. Agron. 1996, 56, 55–114. [Google Scholar] [CrossRef]
- Lu, Q. Evaluation of Aquatic Plants for Phytoremediation of Eutrophic Storm Waters. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2009. [Google Scholar] [CrossRef]
- Hassan, I.; Chowdhury, S.R.; Prihartato, P.K.; Razzak, S.A. Wastewater Treatment Using Constructed Wetland: Current Trends and Future Potential. Processes 2021, 9, 1917. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, X.; Gan, L.; He, Z.; Zhu, J.; Zhang, W.; Gao, Y.; Yang, L. Effects of biochar on the growth of Vallisneria natans in surface flow constructed wetland. Environ. Sci. Pollut. Res. 2021, 28, 66158–66170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, J.; Bai, S.; Ma, F.; Wang, L. Microbial population dynamics in response to bioaugmentation in a constructed wetland system under 10 °C. Bioresour. Technol. 2016, 205, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Y.; Qu, M.; Hao, M.; Yang, D.; Yang, Q.; Wang, X.C.; Dzakpasu, M. Fate of an antibiotic and its effects on nitrogen transformation functional bacteria in integrated vertical flow constructed wetlands. Chem. Eng. J. 2021, 417, 129272. [Google Scholar] [CrossRef]
- Calheiros, C.S.; Duque, A.F.; Moura, A.; Henriques, I.S.; Correia, A.; Rangel, A.O.; Castro, P.M. Changes in the bacterial community structure in two-stage constructed wetlands with different plants for industrial wastewater treatment. Bioresour. Technol. 2009, 100, 3228–3235. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Meng, P.; Pei, H.; Hu, W.; Shao, Y.; Li, Z. How to increase microbial degradation in constructed wetlands: Influencing factors and improvement measures. Bioresour. Technol. 2014, 157, 316–326. [Google Scholar] [CrossRef]
- Badhe, N.; Saha, S.; Biswas, R.; Nandy, T. Role of algal biofilm in improving the performance of free surface, up-flow constructed wetland. Bioresour. Technol. 2014, 169, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mao, W.; Pang, L.; Li, R.; Li, S. Influence of Phragmites communis and Zizania aquatica on rhizosphere soil enzyme activity and bacterial community structure in a surface flow constructed wetland treating secondary domestic effluent in China. Environ. Sci. Pollut. Res. 2020, 27, 26141–26152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Gong, Z.; Li, X. Performance study of vertical flow constructed wetlands for phosphorus removal with water quenched slag as a substrate. Ecol. Eng. 2013, 53, 39–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, X.; Cao, X.; Wang, Y.; Zhao, Z.; Si, Z.; Yuan, S. Modified solid carbon sources with nitrate adsorption capability combined with nZVI improve the denitrification performance of constructed wetlands. Bioresour. Technol. 2019, 294, 122189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, J.; Wang, X.; Liu, Y.; Wang, S.; Kong, F. Interactions of chlorpyrifos degradation and Cd removal in iron-carbon-based constructed wetlands for treating synthetic farmland wastewater. J. Environ. Manag. 2021, 299, 113559. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J.; Zhao, Y.; Mander, U. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Agarwal, S.; Darbar, S.; Saha, S. Challenges in management of domestic wastewater for sustainable development. In Current Directions in Water Scarcity Research; Elsevier: Amsterdam, The Netherlands, 2022; pp. 531–552. [Google Scholar] [CrossRef]
- UNEP. Phytoremediation: An Environmentally Sound Technology for Pollution Prevention, Control and Remediation. An In-troductory Guide to Decision-Makers. Newsletter and Technical Publications Freshwater Management Series No. 2 United Nations Environment Programme Division of Technology, Industry, and Economics. 2019. Available online: http://www.unep.or.jp/Ietc/Publications/Freshwater/FMS2/1.asp (accessed on 18 August 2019).
- Interstate Technology & Regulatory Council. Phytotechnology Technical and Regulatory Guidance and Decision Trees, Revised; ITRC: Washington, DC, USA, 2009. Available online: www.itrcweb.org (accessed on 17 September 2023).
- Nwoko, C. Trends in phytoremediation of toxic elemental and organic pollutants. Afr. J. Biotechnol. 2010, 9, 6010–6016. [Google Scholar] [CrossRef]
- Liu, J.L.; Anderson, J.T.; Zhang, R.; Zhang, Z.M. Potential of aquatic macrophytes and artificial floating island for removing contaminants. Plant Biosyst. 2016, 150, 702–709. [Google Scholar] [CrossRef]
- De Stefani, G.; Tocchetto, D.; Salvato, M.; Borin, M. Performance of a floating treatment wetland for in-stream water amelioration in NE Italy. Hydrobiologia 2011, 674, 157–167. [Google Scholar] [CrossRef]
- Bashar, K.; Noro, K.; Wang, Q.; Tokumura, M.; Mori, I.; Raknuzzaman, M.; Hossain, A.; Amagai, T. Spatiotemporal distribution and pollution assessment of trace metals in the Buriganga River, Bangladesh. J. Water Health 2023, 21, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Pleto, J.V.R.; Migo, V.P.; Arboleda, M.D.M. Preliminary water and sediment quality assessment of the Meycauayan river segment of the Marilao-Meycauayan-Obando river system in Bulacan, the Philippines. J. Health Pollut. 2020, 10, 200609. [Google Scholar] [CrossRef] [PubMed]
- Barinova, S.; Tavassi, M.; Glassman, H.; Nevo, E. Algal indication of pollution in the Lower Jordan River, Israel. Appl. Ecol. Environ. Res. 2010, 8, 19–38. [Google Scholar] [CrossRef]
- Abdel-Satar, A.M.; Ali, M.H.; Goher, M.E. Indices of water quality and metal pollution of Nile River, Egypt. Egypt. J. Aquat. Res. 2017, 43, 21–29. [Google Scholar] [CrossRef]
- Ibragimov, R. Lake Karachay as a Source of Groundwater Pollution. 2020. Available online: https://www.theseus.fi/handle/10024/343214 (accessed on 17 September 2023).
- Cicchella, D.; Giaccio, L.; Lima, A.; Albanese, S.; Cosenza, A.; Civitillo, D.; De Vivo, B. Assessment of the topsoil heavy metals pollution in the Sarno River basin, south Italy. Environ. Earth Sci. 2014, 71, 5129–5143. [Google Scholar] [CrossRef]
- Mbui, D.; Chebet, E.; Kamau, G.; Kibet, J. The State of Water Quality in Nairobi River, Kenya. Asian J. Res. Chem. 2016, 9, 579. [Google Scholar] [CrossRef]
- Fergusson, L. A 12-month Field Trial to Remediate an Exposed “Tailings Beach” in Tasmania. Resour. Environ. 2014, 4, 238–245. [Google Scholar]
- Tripodi, M.A.; Cueto, G.R.; Muschetto, E.; Hancke, D.; Suárez, O.V. Intra-and inter-annual variations in metal concentra-tions in the superficial water of a highly polluted urban basin of Argentina. Environ. Sci. Pollut. Res. 2023, 30, 60838–60853. [Google Scholar] [CrossRef]
- Robinson, R.M. The “Most Polluted River”: The Grand Calumet. In Environmental Advocacy and Local Restorations; Springer International Publishing: Cham, Switzerland, 2023; pp. 187–207. [Google Scholar]
- Magdaleno, A.; Mendelson, A.; de Iorio, A.F.; Rendina, A.; Moretton, J. Genotoxicity of leachates from highly polluted lowland river sediments destined for disposal in landfill. Waste Manag. 2008, 28, 2134–2139. [Google Scholar] [CrossRef]
- Tao, H.; Al-Hilali, A.A.; Ahmed, A.M.; Mussa, Z.H.; Falah, M.W.; Abed, S.A.; Deo, R.; Jawad, A.H.; Maulud, K.N.A.; Latif, M.T.; et al. Statistical and spatial analysis for soil heavy metals over the Murray-Darling river basin in Australia. Chemosphere 2023, 317, 137914. [Google Scholar] [CrossRef]
- Yamamoto, F.Y.; Pauly, G.F.E.; Nascimento, L.S.; Fernandes, G.M.; Santos, M.P.; Figueira, R.C.L.; Cavalcante, R.M.; Grassi, M.T.; Abessa, D.M.S. Explaining the persistence of hazardous chemicals in the Doce River (Brazil) by multiple sources of contamination and a major envi-ronmental disaster. J. Hazard. Mater. Adv. 2023, 9, 100250. [Google Scholar] [CrossRef]
- Yildiz, Y.; Karadag, R.; Cheema, M.; Sayedahmed, M. Ion Selective Electrode Determination of Ammonia Nitrogen in Passaic River Waste Water in New Jersey Essex County Area. Am. J. Anal. Chem. 2022, 13, 96–107. [Google Scholar] [CrossRef]
