Urban Wastewater Mining for Circular Resource Recovery: Approaches and Technology Analysis
Abstract
:1. Introduction
2. Wastewater or “Usedwater”?
2.1. Usedwater Generation and Composition
2.1.1. Centralized vs. Decentralized Used-Water Generation and Management
2.2. Residual Energy and Materials in Used Water
An Inefficient Nutrient Management Paradigm
3. UWW Mining: Approaches and Technologies
3.1. Water Reuse Opportunities
3.2. UWW Energy-Mining Technologies
3.2.1. Thermal Energy
3.2.2. Chemical Energy
3.2.3. Potential Energy
3.3. UWW Nutrient Mining
3.3.1. Ammonia Recovery
| Technology | Pros | Cons | Comments | Ref. |
|---|---|---|---|---|
| Heat pumps | Mature technology. High, steady sewage flows allow consistent recovery. Can be applied in many situations, including segregated GW streams. | Issues with exchangers’ corrosion and fouling can develop in raw sewage applications. Low-grade heat transfer is most efficient in proximity uses. | Applicable both in large sewer networks and WWTPs. Excessive heat extraction may impair biological treatment efficiency. High potential in densely populated areas. | [19,81,82,83,84,85,86,87,88] |
| Thermoelectric generators (TEGs) | Direct electricity generation from fluid-embedded heat possible through the Seebeck effect. | Most efficient at high thermal gradients (low in UWW applications). Expensive technology. | Not mature in the water sector. Efforts to exploit low-gradient, high-flow conditions are ongoing. Present potential is low. | [89,90] |
| Anaerobic Digestion (fermentation) | Mature, most common technology for energy recovery from organic wastes. UASB version applicable to diluted UWW. Energy recovered as biogas or, with process modification, hydrogen. | Lower efficiency at low temperatures. Biogas contains up to 40% CO2 and must be upgraded for general use as a natural gas substitute. | Can be used as the first step in complex sludge biorefinery schemes, with sequential materials recovery. Could completely replace aerobic biodegradation processes in the presence of high concentrations of sewage. Very high potential for improvement. | [51,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108] |
| Bioelectrochemical systems | Direct electricity generation from UWW organics. Possible to achieve hydrogen production at lower costs than pure water electrolysis. | Expensive technology, not yet applied on a large scale. Limited electrical recovery compared to theoretical potential. | TRL still low. More research is needed for successful full-scale applications. Presently, it has low potential. | [109,110,111,112,113,137] |
| Ammonia-fuel recovery | NH4 can be used as C-free fuel, with modification of existing engine technology. More economical to produce than H2, at a higher volumetric energy density. | High combustion temperature needed. Could generate NOx emissions if not properly controlled. | Ammonia-to-energy approach possible on a large scale and in heavy transport vehicles/ships. Good medium-term potential. | [114,115,116,117,124,125,126,127,128,129,130,131,132] |
| Picoturbine hydropower | Electricity generation from liquid streams’ potential energy in high-rise buildings. | Picoturbines can be affected by solids and impurities in the flow. Needs buildings’ internal plumbing adaptation. | New picoturbine types developed to allow use with BW. Application to less contaminated GW could be an efficient solution. Good potential in highly dense, vertically developed urban areas. | [119,120] |
3.3.2. Phosphorous Recovery
3.3.3. Other Nutrient Recovery Approaches
3.4. UWW Chemicals and Materials Mining
3.4.1. Cellulose, a Used Water-Embedded Resource
3.4.2. Protein Recovery
3.4.3. UWW-Based Biorefineries
3.4.4. Recovery of Metals from UWW
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- U.N. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423); Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- IRP. The Weight of Cities: Resource Requirements of Future Urbanization. A Report by the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2018. [Google Scholar]
- Michielin, D. From Waste to Resource: The Rise of Urban Mining. Foresight. 2023. Available online: https://www.climateforesight.eu/articles/raw-materials-urban-mining/ (accessed on 4 October 2023).