- Kirschner, A.K.T.; Reischer, G.H.; Jakwerth, S.; Savio, D.; Ixenmaier, S.; Toth, E.; Sommer, R.; Mach, R.L.; Linke, R.; Eiler, A.; et al. Multiparametric monitoring of microbial faecal pollution reveals the dominance of human contamination along the whole Danube River. Water Res. 2017, 124, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kumar, R.; Satapathy, S.C.; Al-Ansari, N.; Singh, K.K.; Mahapatra, R.P.; Agarwal, A.K.; Van Le, H.; Pham, B.T. Analysis of water pollution using different physicochemical parameters: A study of Yamuna River. Front. Environ. Sci. 2020, 8, 581591. [Google Scholar] [CrossRef]
- Dwivedi, S.; Mishra, S.; Tripathi, R.D. Ganga water pollution: A potential health threat to inhabitants of Ganga basin. Environ. Int. 2018, 117, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mairaj, M.; Panhwar, S.K.; Qamar, N.; Rashid, S. Indus river estuary: An assessment of potential risk of contaminants and ecosystem susceptibility. SN Appl. Sci. 2021, 3, 730. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Fu, Z.; Li, Y.; Ni, H.; Chen, S.; Zhou, Y.; Jin, B.; Shi, Z. Identifying heavy metal pollution hot spots in soil-rice systems: A case study in South of Yangtze River Delta, China. Sci. Total Environ. 2019, 658, 614–625. [Google Scholar] [CrossRef]
- Li, Z.-H.; Li, Z.-P.; Tang, X.; Hou, W.-H.; Li, P. Distribution and risk assessment of toxic pollutants in surface water of the lower yellow river, China. Water 2021, 13, 1582. [Google Scholar] [CrossRef]
- Ding, L.; Tao, J.; Tang, B.; Sun, J.; Ding, C.; He, D. Anguillids in the upper Nu–Salween River, South-East Asia: Species composition, distributions, natal sources and conservation implications. Mar. Freshw. Res. 2023, 74, 614–624. [Google Scholar] [CrossRef]
- Belinawati RA, P.; Soesilo TE, B.; Asteria, D.; Harmain, R. Sustainability: Citarum River, government role on the face of SDGs (water and sanitation). E3S Web Conf. 2018, 52, 00038. [Google Scholar] [CrossRef]
- Roosmini, D.; Septiono, M.A.; Putri, N.E.; Shabrina, H.M.; Salami IR, S.; Ariesyady, H.D. River water pollution condition in upper part of Brantas River and Bengawan Solo River. IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012059. [Google Scholar] [CrossRef]
- Franklin, R.L.; Fávaro DI, T.; Damatto, S.R. Trace metal and rare earth elements in a sediment profile from the Rio Grande Reservoir, Sao Paulo, Brazil: Determination of anthropogenic contamination, dating, and sedimentation rates. J. Radioanal. Nucl. Chem. 2016, 307, 99–110. [Google Scholar] [CrossRef]
- Miara, A.; Vörösmarty, C.J.; Macknick, J.E.; Tidwell, V.C.; Fekete, B.; Corsi, F.; Newmark, R. Thermal pollution impacts on rivers and power supply in the Mississippi River watershed. Environ. Res. Lett. 2018, 13, 034033. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Pramanick, P.; Zaman, S.; Mitra, A. Phytoremediation of heavy metals by the dominant mangrove associate species of indian sundarbans. J. Environ. Eng. Landsc. Manag. 2021, 29, 391–402. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas Rhizobacteria of Avicennia marina of Indian Sundarbans Promote Rice Growth Under Saline and Heavy Metal Stresses Through Exopolysaccharide Production. Front. Microbiol. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Muhammad, Z.; Majeed, M.; Aziz, R.; Khan, A.; Mangrio, W.M.; Abdo, H.G.; Almohamad, H.; Al Dughairi, A.A. Vegetation diversity pattern during spring season in relation to topographic and edaphic variables in sub-tropical zone. Bot. Stud. 2023, 64, 25. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Vajpayee, P.; Srivastava, S.; Srivastava, P.K. Revelation of bioremediation approaches for hexachlorocyclohexane degradation in soil. World J. Microbiol. Biotechnol. 2023, 39, 243. [Google Scholar] [CrossRef]
- Nissim, W.G.; Palm, E.; Mancuso, S.; Azzarello, E. Trace element phytoextraction from contaminated soil: A case study under Mediterranean climate. Environ. Sci. Pollut. Res. 2018, 25, 9114–9131. [Google Scholar] [CrossRef]
- Agarwal, S.; Mukherjee, P.; Pramanick, P.; Mitra, A. Seasonal Variations in Bioaccumulation and Translocation of Toxic Heavy Metals in the Dominant Vegetables of East Kolkata Wetlands: A Case Study with Suggestive Ecorestorative Strategies. Appl. Biochem. Biotechnol. 2023, 195, 2332–2358. [Google Scholar] [CrossRef]
- Shanker, A.K.; Cervantes, C.; Lozatavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Barya, M.P.; Kumar, A.; Thakur, T.K. Utilization of constructed wetland for the removal of heavy metal through fly ash bricks manufactured using harvested plant biomass. Ecohydrology 2022, 15, e2424. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The Genetic Basis of Metal Hyperaccumulation in Plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Gajić, G.; Djurdjević, L.; Kostić, O.; Jarić, S.; Mitrović, M.; Pavlović, P. Ecological Potential of Plants for Phytoremediation and Ecorestoration of Fly Ash Deposits and Mine Wastes. Front. Environ. Sci. 2018, 6, 124. [Google Scholar] [CrossRef]
- Pandey, A.; Khobra, R.; Mamrutha, H.M.; Wadhwa, Z.; Krishnappa, G.; Singh, G.; Singh, G.P. Elucidating the Drought Responsiveness in Wheat Genotypes. Sustainability 2022, 14, 3957. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N.; Singh, R.P.; Singh, D. Rhizoremediation potential of spontaneously grown Typha latifolia on fly ash basins: Study from the field. Ecol. Eng. 2014, 71, 722–727. [Google Scholar] [CrossRef]
- Hansel, C.M.; Fendorf, S.; Sutton, S.; Newville, M. Characterization of Fe Plaque and Associated Metals on the Roots of Mine-Waste Impacted Aquatic Plants. Environ. Sci. Technol. 2001, 35, 3863–3868. [Google Scholar] [CrossRef]
- Christensen, K.K.; Sand-Jensen, K. Precipitated iron and manganese plaques restrict root uptake of phosphorus in Lobelia dort-manna. Can. J. Bot. 1998, 76, 2158–2163. [Google Scholar]
- Liu, W.-J.; Zhu, Y.-G.; Smith, F. Effects of Iron and Manganese Plaques on Arsenic Uptake by Rice Seedlings (Oryza sativa L.) Grown in Solution Culture Supplied with Arsenate and Arsenite. Plant Soil 2005, 277, 127–138. [Google Scholar] [CrossRef]
- Surriya, O.; Saleem, S.S.; Waqar, K.; Kazi, A.G. Phytoremediation of Soils: Prospects and Challenges. In Soil Remediation and Plants: Prospects and Challenges; Academic Press: Cambridge, MA, USA, 2015; pp. 1–36. [Google Scholar] [CrossRef]
- Bhat, N.A.; Bhat, A.A.; Singh, B.P.; Guha, D.B. Heavy metal contamination in agricultural soils of NW Himalayas: With a perspective of spatial distribution, environmental contamination and health risk assessment. Arab. J. Geosci. 2021, 14, 2608. [Google Scholar] [CrossRef]
- Dutta, J.; Zaman, S.; Thakur, T.K.; Kaushik, S.; Mitra, A.; Singh, P.; Kumar, R.; Zuan, A.T.K.; Samdani, M.S.; Alharbi, S.A.; et al. Assessment of the bioaccumulation pattern of Pb, Cd, Cr and Hg in edible fishes of East Kolkata Wetlands, India. Saudi J. Biol. Sci. 2022, 29, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Pandey, V.C. Role of microbes in grass-based phytoremediation. In Phytoremediation Potential of Perennial Grasses; Pandey, V.C., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 303–327. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Correa-García, S.; Pande, P.; Séguin, A.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of petroleum hydrocarbons: A model system for plant microbiome manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Zuzolo, D.; Guarino, C.; Tartaglia, M.; Sciarrillo, R. Plant-Soil-Microbiota Combination for the Removal of Total Petroleum Hydrocarbons (TPH): An In-Field Experiment. Front. Microbiol. 2021, 11, 621581. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Soole, K.; Bentham, R. Hydrocarbon Phytoremediation in the Family Fabacea—A review. Int. J. Phytoremediation 2011, 13, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Rupassara, S.; Larson, R.; Sims, G.; Marley, K. Degradation of Atrazine by Hornwort in Aquatic Systems. Bioremediation J. 2002, 6, 217–224. [Google Scholar] [CrossRef]
- Limmer, M.A.; Burken, J.G. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Tjadraatmadja, G.; Diaper, C. Sources of Critical Contaminants in Domestic Wastewater—A Literature Review; CSIRO: Water for a Healthy Country National Research Flagship: Canberra, Australia, 2006.
- Domestic Wastewater Sources and Its Characteristics. Available online: https://cgi.tu-harburg.de/~awwweb/wbt/emwater/lessons/lesson_a1/lm_pg_1066.html (accessed on 17 September 2023).