- EC. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; COM/2020/98 final; EC: Brussels, Belgium, 2020. [Google Scholar]
- Vörösmarty, C.J.; Sahagian, D. Anthropogenic Disturbance of the Terrestrial Water Cycle. BioScience 2000, 50, 753–765. [Google Scholar] [CrossRef]
- EEA. European Environmental Agency. Data and Maps. Available online: https://www.eea.europa.eu/data-and-maps/figures/water-stress-in-europe/figure_4_3_3_left_graphic.eps (accessed on 25 June 2023).
- Hughes, J.; Cowper-Heays, K.; Olesson, E.; Bell, R.; Stroombergen, A. Impacts and implications of climate change on wastewater systems: A New Zealand perspective. Clim. Risk Manag. 2020, 31, 100262. [Google Scholar] [CrossRef]
- EIB. Wastewater as a Resource; European Investment Bank: Luxembourg, 2022. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Bojanowska-Czajka, A.; Trojanowicz, M. Comparison of different advanced degradation processes for the removal of the pharmaceutical compounds diclofenac and carbamazepine from liquid solutions. Environ. Sci. Pollut. Res. 2018, 25, 27704–277231. [Google Scholar] [CrossRef] [PubMed]
- Viviano, G.; Valsecchi, S.; Polesello, S.; Capodaglio, A.; Tartari, G.; Salerno, F. Combined Use of Caffeine and Turbidity to Evaluate the Impact of CSOs on River Water Quality. Water Air Soil Pollut. 2017, 228, 330. [Google Scholar] [CrossRef]
- USDA. Streams through the City: Water Quality and Quantity. Available online: https://www.srs.fs.usda.gov/compass/2015/02/19/streams-through-the-city-water-quality-and-quantity/#:~:text=Urbanization%20affects%20water%20quality%20because,the%20increase%20in%20impervious%20surfaces (accessed on 15 September 2022).
- Capodaglio, A.G.; Muraca, A.; Becchi, G. Accounting for water quality effects of future urbanization: Diffuse pollution loads estimates and control in Mantua’s Lakes (Italy). Water Sci. Technol. 2003, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- UN-WATER. Wastewater: The Untapped Resource; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Ghernaout, D.; Elboughdiri, N.; Al Arni, S. Water Reuse (WR): Dares, Restrictions, and Trends. Appl. Eng. 2019, 3, 159–170. [Google Scholar]
- Singh, S.; Yadav, R.; Kathi, S.; Singh, A.N. Treatment of harvested rainwater and reuse: Practices, prospects, and challenges. In Advances in Environmental Pollution Research, Cost Effective Technologies for Solid Waste and Wastewater Treatment; Chapter 14; Kathi, S., Devipriya, S., Thamaraiselvi, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 161–178. [Google Scholar] [CrossRef]
- Tampo, L.; Mande, S.L.A.S.; Adekanmbi, A.O.; Boguido, G.; Akpataku, K.V.; Ayah, M.; Tchakala, I.; Gnazou, M.D.T.; Bawa, L.M.; Djaneye-Boundjou, G.; et al. Treated wastewater suitability for reuse in comparison to groundwater and surface water in a peri-urban area: Implications for water quality management. Sci. Total Environ. 2022, 815, 152780. [Google Scholar] [CrossRef]
- Yin, H.; Qiu, P.; Qian, Y.; Kong, Z.; Zheng, X.; Tang, Z.; Guo, H. Textile Wastewater Treatment for Water Reuse: A Case Study. Processes 2019, 7, 34. [Google Scholar] [CrossRef]
- Mainardis, M.; Cecconet, D.; Moretti, A.; Freguia, S.; Capodaglio, A.G. Wastewater fertigation in agriculture: Issues and opportunities for improved water management and circular economy. Environ. Pollut. 2022, 296, 118755. [Google Scholar] [CrossRef]