- Henze, M.; Ledin, A. Types, Characteristics and quantities of classic, combined domestic wastewaters. In Decentralised Sanitation and Reuse: Concepts, Systems and Implementation; IWA Publishing: London, UK, 2001; pp. 59–72. [Google Scholar]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A Review on the Sustainability of Constructed Wetlands for Wastewater Treatment: Design and Operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.C.; Nguyen, D.D.; Tran, Q.B.; Nguyen, T.T.H.; Tran, T.K.A.; Tran, T.C.P.; Nguyen, T.H.G.; Tran, T.N.T.; La, D.D.; Chang, S.W.; et al. Two-step system consisting of novel vertical flow and free water surface constructed wetland for effective sewage treatment and reuse. Bioresour. Technol. 2020, 306, 123095. [Google Scholar] [CrossRef]
- Alegria, H.A.; Bidleman, T.F.; Shaw, T.J. Organochlorine pesticides in ambient air of Belize, Central America. Environ. Sci. Technol. 2000, 34, 1953–1958. [Google Scholar] [CrossRef]
- Ye, F.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.C.; Xiong, J.; Liu, Y.; Liu, H.; Ding, F.; Wen, T. Engineering application of constructed wetland for tertiary treatment of tail water from wastewater treatment plants in industrial zone. Environ. Eng. 2017, 35, 11–14. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Haynes, R.J. Sorption of heavy metals by inorganic and organic components of solid wastes: Significance to use of wastes as low-cost adsorbents and immobilizing agents. Crit. Rev. Environ. Sci. Technol. 2010, 40, 909–977. [Google Scholar] [CrossRef]
- Nivala, J.; Headley, T.; Wallace, S.; Bernhard, K.; Brix, H.; van Afferden, M.; Müller, R.A. Comparative analysis of constructed wetlands: The design and construction of the ecotechnology research facility in Langenreichenbach, Germany. Ecol. Eng. 2013, 61, 527–543. [Google Scholar] [CrossRef]
- Guittonny-Philippe, A.; Masotti, V.; Höhener, P.; Boudenne, J.-L.; Viglione, J.; Laffont-Schwob, I. Constructed wetlands to reduce metal pollution from industrial catchments in aquatic Mediterranean ecosystems: A review to overcome obstacles and suggest potential solutions. Environ. Int. 2014, 64, 1–16. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L.; Vymazal, J.; Brix, H.; Cooper, P.; Haberl, R. Constructed Wetlands for Pollution Control-Processes, Per-Formance, Design and Operation; IWA Scientific and Technical Report No. 8.; IWA Publishing: London, UK, 2000. [Google Scholar]
- Vymazal, J. Constructed wetlands, surface flow. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2008; Volume 1, pp. 765–776. [Google Scholar]
- Maiga, Y.; von Sperling, M.; Mihelcic, J.R. Constructed Wetlands. In Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project). (Mihelcic JR, Verbyla ME (eds), Part 4: Management of Risk from Excreta and Wastewater-Section: Sanitation System Technologies, Pathogen Reduction in Sewered System Technologies); Rose, J.B., Jiménez-Cisneros, B., Eds.; Michigan State University: East Lansing, MI, USA, 2017. [Google Scholar] [CrossRef]
- Ge, G.; Kou, K.; Chen, A. Research status on the contribution of plant a substrate to heavy metals removal in wetland system. Contemp. Chem. Ind. 2013, 42, 1006–1008. [Google Scholar]
- Vymazal, J. Constructed wetlands for wastewater treatment in the Czech Republic. Water Sci. Technol. 2001, 44, 369–374. [Google Scholar] [CrossRef]
- Vymazal, J. Types of constructed wetlands for wastewater treatment: Their potential for nutrient removal. In Transformations of Nutrients in Natural and Constructed Wetlands; Vymazal, J., Ed.; Backhuys Publishers: Leiden, The Netherlands, 2001; pp. 1–93. [Google Scholar]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Crites, R.W.; Middlebrooks, E.J.; Reed, S.C. Natural Wastewater Treatment Systems; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Mander, Ü.; Jenssen, P.D. (Eds.) Constructed Wetlands for Wastewater Treatment in Cold Climates; WIT Press: Southampton, UK, 2003. [Google Scholar]
- Akratos, C.S.; Tsihrintzis, V.A. Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2007, 29, 173–191. [Google Scholar] [CrossRef]
- Bahlo, K.E.; Wachs, F.G. Purification of domestic sewage with and without faeces by vertical intermittent filtration in reed and rush beds. In Constructed Wetlands in Water Pollution Control; Cooper, P.F., Findlater, B.C., Eds.; Pergamon Press: Oxford, UK, 1990; pp. 215–221. [Google Scholar]
- Vymazal, J. Vegetation development in subsurface flow constructed wetlands in the Czech Republic. Ecol. Eng. 2013, 61, 575–581. [Google Scholar] [CrossRef]
- Available online: https://www.azuwater.com/category/tertiary-treatment/constructed-wetland-phytoremediation/ (accessed on 17 September 2023).
- Hoffmann, H.; Platzer, C.; von Münch, E.; Winker, M. Technology Review of Constructed Wetlands-Subsurface Flow Constructed Wetlands for Greywater and Domestic Wastewater Treatment; Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ) GmbH: Eschborn, Germany, 2011. [Google Scholar] [CrossRef]
- Cooper, P.F.; Job, G.D.; Green, M.B.; Shutes, R.B.E. Reed Beds and Constructed Wetlands for Wastewater Treatment; Water Research Centre Swindon: Swindon, UK, 1996. [Google Scholar]
- Horner, J.E.; Castle, J.W.; Rodgers, J.H.; Gulde, C.M.; Myers, J.E. Design and Performance of Pilot-Scale Constructed Wetland Treatment Systems for Treating Oilfield Produced Water from Sub-Saharan Africa. Water Air Soil Pollut. 2012, 223, 1945–1957. [Google Scholar] [CrossRef]
- Tanner, C.C. Plants for constructed wetland treatment systems–A comparison of the growth and nutrient uptake of eight emergent species. Ecol. Eng. 1996, 7, 59–83. [Google Scholar] [CrossRef]
- Gingerich, R.T.; Panaccione, D.G.; Anderson, J.T. The role of fungi and invertebrates in litter decomposition in mitigated and reference wetlands. Limnologica 2015, 54, 23–32. [Google Scholar] [CrossRef]
- Sirianuntapiboon, S.; Kongchum, M.; Jitmaikasem, W. Effects of hydraulic retention time and media of constructed wetland for treatment of domestic wastewater. Afr. J. Agric. Res. 2006, 1, 027–037. [Google Scholar]
- Wang, Y.; Shen, L.; Wu, J.; Zhong, F.; Cheng, S. Step-feeding ratios affect nitrogen removal and related microbial communities in multi-stage vertical flow constructed wetlands. Sci. Total. Environ. 2020, 721, 137689. [Google Scholar] [CrossRef]
- Si, Z.; Wang, Y.; Song, X.; Cao, X.; Zhang, X.; Sand, W. Mechanism and performance of trace metal removal by continuous-flow constructed wetlands coupled with a micro-electric field. Water Res. 2019, 164, 114937. [Google Scholar] [CrossRef]
- Bijalwan, A.; Thakur, T. Effect of IBA and age of cuttings on rooting behaviour of Jatropha curcas L. in different seasons in Western Himalaya, India. Afr. J. Plant Sci. 2010, 4, 387–390. [Google Scholar]
- Bessa, V.; Moreira, I.; Tiritan, M.; Castro, P. Enrichment of bacterial strains for the biodegradation of diclofenac and carbamazepine from activated sludge. Int. Biodeterior. Biodegrad. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Kumar, S.; Bijalwan, A.; Singh, B.; Rawat, D.; Yewale, A.G.; Riyal, M.K.; Thakur, T.K. Comparison of Carbon Sequestration Potential of Quercus leucotrichophora Based Ag-roforestry Systems and Natural Forest in Central Himalaya, India. Water Air Soil Pollut. 2021, 232, 350. [Google Scholar] [CrossRef]
- Vassallo, A.; Miceli, E.; Fagorzi, C.; Castronovo, L.M.; Del Duca, S.; Chioccioli, S.; Venditto, S.; Coppini, E.; Fibbi, D.; Fani, R. Temporal Evolution of Bacterial Endophytes Associated to the Roots of Phragmites australis Exploited in Phytodepuration of Wastewater. Front. Microbiol. 2020, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Long, Y.; Yu, G.; Wang, G.; Zhou, Z.; Li, P.; Zhang, Y.; Yang, K.; Wang, S. A Review on Microorganisms in Constructed Wetlands for Typical Pollutant Removal: Species, Function, and Diversity. Front. Microbiol. 2022, 13, 845725. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Mishra, J.; Dwivedi, S.K.; Arora, N.K. Microbial Enzymes in Biocontrol of Phytopathogens. In Microbial Enzymes: Roles and Applications in Industries. Microorganisms for Sustainability; Arora, N., Mishra, J., Mishra, V., Eds.; Springer: Singapore, 2020; pp. 259–285. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Tan, S.K.; Gersberg, R.M.; Zhu, J.; Sadreddini, S.; Li, Y. Nutrient removal in tropical subsurface flow constructed wetlands under batch and continuous flow conditions. J. Environ. Manag. 2012, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Caselles-Osorio, A.; Garcia, J. Effect of physico-chemical pretreatment on the removal efficiency of horizontal subsurface-flow constructed wetlands. Environ. Pollut. 2007, 146, 55–63, ISSN 0269-7491. [Google Scholar] [CrossRef] [PubMed]
- Brix, H.; Schierup, H. Danish experience with sewage treatment in constructed wetlands. In Constructed Wetlands for Wastewater Treatment; Hammer, D.A., Ed.; Lewis Publishers: Chelsea, MI, USA, 1989; pp. 565–573. [Google Scholar]
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of Plants in a Constructed Wetland: Current and New Perspectives. Water 2013, 5, 405–419. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. Chemistry for Environmental Engineering and Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; IWA Publishing: London, UK, 1996. [Google Scholar]
- Akinbile, C.O.; Yusoff, M.S. Water Hyacinth (Eichhornia crassipes) and Lettuce (Pistia stratiotes) effectiveness in aquaculture wastewater treatment in Malaysia. Int. J. Phytoremediation 2012, 14, 201–211. [Google Scholar] [CrossRef]
- US, EPA. Manual: Constructed Wetlands Treatment of Municipal Wastewaters; EPA/625/R-99/010; U.S. EPA Office of Research and Development: Cincinnati, OH, USA, 2000.
- Zhang, M.; Wang, Z.-J.; Huang, J.-C.; Sun, S.; Cui, X.; Zhou, W.; He, S. Salinity-driven nitrogen removal and its quantitative molecular mechanisms in artificial tidal wetlands. Water Res. 2021, 202, 117446. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.-L.; Liu, Y.-W.; Li, X.; Zhu, W.-B. Quantitative ecology associations between heterotrophic nitrification-aerobic denitrification, nitrogen-metabolism genes, and key bacteria in a tidal flow constructed wetland. Bioresour. Technol. 2021, 337, 125449. [Google Scholar] [CrossRef]
- Ojoawo, S.O.; Udayakumar, G.; Naik, P. Phytoremediation of Phosphorus and Nitrogen with Canna × generalis Reeds in Domestic Wastewater through NMAMIT Constructed Wetland. Aquat. Procedia 2015, 4, 349–356. [Google Scholar] [CrossRef]
- Hu, Y.; He, F.; Ma, L.; Zhang, Y.; Wu, Z. Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour. Technol. 2016, 207, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Ding, A.; Zheng, L.; Lu, C.; Wang, J.; Huang, B.; Xiu, H. Seasonal variation in populations of nitrogen-transforming bacteria and correlation with nitrogen removal in a full-scale horizontal flow constructed wetland treating Polluted River water. Geomicrobiol. J. 2016, 33, 338–346. [Google Scholar] [CrossRef]
- Zhao, Y.; Cao, X.; Song, X.; Zhao, Z.; Wang, Y.; Si, Z.; Lin, F.; Chen, Y.; Zhang, Y. Montmorillonite supported nanoscale zero-valent iron immobilized in sodium alginate (SA/Mt-NZVI) enhanced the nitrogen removal in vertical flow constructed wetlands (VFCWs). Bioresour. Technol. 2018, 267, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Maniquiz-Redillas, M.C.; Choi, J.; Kim, L.-H. Nitrogen mass balance in a constructed wetland treating piggery wastewater effluent. J. Environ. Sci. 2014, 26, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Yang, Y.-L.; Liu, Y.-W.; Yin, W.-C.; Fan, X.-Y. The synergy of porous substrates and functional genera for efficient nutrients removal at low temperature in a pilot-scale two-stage tidal flow constructed wetland. Bioresour. Technol. 2021, 319, 124135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Dissimilatory nitrate reduction processes and corresponding nitrogen loss in tidal flow constructed wetlands. J. Clean. Prod. 2021, 295, 126429. [Google Scholar] [CrossRef]
- Wang, W.; Su, Y.; Wang, B.; Wang, Y.; Zhuang, L.; Zhu, G. Spatiotemporal shifts of ammonia-oxidizing archaea abundance and structure during the restoration of a multiple pond and plant-bed/ditch wetland. Sci. Total. Environ. 2019, 684, 629–640. [Google Scholar] [CrossRef]
- Zhao, L.; Fu, G.; Wu, J.; Pang, W.; Hu, Z. Bioaugmented constructed wetlands for efficient saline wastewater treatment with multiple denitrification pathways. Bioresour. Technol. 2021, 335, 125236. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, L.; Lu, H.; Lu, S.; Li, J.; Guo, X.; Zhao, X. Nitrogen removal from summer to winter in a field pilot-scale multistage constructed wetland-pond system. J. Environ. Sci. 2022, 111, 249–262. [Google Scholar] [CrossRef]
- Liu, T.; Lu, S.; Wang, R.; Xu, S.; Qin, P.; Gao, Y. Behavior of selected organophosphate flame retardants (OPFRs) and their influence on rhizospheric microorganisms after short-term exposure in integrated vertical-flow constructed wetlands (IVCWs). Sci. Total. Environ. 2020, 710, 136403. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Liu, W.; Zhang, Y.; Zhou, Q.; Wu, Z.; He, F. A stable simultaneous anammox, denitrifying anaerobic methane oxidation and denitrification process in integrated vertical constructed wetlands for slightly polluted wastewater. Environ. Pollut. 2020, 262, 114363. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Lv, D.; Li, Y.; Wu, J. Nitrogen and phosphorus removal performance and bacterial communities in a multi-stage surface flow constructed wetland treating rural domestic sewage. Sci. Total. Environ. 2020, 709, 136235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S. Depth-specific distribution and significance of nitrite-dependent anaerobic methane oxidation process in tidal flow constructed wetlands used for treating river water. Sci. Total. Environ. 2020, 716, 137054. [Google Scholar] [CrossRef] [PubMed]
- Kraiem, K.; Kallali, H.; Wahab, M.A.; Fra-Vazquez, A.; Mosquera-Corral, A.; Jedidi, N. Comparative study on pilots between ANAMMOX favored conditions in a partially saturated vertical flow constructed wetland and a hybrid system for rural wastewater treatment. Sci. Total. Environ. 2019, 670, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bu, C.; Ahmad, H.A.; Guimbaud, C.; Gao, B.; Qiao, Z.; Ding, S.; Ni, S.-Q. The distribution of dissimilatory nitrate reduction to ammonium bacteria in multistage constructed wetland of Jining, Shandong, China. Environ. Sci. Pollut. Res. 2021, 28, 4749–4761. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Roberts, K.L.; Grace, M.R.; Kessler, A.J.; Cook, P.L.M. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci. Total Environ. 2019, 666, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, J.-C.; Sun, S.; Rehman, M.M.U.; He, S.; Zhou, W. Nitrogen removal through collaborative microbial pathways in tidal flow constructed wetlands. Sci. Total. Environ. 2021, 758, 143594. [Google Scholar] [CrossRef]
- Si, Z.; Song, X.; Wang, Y.; Cao, X.; Zhao, Y.; Wang, B.; Chen, Y.; Arefe, A. Intensified heterotrophic denitrification in constructed wetlands using four solid carbon sources: Denitrification efficiency and bacterial community structure. Bioresour. Technol. 2018, 267, 416–425. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Wang, H.-C.; Guadie, A.; Ajibade, T.F.; Fang, Y.-K.; Sharif, H.M.A.; Liu, W.-Z.; Wang, A.-J. Total nitrogen removal in biochar amended non-aerated vertical flow constructed wetlands for secondary wastewater effluent with low C/N ratio: Microbial community structure and dissolved organic carbon release conditions. Bioresour. Technol. 2021, 322, 124430. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Song, X.; Jiang, X.; Wang, Y.; Cao, X.; Si, Z.; Pan, F. Effects of nZVI dosing on the improvement in the contaminant removal performance of constructed wetlands under the dye stress. Sci. Total. Environ. 2020, 703, 134789. [Google Scholar] [CrossRef]
- Du, L.; Chen, Q.; Liu, P.; Zhang, X.; Wang, H.; Zhou, Q.; Xu, D.; Wu, Z. Phosphorus removal performance and biological dephosphorization process in treating reclaimed water by Integrated Vertical-flow Constructed Wetlands (IVCWs). Bioresour. Technol. 2017, 243, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, J.; Xie, H.; Hao, D.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q.; Wang, B. Phosphorus removal performance of microbial-enhanced constructed wetlands that treat saline wastewater. J. Clean. Prod. 2021, 288, 125119. [Google Scholar] [CrossRef]
- Mitsch, J.W.; Gosselink, J.G. Wetlands; Van Nostrand Reinhold Company: New York, NY, USA, 1986. [Google Scholar]
- Babatunde, A.; Zhao, Y.; Burke, A.; Morris, M.; Hanrahan, J. Characterization of aluminium-based water treatment residual for potential phosphorus removal in engineered wetlands. Environ. Pollut. 2009, 157, 2830–2836. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fan, J.; Zhang, J.; Shen, Y. Enhanced phosphorus removal in intermittently aerated constructed wetlands filled with various construction wastes. Environ. Sci. Pollut. Res. 2017, 24, 22524–22534. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yu, C.; Liu, J.; Ye, C.; Zhou, X.; Chen, L. Performance of an ultraviolet Mutagenetic polyphosphate-accumulating bacterium PZ2 and its application for wastewater treatment in a newly designed constructed wetland. Appl. Biochem. Biotechnol. 2017, 181, 735–747. [Google Scholar] [CrossRef]