- Cecconet, D.; Raček, J.; Callegari, A.; Hlavínek, P. Energy Recovery from Wastewater: A Study on Heating and Cooling of a Multipurpose Building with Sewage-Reclaimed Heat Energy. Sustainability 2020, 12, 116. [Google Scholar] [CrossRef]
- Tomei, M.C.; Stazi, V.; Daneshgar, S.; Capodaglio, A.G. Holistic Approach to Phosphorus Recovery from Urban Wastewater: Enhanced Biological Removal Combined with Precipitation. Sustainability 2020, 12, 575. [Google Scholar] [CrossRef]
- Barros, Ó.; Costa, L.; Costa, F.; Lago, A.; Rocha, V.; Vipotnik, Z.; Silva, B.; Tavares, T. Recovery of Rare Earth Elements from Wastewater Towards a Circular Economy. Molecules 2019, 24, 1005. [Google Scholar] [CrossRef] [PubMed]
- Faragò, M.; Damgaard, A.; Agertved Madsen, J.; Andersen, J.K.; Thornberg, D.; Andersen, M.H.; Rygaard, M. From wastewater treatment to water resource recovery: Environmental and economic impacts of full-scale implementation. Water Res. 2021, 204, 117554. [Google Scholar] [CrossRef]
- Qadir, M.; Drechsel, P.; Jiménez Cisneros, B.; Kim, Y.; Pramanik, A.; Mehta, P.; Olaniyan, O. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum 2020, 44, 40–51. [Google Scholar] [CrossRef]
- Beder, S. Technological Paradigms: The Case of Sewerage Engineering. Technol. Stud. 1997, 4, 167–188. [Google Scholar]
- USEPA. Energy Efficiency for Water Utilities. US Environmental Protection Agency. Available online: https://www.epa.gov/sustainable-water-infrastructure/energy-efficiency-water-utilities (accessed on 16 October 2022).
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Opher, T.; Friedler, E. Comparative LCA of decentralized wastewater treatment alternatives for non-potable urban reuse. J. Environ. Manag. 2016, 182, 464–476. [Google Scholar] [CrossRef]
- Bernal, D.; Restrepo, I.; Grueso-Casquete, S. Key criteria for considering decentralization in municipal wastewater management. Heliyon 2021, 7, e06375. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.T.; Narayanan, N.C.; Cheng, Y.L. Cost comparison of centralized and decentralized wastewater management systems using optimization model. J. Environ. Manag. 2018, 213, 90–97. [Google Scholar] [CrossRef]
- Maurer, M.; Rothenberger, D.; Larsen, T.A. Decentralised wastewater treatment technologies from a national perspective: At what cost are they competitive? Water Sci. Technol. 2005, 5, 145–154. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Molognoni, D. Online monitoring of priority and dangerous pollutants in natural and urban waters: A state-of-the-art review. Manag. Environ. Qual. 2016, 27, 507–536. [Google Scholar] [CrossRef]
- Yuan, G.; Olsson, G.; Cardell-Oliver, R.; van Schagen, K.; Marchi, A.; Deletic, A.; Urich, C.; Rauch, W.; Liu, Y.; Jiang, G. Sweating the assets–The role of instrumentation, control and automation in urban water systems. Water Res. 2019, 155, 381–402. [Google Scholar] [CrossRef] [PubMed]
- Tervahauta, T.; Trang, H.; Hernández, L.; Zeeman, G.; Buisman, C.J.N. Prospects of Source-Separation-Based Sanitation Concepts: A Model-Based Study. Water 2013, 5, 1006–1035. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Taking the water out of “wastewater”: An ineluctable oxymoron for urban water cycle sustainability. Water Environ. Res. 2020, 92, 2030–2040. [Google Scholar] [CrossRef]
- Garrido-Baserba, M.; Vinardell, S.; Molinos-Senante, M.; Rosso, D.; Poch, M. The Economics of Wastewater Treatment Decentralization: A Techno-economic Evaluation. Environ. Sci. Technol. 2018, 52, 8965–8976. [Google Scholar] [CrossRef]