- Lv, R.; Wu, D.; Ding, J.; Yuan, X.; Zhou, G.; Zhang, Y.; Kong, Q.; Zhao, C.; Du, Y.; Xu, F.; et al. Long-term performance and microbial mechanism in intertidal wetland sediment introduced constructed wetlands treating saline wastewater. J. Clean. Prod. 2021, 310, 127409. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, J.; Guo, Y.; Guan, W.; Cao, C.; Yan, C.; Wang, M. Long-term effects of silver nanoparticles on performance of phosphorus removal in a laboratory-scale vertical flow constructed wetland. J. Environ. Sci. 2020, 87, 319–330. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Liu, F.; Li, Y.; Li, Y.; Xiao, R.; Wu, J. Bacterial community dynamics in a Myriophyllum elatinoides purification system for swine wastewater in sediments. Appl. Soil Ecol. 2017, 119, 56–63. [Google Scholar] [CrossRef]
- Wu, H.; Gao, X.; Wu, M.; Zhu, Y.; Xiong, R.; Ye, S. The efficiency and risk to groundwater of constructed wetland system for domestic sewage treatment-A case study in Xiantao, China. J. Clean. Prod. 2020, 277, 123384. [Google Scholar] [CrossRef]
- Yu, G.; Wang, G.; Li, J.; Chi, T.; Wang, S.; Peng, H.; Chen, H.; Du, C.; Jiang, C.; Liu, Y.; et al. Enhanced Cd2+ and Zn2+ removal from heavy metal wastewater in constructed wetlands with resistant microorganisms. Bioresour. Technol. 2020, 316, 123898. [Google Scholar] [CrossRef] [PubMed]
- Thakur, T.K.; Dutta, J.; Upadhyay, P.; Patel, D.K.; Thakur, A.; Kumar, M.; Kumar, A. Assessment of land degradation and restoration in coal mines of central India: A time series analysis. Ecol. Eng. 2021, 175, 106493. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, F.; Chen, Y.; Wu, J.; Cheng, S. The Interaction Effects of Aeration and Plant on the Purification Performance of Horizontal Subsurface Flow Constructed Wetland. Int. J. Environ. Res. Public Health 2022, 19, 1583. [Google Scholar] [CrossRef] [PubMed]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Cristani, M.; Naccari, C.; Nostro, A.; Pizzimenti, A.; Trombetta, D.; Pizzimenti, F. Possible use of Serratia marcescens in toxic metal biosorption (removal). Environ. Sci. Pollut. Res. 2012, 19, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tigane, T.; Li, X.; Truu, M.; Truu, J.; Mander, Ü. Hexachlorobenzene dechlorination in constructed wetland mesocosms. Water Res. 2013, 47, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial Endophytes: Potential Role in Developing Sustainable Systems of Crop Production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Syranidou, E.; Christofilopoulos, S.; Gkavrou, G.; Thijs, S.; Weyens, N.; Vangronsveld, J.; Kalogerakis, N. Exploitation of Endophytic Bacteria to Enhance the Phytoremediation Potential of the Wetland Helophyte Juncus acutus. Front. Microbiol. 2016, 07, 1016. [Google Scholar] [CrossRef]
- Dotro, G.; Langergraber, G.; Molle, P.; Nivala, J.; Puigagut Juárez, J.; Stein, O.R.; von Sperling, M. Treatment Wetlands; Biological Wastewater Treatment Series (Volume 7); IWA Publishing: London, UK, 2017. [Google Scholar]
- Ansola, G.; González, J.M.; Cortijo, R.; de Luis, E. Experimental and full–scale pilot plant constructed wetlands for municipal wastewaters treatment. Ecol. Eng. 2003, 21, 43–52. [Google Scholar] [CrossRef]
- Kaseva, M. Performance of a sub-surface flow constructed wetland in polishing pre-treated wastewater—A tropical case study. Water Res. 2004, 38, 681–687. [Google Scholar] [CrossRef]
- Mbuligwe, S.E. Comparative effectiveness of engineered wetland systems in the treatment of anaerobically pre-treated domestic wastewater. Ecol. Eng. 2004, 23, 269–284. [Google Scholar] [CrossRef]
- Solano, M.; Soriano, P.; Ciria, M. Constructed Wetlands as a Sustainable Solution for Wastewater Treatment in Small Villages. Biosyst. Eng. 2004, 87, 109–118. [Google Scholar] [CrossRef]
- Slak, A.S.; Bulc, T.G.; Vrhovsek, D. Comparison of nutrient cycling in a surface-flow constructed wetland and in a facultative pond treating secondary effluent. Water Sci. Technol. 2005, 51, 291–298. [Google Scholar] [CrossRef]
- Jinadasa, K.B.S.N.; Tanaka, N.; Mowjood, M.I.M.; Werellagama, D.R.I.B. Free water surface constructed wetlands for domestic wastewater treatment: A tropical case study. Chem. Ecol. 2006, 22, 181–191. [Google Scholar] [CrossRef]
- Song, Z.; Zheng, Z.; Li, J.; Sun, X.; Han, X.; Wang, W.; Xu, M. Seasonal and annual performance of a full-scale constructed wetland system for sewage treatment in China. Ecol. Eng. 2006, 26, 272–282. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Liu, P.; Yang, Y.-S.; Chen, W.-R. Phytoremediation of urban wastewater by model wetlands with ornamental hydrophytes. J. Environ. Sci. 2007, 19, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Katsenovich, Y.P.; Hummel-Batista, A.; Ravinet, A.J.; Miller, J.F. Performance evaluation of constructed wetlands in a tropical region. Ecol. Eng. 2009, 35, 1529–1537. [Google Scholar] [CrossRef]
- Konnerup, D.; Koottatep, T.; Brix, H. Treatment of domestic wastewater in tropical, subsurface flow constructed wetlands planted with Canna and Heliconia. Ecol. Eng. 2009, 35, 248–257. [Google Scholar] [CrossRef]
- Morari, F.; Giardini, L. Municipal wastewater treatment with vertical flow constructed wetlands for irrigation reuse. Ecol. Eng. 2009, 35, 643–653. [Google Scholar] [CrossRef]
- Xinshan, S.; Qin, L.; Denghua, Y. Nutrient Removal by Hybrid Subsurface Flow Constructed Wetlands for High Concentration Am-monia Nitrogen Wastewater. Procedia Environ. Sci. 2010, 2, 1461–1468. [Google Scholar] [CrossRef]
- Zhai, J.; Xiao, H.W.; Kujawa-Roeleveld, K.; He, Q.; Kerstens, S.M. Experimental study of a novel hybrid constructed wetland for water reuse and its application in Southern China. Water Sci. Technol. 2011, 64, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Hui, Z.; Chao, X.; Nie, E.; Li, H.J.; He, J.; Zheng, Z. Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol. Eng. 2011, 37, 1546–1554. [Google Scholar] [CrossRef]
- Zurita, F.; Belmont, M.A.; De Anda, J.; White, J.R. Seeking a way to promote the use of constructed wetlands for domestic wastewater treatment in developing countries. Water Sci. Technol. 2011, 63, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elela, S.I.; Hellal, M.S. Municipal wastewater treatment using vertical flow constructed wetlands planted with Canna, Phragmites and Cyprus. Ecol. Eng. 2012, 47, 209–213. [Google Scholar] [CrossRef]
- Chang, J.-J.; Wu, S.-Q.; Dai, Y.-R.; Liang, W.; Wu, Z.-B. Treatment performance of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol. Eng. 2012, 44, 152–159. [Google Scholar] [CrossRef]
- Villara, M.M.P.; Domínguez, E.R.; Tack, F.; Ruiz, J.M.H.; Morales, R.S.; Arteaga, L.E. Vertical subsurface wetlands for wastewater purification. Procedia Eng. 2012, 42, 1960–1968. [Google Scholar] [CrossRef]
- Abou-Elela, S.I.; Golinielli, G.; Abou-Taleb, E.M.; Hellal, M.S. Municipal wastewater treatment in horizontal and vertical flows constructed wetlands. Ecol. Eng. 2013, 61, 460–468. [Google Scholar] [CrossRef]
- Rai, U.; Tripathi, R.; Singh, N.; Upadhyay, A.; Dwivedi, S.; Shukla, M.; Mallick, S.; Singh, S.; Nautiyal, C. Constructed wetland as an ecotechnological tool for pollution treatment for conservation of Ganga river. Bioresour. Technol. 2013, 148, 535–541. [Google Scholar] [CrossRef]
- Shao, Y.; Pei, H.; Hu, W.; Chanway, C.P.; Meng, P.; Ji, Y.; Li, Z. Bioaugmentation in lab scale constructed wetland microcosms for treating polluted river water and domestic wastewater in northern China. Int. Biodeterior. Biodegrad. 2014, 95, 151–159. [Google Scholar] [CrossRef]
- Sharma, G.; Priya; Brighu, U. Performance Analysis of Vertical up-flow Constructed Wetlands for Secondary Treated Effluent. APCBEE Procedia 2014, 10, 110–114. [Google Scholar] [CrossRef]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, C.S.C.; Bessa, V.S.; Mesquita, R.B.R.; Brix, H.; Rangel, A.O.S.S.; Castro, P.M.L. Constructed wetland with a polyculture of ornamental plants for wastewater treatment at a rural tourism facility. Ecol. Eng. 2015, 79, 1–7, ISSN 0925-8574. [Google Scholar] [CrossRef]
- Lu, S.; Pei, L.; Bai, X. Study on method of domestic wastewater treatment through new-type multi-layer artificial wetland. Int. J. Hydrog. Energy 2015, 40, 11207–11214. [Google Scholar] [CrossRef]
- Xu, D.; Wang, L.; Li, H.; Li, Y.; Howard, A.; Guan, Y.; Li, J.; Xu, H. The forms and bioavailability of phosphorus in integrated vertical flow constructed wetland with earthworms and different substrates. Chemosphere 2015, 134, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Abdelhakeem, S.G.; Aboulroos, S.A.; Kamel, M.M. Performance of a vertical subsurface flow constructed wetland under different operational conditions. J. Adv. Res. 2016, 7, 803–814. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Cui, L.; Yu, G. Optimization of operating parameters of hybrid vertical down-flow constructed wetland systems for domestic sewerage treatment. Environ. Manag. 2016, 180, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Peng, L.; Zhang, S.; Heal, K.V.; Zhao, J.; Zhu, D. Effects of plants and temperature on nitrogen removal and microbiology in pilot-scale horizontal subsurface flow constructed wetlands treating domestic wastewater. Ecol. Eng. 2017, 108, 70–77. [Google Scholar] [CrossRef]
- Ali, M.; Rousseau, D.P.; Ahmed, S. A full-scale comparison of two hybrid constructed wetlands treating domestic wastewater in Pakistan. J. Environ. Manag. 2018, 210, 349–358. [Google Scholar] [CrossRef]
- Benvenuti, T.; Hamerski, F.; Giacobbo, A.; Bernardes, A.M.; Zoppas-Ferreira, J.; Rodrigues, M.A. Constructed floating wetland for the treatment of domestic sewage: A real-scale study. Environ. Chem. Eng. 2018, 6, 5706–5711. [Google Scholar] [CrossRef]
- Elfanssi, S.; Ouazzani, N.; Latrach, L.; Hejjaj, A.; Mandi, L. Phytoremediation of domestic wastewater using a hybrid constructed wetland in mountainous rural area. Int. J. Phytoremediation 2018, 20, 75–87. [Google Scholar] [CrossRef]
- Mishra, V.K.; Otter, P.; Shukla, R.; Goldmaier, A.; Alvarez, J.A.; Khalil, N.; Avila, C.; Arias, C.; Ameršek, I. Application of horizontal flow constructed wetland and solar driven disinfection technologies for wastewater treatment in India. Water Pract. Technol. 2018, 13, 469–480. [Google Scholar] [CrossRef]
- Worku, A.; Tefera, N.; Kloos, H.; Benor, S. Constructed wetlands for phytoremediation of industrial wastewater in Addis Ababa, Ethiopia. Nanotechnol. Environ. Eng. 2018, 3, 9. [Google Scholar] [CrossRef]
- Yadav, A.; Chazarenc, F.; Mutnuri, S. Development of the “French system” vertical flow constructed wetland to treat raw domestic wastewater in India. Ecol. Eng. 2018, 113, 88–93. [Google Scholar] [CrossRef]
- García-Ávila, F.; Avilés-Añazco, A.; Sánchez-Cordero, E.; Valdiviezo-Gonzáles, L.; Ordoñez, M.D.T. The challenge of improving the efficiency of drinking water treatment systems in rural areas facing changes in the raw water quality. S. Afr. J. Chem. Eng. 2021, 37, 141–149. [Google Scholar] [CrossRef]
- Barya, M.P.; Gupta, D.; Thakur, T.K.; Shukla, R.; Singh, G.; Mishra, V.K. Phytoremediation performance of Acorus calamus and Canna indica for the treatment of primary treated domestic sewage through vertical subsurface flow constructed wetlands: A field-scale study. Water Pract. Technol. 2020, 15, 528–539. [Google Scholar] [CrossRef]
- Xiong, C.; Tam, N.F.; Dai, Y.; Zhang, X.; Li, R.; Zheng, Y.; Wang, L.; Yang, Y. Enhanced performance of pilot-scale hybrid constructed wetlands with A/O reactor in raw domestic sewage treatment. J. Environ. Manag. 2020, 258, 110026. [Google Scholar] [CrossRef] [PubMed]
- Aregu, M.B.; Asfaw, S.L.; Khan, M.M. Developing horizontal subsurface flow constructed wetland using pumice and Chrysopogon zizanioides for tannery wastewater treatment. Environ. Syst. Res. 2021, 10, 33. [Google Scholar] [CrossRef]
- Shukla, R.; Gupta, D.; Singh, G.; Mishra, V.K. Performance of horizontal flow constructed wetland for secondary treatment of domestic wastewater in a remote tribal area of Central India. Sustain. Environ. Res. 2021, 31, 13. [Google Scholar] [CrossRef]
- Gao, J.; Li, Q.; Zhang, J.; Wang, S.; Song, B.; Huang, Z. Purification of Micro-Polluted Lake Water by Biofortification of Vertical Subsurface Flow Constructed Wetlands in Low-Temperature Season. Water 2022, 14, 896. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, R.; Rene, E.R.; Qi, X.; Hao, Q.; Du, Y.; Zhao, C.; Xu, F.; Kong, Q. Characterization of microbial community and resistance gene (CzcA) shifts in up-flow constructed wetlands-microbial fuel cell treating Zn (II) contaminated wastewater. Bioresour. Technol. 2020, 302, 122867. [Google Scholar] [CrossRef]
- Caetano, N.S.; Xu, S.; Banu, J.R.; Sani, R.K.; Karthikeyan, O.P. Editorial: Biomass, Bioenergy and Biofuels for Circular Bioeconomy. Front. Energy Res. 2022, 10, 851047. [Google Scholar] [CrossRef]
- Thakur, T.K.; Dutta, J.; Bijalwan, A.; Swamy, S.L. Evaluation of decadal land degradation dynamics in old coal mine areas of Central India. Land Degrad. Dev. 2022, 33, 3209–3230. [Google Scholar] [CrossRef]
- Thakur, T.K.; Eripogu, K.K.; Thakur, A.; Kumar, A.; Bakshi, S.; Swamy, S.L.; Bijalwan, A.; Kumar, M. Disentangling forest dynamics for litter biomass production in biosphere reserve of Central India. Front. Environ. Sci. 2022, 10, 940614. [Google Scholar] [CrossRef]
- Kumar, Y.; Thakur, T.K.; Thakur, A. Socio-Cultural Paradigm of Agroforestry in India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1371–1377. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, B.; Chandraker, S.K.; Padwar, G.K.; Dubey, A.K.; Thakur, T.; Sahu, M.L. Mahua (Madhucaindica) (Koenig) J.F. Macribide) A Nature, Reward to Tribal Ecosystem of Central India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1519–1526. [Google Scholar] [CrossRef]
- Bijalwan, A.; Verma, P.; Dobriyal, M.J.R.; Patil, A.K.; Thakur, T.K.; Sharma, C.M. Trends and Insights of Agroforestry Practices in Madhya Pradesh, India. Curr. Sci. 2019, 117, 597–605. [Google Scholar] [CrossRef]
- Thakur, T.K. Diversity, composition and structure of understorey vegetation in the tropical forest of Achanakmaar Biosphere Reserve, India. Environ. Sustain. 2018, 1, 279–293. [Google Scholar] [CrossRef]
- Hettiarachchi, H.; Ardakanian, R. Safe Use of Wastewater in Agriculture: Good Practice Examples; UNU-FLORES: Dresden, Germany, 2016. [Google Scholar]
- Ansari, A.A.; Khan, F.A. Nutrients phytoremediation of eutrophic waters using Eichhornia crassipes in a controlled environment. Int. J. Environ. Sci. 2011, 2, 241–246. [Google Scholar] [CrossRef]
- Mkandawire, M.; Dudel, E.G. Are Lemna spp. effective phytoremediation agents. Bioremediation Biodivers. Bioavailab. 2007, 1, 56–71. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Berti, W.R. Remediation of contaminated soils with green plants: An overview. Vitr. Cell. Dev. Biol.-Plant 1993, 29, 207–212. [Google Scholar] [CrossRef]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Abbassi, R. Constructed Wetland Coupled Microbial Fuel Cell Technology. In Microbial Electrochemical Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1021–1036. [Google Scholar]








| Sl. No. | Physico-Chemical and Biological Analysis Parameters | Unit | Permissible Standard Limit(s) | References |
|---|---|---|---|---|
| 1. | Alkalinity | eqv/m3 ** | 37 * | [19,79,80] |
| 2. | Conductivity | mS/m *** | 120 * | |
| 3. | pH | – | 5.5–9.0 | |
| 4. | Temperature | °C | 22–32 # | |
| 5. | Ammoniacal nitrogen (NH3-N) | mg/L | 50 | |
| 6. | BOD3 (3-days Biological Oxygen Demand at 27 °C) | mg O2/L | 350 | |
| 7. | BOD5 (5-days Biological Oxygen Demand at 27 °C) | 350 * | ||
| 8. | COD (Chemical Oxygen Demand) | 740 * | ||
| 9. | Fats, oil, and grease | g/m3 | 100 * | |
| 10. | Oil and grease | mg/L | 20 | |
| 11. | Phenolic compounds | 5.0 | ||
| 12. | Suspended Solids (SS) | g SS/m3 | 450 * | |
| 13. | Total Nitrogen (TN) | g N/m3 | 80 * | |
| 14. | Total Organic Carbon (TOC) | g C/m3 | 250 * | |
| 15. | Total Phosphorus (TP) | g P/m3 | 23 * | |
| 16. | Total Suspended Solids (TSS) | mg/L | 600 | |
| 17. | Volatile Suspended Solids (VSS) | VSS/m3 | 320 * | |
| 18. | Arsenic (As) | mg/L | 0.01 | |
| 19. | Cadmium (Cd) | 1.0 | ||
| 20. | Copper (Cu) | 3.0 | ||
| 21. | Cyanide (CN) | 2.0 | ||
| 22. | Fluoride (F) | 15.0 | ||
| 23. | Iron (Fe) | 3.0 | ||
| 24. | Lead (Pb) | 1.0 | ||
| 25. | Manganese (Mn) | 2.0 | ||
| 26. | Mercury (Hg) | 0.01 | ||
| 27. | Nickel (Ni) | 3.0 | ||
| 28. | Selenium (Se) | 0.05 | ||
| 29. | Total chromium (Cr) | 2.0 | ||
| 30. | Vanadium (Vn) | 0.2 | ||
| 31. | Zinc (Zn) | 15.0 | ||
| 32. | Bioassay | – | 90% fish survival after 96 h in cent percent effluent |
| Sl. No. | Latin and Common Names of Plant (Macrophyte) Species | Percentage Removal of Inorganic and Organic Pollutant(s) (%) | Percentage Removal of Pollutant Indicator Organism(s)/Pathogen(s) (%) | Microbes Involved in the Removal of Nutrient(s)/Pollutant(s) | Constructed-Wetland-Based Phytoremediation Set-Up(s)/System(s) Used | Type of Treated Wastewater | Reference(s) |
|---|---|---|---|---|---|---|---|
| 1. | Salix atrocinerea Brot. (grey willow) and Typha latifolia L. (cattail) | BOD5 (87.5) COD (89.0) SS (66.5) | Fecal bacteria (99.9) | - | Full–scale, pilot-plant constructed wetland | Domestic wastewater | [169] |
| 2. | Control unit (A): Phragmites mauritianus Kunth (reed grass) and T. latifolia | COD (33.6, 56.3, and 60.7) NH4+-N (11.2, 25.2, and 23.0) NO2-N (23.9, 38.5, and 23.1) NO3−-N (32.2, 40.3, and 44.3) | TC and FC (43.0–72.0) | - | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [170] |
| 3. | Control unit (A): Colocasia esculenta (L.) Schott (taro) and T. latifolia | COD (64.7, 74.8, and 79.4) NH3 (74.0–75.0) P4 (72.0–77.0) SO42− (74.0–75.0) | - | - | Engineered wetland | Domestic wastewater | [171] |
| T. latifolia | NH3 (74.0) PO42− (69.0) SO42− (72.0) | - | - | ||||
| 4. | Phragmites sp. | BOD5 (93.0) COD (88.0) TDS (93.0) TSS (93.0) | FC (76.0–99.0) Fecal Streptococci (49.0–85.0) Note: These were removed in two phases in four distinct seasons | - | Subsurface-flow constructed wetland | Municipal wastewater | [172] |
| Typha sp. | BOD5 (63.0) COD (50) TDS (58.0) TSS (58.0) | FC (50.0–99.0) Fecal Streptococci (33.0–85.0) Note: These were removed in two phases in four distinct seasons | - | ||||
| 5. | Pontederia crassipes Mart. [formerly Eichhornia crassipes (Mart.) Solms] (common water hyacinth) and Phragmites australis (Cav.) Trin. ex Steud. (common reed) | BOD5 (72.1) COD (67.2) Org-N (59.3) SS (64.6) Settleable solids (91.8) TN (38.0) TP (43.0) | - | - | Surface-flow constructed wetland | Secondary-treated domestic wastewater | [173] |
| 6. | Typha angustifolia L. and Scirpus grossus L.f. (club-rush or bulrush) | BOD5 (68.2) NH4+-N (74.4) NO3−-N (50.0) TP (19.0) TSS (71.9) | - | - | Free-water surface wetland | Secondary-treated municipal wastewater | [174] |
| 7. | Lemna minor L. (common duckweed), P. australis, Schoenoplectus tabernaemontani (C.C. Gmel.) Palla (syn. Scirpus validus Vahl) (softstem bulrush), and Typha orientalis C. Presl (cumbungi) | BOD5 (70.4) NH3-N (40.6) SS (71.8) TP (29.6) | TC and FC (99.7 and 99.6, respectively) | - | Constructed wetland | Sewage water | [175] |
| 8. | A Acorus gramineus Sol. ex Aiton (Japanese sweet flag), B Iris pseudacorus L. (yellow flag) | BOD5 (A 71.3, B 72.5) COD (A 61.71, B 61.5) TN (11.24–21.95) TP (33.15) | - | - | Constructed wetland | Domestic wastewater | [176] |
| 9. | A Iris pseudacorus L. and B Acorus gramineus Soland | BOD5 (72.5, 71.3) COD (61.1, 61.7) TN (70.9, 70.7) TP (86.9, 84.8) A HMs (Cd, Cr, and Pb)—15.3, 21.3, and 24.5, respectively) | - | - | Model wetlands | Rural or urban domestic wastewater | [176] |
| 10. | T. angustifolia | BOD5 (80.78) NH4+-N (95.75) TN (66.5) TP (58.59) | - | - | Free-water surface wetland | Secondary-treated municipal wastewater | [177] |
| 11. | Canna and Heliconia | TSS (88.0) COD (42–83) | - | - | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [178] |
| 12. | P. australis and T. latifolia | BOD5 (>86.0) COD (>86.0) | - | - | Vertical-flow constructed wetland | Domestic wastewater | [179] |
| 13. | Canna | BOD5 (94.0) TN (93.0) | - | - | Vertical subsurface-flow constructed wetland and horizontal subsurface-flow constructed wetland | Sewage water | [180] |
| 14. | Cyperus alternifolius L. (umbrella papyrus) | COD (83.6) NH4+-N (71.4) TN (64.5) TP (68.1) TSS (99.0) | - | - | Hybrid-flow constructed wetland | Municipal wastewater | [181] |
| 15. | Acorus calamus Linn. (sweet flag) | COD (73.0–93.0) TN (46.0–87.0) TOC (40.0–66.0) TP (75.0–90.0) | - | - | Vertical-flow constructed wetland | Domestic wastewater | [182] |
| 16. | Anthurium andraeanum Linden (flamingo flower), Strelitzia reginae Aiton (crane flower), Zantedeschia aethiopica (L.) Spreng. (calla lily), and Agapanthus africanus L. (African lily) | BOD5 (81.94) TN (49.38) TP (50.14) TSS (61.56) | TC (99.30) | - | Vertical subsurface-flow constructed wetland | Secondary-treated municipal wastewater | [183] |
| 17. | Canna, Cyperus papyrus L. (papyrus or Nile grass), and P. australis | BOD5 (90) COD (88.0) TSS (92) | TC, FC, and E. coli (94.0–99.0) | - | Vertical-flow constructed wetland | Municipal wastewater | [184] |
| 18. | Canna indica L. (Indian shot) and T. orientalis | BOD5 (62.8) NH4+-N (80.72) NO3−-N (12.8) TN (51.1) | - | - | Hybrid-flow constructed wetland | Municipal wastewater | [185] |
| 19. | Scirpus alternifolios (umbrella papyrus) | BOD5 (84.9) COD (89.8) NH4-N (82.2) TKN (82.7) TP (76.5) TSS (98.1) | - | - | Vertical subsurface-flow constructed wetland | Wastewater | [186] |
| 20. | T. angustifolia | NH4+-N (95.2) TP (69.6) | - | - | Subsurface-flow constructed wetland | Artificial wastewater | [120] |
| 21. | Canna and P. australis | BOD5 (92.8, 93.6) COD (91.5) NH3 (62.3, 57.1) TSS (92.3, 94.0) | - | - | Vertical-flow and horizontal-flow constructed wetlands | Municipal wastewater | [187] |
| 22. | Alternanthera sessilis (L.) R.Br. ex DC. (Brazilian spinach), C. esculenta, P. australis, Pistia stratiotes L. (water lettuce), Persicaria hydropiper (L.) Delarbre (syn. Polygonum hydropiper L.) (water pepper), and T. latifolia | BOD5 (90.0) NH4-N (86.0) NO3-N (84.0) TDS (78.0) TE (As, Co, Cr, Cu, Mn, Ni, Pb, and Zn—85.0, 49.0, 35.0, 95.0, 87.0, 39.0, 92.0, and 55.0, respectively) TSS (65.0) | - | - | Subsurface-flow constructed wetland | Sewage wastewater | [188] |
| 23. | T. latifolia | COD (53.0–70.0) NH3 (12.0–15.0) P (18.0–25.0) | - | - | Surface, up-flow constructed wetland | Sewage wastewater | [12] |
| 24. | Phragmites | CODCr (75.7) NH3-N (96.8) TN (96.7) TP (90.4) | - | N was removed by Paenibacillus sp., Pseudomonas oleovorans, Pseudomonas pseudoalcaligenes, Pseudomonas stutzeri (LZ-4), Pseudomonas stutzeri (LZ-14), Pseudomonas stutzeri (XP-2), and Pseudomonas pseudoalcaligenes (Note: These microbes remove N from wetlands with processes like adsorption, filtration, precipitation, sedimentation, and volatization); Pseudomonas mendocina LR contributed to the maximal N removal (97.35%) | Laboratory-scale constructed wetland microcosm | River water and domestic wastewater | [189] |
| 25. | Canna and Phragmites | NH4+-N, NO3−-N and TKN (52.99) | - | - | Vertical-flow constructed wetland | Secondary-treated sewage wastewater | [190] |
| 26. | I. pseudacorus, P. australis, and T. latifolia | BOD5 (41.0) Dissolved P (59.0) HM (Pb—98.0) NH4+-N (66.0) TP (46.0) SS (66) | - | - | Constructed wetland | Mine water and Sewage | [191] |
| 27. | Agapanthus africanus (L.) Hoffman. (African lily), Canna ffuses, C. indica, Watsonia borbonica (Pourr.) Goldblatt (Cape bugle lily), and Z. aethiopica | BOD5 (90.0) NH4+ (84.0) PO42− (92.0) | - | - | Horizontal subsurface-flow constructed wetland | Sewage water | [192] |
| 28. | Aquatic plants | BOD5 (87.9) CODCr (90.6) NH3-N (66.7) TN (63.4) TP (92.6) | - | - | New-type, multi-layer artificial wetland | Domestic wastewater | [193] |
| 29. | Canna × generalis | NO3− (51.9) P (8.9) Phenolic compounds (1.0) | - | - | Constructed wetland | Domestic wastewater | [129] |
| 30. | A. calamus, C. indica, Iris japonica Thunb. (butterfly flower), P. australis, T. angustifolia, and Zizania caduciflora (Trin.) Hand.-Mazz. (wild rice) | TP (79.6, 87.9, 90.3, and 93.2) | - | - | Integrated vertical-flow constructed wetland | Synthetic domestic wastewater | [194] |
| 31. | P. australis | BOD5 (84.0) COD (75.0) NH4+ (32.0) TP (22.0) TSS (75.0) | - | - | Vertical subsurface-flow constructed wetland | Sewage water | [195] |
| 32. | C. indica | BOD5 (88.11, 80.51, and 89.78) NH4+-N (94.81, 39.39) TN (56.17, 50.0, and 55.06) TP (94.82, 93.04, and 93.31) | - | N was removed by denitrifying bacteria | Hybrid vertical down-flow constructed wetland | Domestic wastewater | [196] |
| 33. | A. calamus and P. australis | TN (45.2) | - | N was removed by a large number of rhizospheric bacteria (out of that, 17.9–26.8% non-rhizospheric bacteria removed N from the soil) | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [197] |
| 34. | Centella asiatica (L.) Urb. (Indian pennywort), E. crassipes, P. australis, T. latifolia, and Chrysopogon zizanioides (L.) Roberty (vetiver grass) | BOD5 (81.0 and 82.0) TKN (63.0 and 69.0) TSS (79.0 and 89.0) | - | - | Hybrid constructed wetland | Domestic wastewater | [198] |
| 35. | Typha domingensis Pers. (southern cattail) | BOD5 (56.0) TKN (41.0) TP (37.0) TSS (78.0) | - | - | Constructed floating wetland | Domestic sewage | [199] |
| 36. | P. australis | BOD5 (93.0) COD (91.0) TN (67.0) TP (62.0) TSS (95.0) | TC, FC, and fecal Streptococci (64.0, 63.0, and 61.0, respectively) | - | Hybrid constructed wetland | Wastewater | [200] |
| 37. | Typha and Commelina benghalensis L. (Benghal dayflower) | NO3− (84.0) PO43− (77.0) | TC and FC, E. coli, Enterococcus, Clostridium, and Salmonella (65.0–70.0) | - | Horizontal-flow constructed wetland | Primary and secondary-treated sewage | [201] |
| 38. | Pennisetum purpureum Schumach. (Napier grass) and T. latifolia | BOD5 (up to 87) (inlet BOD5 of 748–1642 mg L−1) COD (up to 81) (inlet COD of 835–2602 mg L−1) | - | - | Horizontal subsurface-flow constructed wetlands | Industrial (brewery) wastewater | [202] |
| 39. | C. indica and Typha angustata Bory & Chaub. (accepted name: Typha domingensis Pers.) | BOD, COD, NH3-N, TDS, TKN, TP, and TVS (A 65.0–B 62.0, A 64.0–B 61.0, A 21.0–B 58.0, A 34.0–B 33.0, A 15.0–B 35.0, and A 54.0– B 40.0) [at first stage] and (A 88.0–B 84.0, A 90.0–B 90.0, A 52.0–B 82.0, A 58.0–B 61.0, A 50.0–B 47.0, and A 71.0–B 64.0) [for the second stage reactor] Note: The nutrient removal was measured at two different hydraulic loadings at A 0.150 m day−1 and at B 0.225 m day−1 | - | - | Two-stage vertical-flow constructed wetland | Domestic wastewater | [203] |
| 40. | C. papyrus and P. australis | BOD5 (80.69) COD (69.87) NH3-N (69.69) TP (50.0) | TC and FC (98.08 and 95.61, respectively) | - | Vertical-flow subsurface constructed wetlands | Municipal wastewater | [204] |
| 41. | A. calamus and C. indica | BOD5 (78.74 and 81.79) TDS (18.96 and 22.31) TN (56.33 and 60.37) PO42− (79.57 and 81.53) | - | - | Pilot-scale vertical subsurface-flow constructed wetland | Primary-treated domestic sewage | [205] |
| 42. | Myriophyllum elatinoides Gaudich. (water milfoil) | NH4+ (91.35) NO3− (95.16) TN (90.36) TP (96.14) | - | Bacteroides and Firmicutes carried out denitrification; N was removed by Pseudomonas, Dechloromonas, Desulfobacca, and Desulfomicrobium; PO42− was removed by Chlorobaculum, Methanobacterium, and Rhodoblastus | Multi-stage, surface-flow constructed wetland | Domestic sewage | [141] |
| 43. | C. esculenta and Dracaena sanderiana Sander ex Mast. (Chinese water bamboo) | BOD5/ (74.0) NH4-N (90.0) TSS (76.0) | TC (59.0) | - | Novel vertical-flow and free-water surface constructed wetland | Dormitory sewage | [83] |
| 44. | A. calamus and reeds | TN (15.0) TP (18.0) | - | Bioremediation and degradation of diesel, petroleum, and other alkanes could be achieved by Tistrella; N was removed by Achromobacter, Aeromicrobium, Aquicella, Azospirillum, Fluviicola, Halomonas, Limnohabitans, Methylophilacterium, Perlucidibaca, Pseudomonas, Rhodobacter, Rhodospirillaceae, and Variovorax; S compounds were removed by Desulfovibrio and Rhodocista | Constructed wetland | Domestic sewage | [159] |
| 45. | C. indica, C. alternifolius, and Thalia dealbata Fraser ex Roscoe (powdery alligator-flag) | COD (95.2) NH4-N (98.1) PO4−-P (85.3) TN (87.9) TP (86.1) | - | - | Hybrid constructed wetland | Domestic sewage | [206] |
| 46. | Chrysopogon zizanioides L. (vetiver and khus) | BOD5 (83.36) COD (92.34) NH4-N (89.41) NO3-N (90.72) PO4−-P (92.81) TCr (95.20) TN (93.54) TSS (94.66) | - | - | Horizontal subsurface-flow constructed wetland | Tannery wastewater | [207] |
| 47. | A Commelina benghalensis L. (Benghal dayflower) and B T. latifolia | A,B BOD (61.0 and 59.0) A,B COD (58.0 and 53.0) A,B NH4+ (60.3 and 51.5) A,B NO3-N (60.3 and 51.5) A,B PO42−(61.0 and 64.0) | A,B TC (41.0 and 39.0) A,B FC (50.0 and 30.0) A,B E. coli (45.0 and 35.0) | - | Horizontal-flow constructed wetland | Domestic wastewater | [207] |
| 48. | Oenanthe javanica DC. (water celery) | COD (38.65) NH4+-N (28.20) TN (18.82) TP (14.57) | - | Purification of micro-polluted water was improved indirectly by inoculating low temperature-resistant Bacillus spp., via altering the community structure of the wetland microbes (i.e., through stimulating beneficial microbes for treating sewage while inhibiting microbial pathogens) | Vertical subsurface-flow constructed wetland | Micro-polluted lake water | [208] |
| 49. | A Canna indica L. (Indian shot) and B Iris sibrica L. (Siberian flag) | A,B COD (83.6 and 66.3) A,B NH4+-N (82.7 and 44.1) A,B TN (76.8 and 43.8) | - | - | Horizontal subsurface-flow constructed wetland | Domestic wastewater | [162] |
| 50. | P. australis | TP (36.2– 87.5) | - | - | Microbial-enhanced constructed wetlands | P-rich Saline wastewater | [209] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, T.K.; Barya, M.P.; Dutta, J.; Mukherjee, P.; Thakur, A.; Swamy, S.L.; Anderson, J.T. Integrated Phytobial Remediation of Dissolved Pollutants from Domestic Wastewater through Constructed Wetlands: An Interactive Macrophyte-Microbe-Based Green and Low-Cost Decontamination Technology with Prospective Resource Recovery. Water 2023, 15, 3877. https://doi.org/10.3390/w15223877
Thakur TK, Barya MP, Dutta J, Mukherjee P, Thakur A, Swamy SL, Anderson JT. Integrated Phytobial Remediation of Dissolved Pollutants from Domestic Wastewater through Constructed Wetlands: An Interactive Macrophyte-Microbe-Based Green and Low-Cost Decontamination Technology with Prospective Resource Recovery. Water. 2023; 15(22):3877. https://doi.org/10.3390/w15223877
Chicago/Turabian StyleThakur, Tarun Kumar, Mahesh Prasad Barya, Joystu Dutta, Pritam Mukherjee, Anita Thakur, Singam Laxmana Swamy, and James T. Anderson. 2023. "Integrated Phytobial Remediation of Dissolved Pollutants from Domestic Wastewater through Constructed Wetlands: An Interactive Macrophyte-Microbe-Based Green and Low-Cost Decontamination Technology with Prospective Resource Recovery" Water 15, no. 22: 3877. https://doi.org/10.3390/w15223877