- Valencio, I.P.; Gonçalves, O.M. Drainage and sewage system performance–Consequences of reductions in toilet flush volume. Build. Serv. Eng. Res. Technol. 2019, 40, 576–594. [Google Scholar] [CrossRef]
- Shafiqul, I.M. Comparative evaluation of vacuum sewer and gravity sewer systems. Int. J. Syst. Assur. Eng. Manag. 2017, 8, 37–53. [Google Scholar] [CrossRef]
- Lv, Z.; Song, Y.; Chen, C.; Jiang, B.; Sun, H.; Lyu, Z. A novel step-by-step optimization method for interplant water networks. J. Environ. Manag. 2018, 213, 255–270. [Google Scholar] [CrossRef]
- Wen, C.; Dai, Z.; Cheng, F.; Cheng, H.; Yang, Z.; Cai, Q.; Zha, X.; Lu, X. Review on research achievements of blackwater anaerobic digestion for enhanced resource recovery. Environ. Dev. Sustain. 2022. [Google Scholar] [CrossRef]
- Xu, J.; Yang, L.; Zhou, X. A systematical review of blackwater treatment and resource recovery: Advance in technologies and applications. Resour. Conserv. Recycl. 2023, 197, 107066. [Google Scholar] [CrossRef]
- Sydney Water. Sewer Mining. How to Set Up a Sewer Mining Scheme. Available online: http://www.sydneywater.com.au/SW/plumbing-building-developing/plumbing/recycled-water (accessed on 24 June 2023).
- Makropoulos, C.; Rozos, E.; Tsoukalas, I.; Plevri, A.; Karakatsanis, G.; Karagiannidis, L.; Makri, E.; Lioumis, C.; Noutsopoulos, C.; Mamais, D.; et al. Sewer-mining: A water reuse option supporting circular economy, public service provision and entrepreneurship. J. Environ. Manag. 2018, 216, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.F. Energy in Wastewater Treatment; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1982. [Google Scholar]
- Shizas, I.; Bagley, D.M. Experimental Determination of Energy Content of Unknown Organics in Municipal Wastewater Streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- Ali, S.F.; Gillich, A. Opportunities to decarbonize heat in the UK using Urban Wastewater Heat Recovery. Build. Serv. Eng. Res. Technol. 2021, 42, 715–732. [Google Scholar]
- European Parliament. European Parliament Resolution, 24th March 2022 “Need for an Urgent EU Action Plan to Ensure Food Security Inside and Outside the EU in Light of the Russian Invasion of Ukraine”; P9_TA(2022)0099; European Parliament: Strasbourg, France, 2022.
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Nancharaiah, Y.; Mohan, S.V.; Lens, P. Recent advances in nutrient removal and recovery in biological and bioelectrochemical systems. Bioresour. Technol. 2016, 215, 173–185. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Popp, A.; Lotze-Campen, H.; Dietrich, J.P.; Rolinski, S.; Weindl, I.; Schmitz, C.; Muller, C.; Bonsch, M.; Humpenoder, F.; et al. Reactive nitrogen requirements to feed the world in 2050 and potential to mitigate nitrogen pollution. Nat. Commun. 2014, 5, 3858. [Google Scholar] [CrossRef]
- Law, Y.; Ye, L.; Pan, Y.; Yuan, Z. Nitrous oxide emissions from wastewater treatment processes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the Urban Water Cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The potential phosphorus crisis: Resource conservation and possible escape technologies: A review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Shin, C.; Szczuka, A.; Liu, M.J.; Mendoza, L.; Jiang, R.; Tilmans, S.H.; Tarpeh, W.A.; Mitch, W.A.; Criddle, C.S. Recovery of Clean Water and Ammonia from Domestic Wastewater: Impacts on Embodied Energy and Greenhouse Gas Emissions. Environ. Sci. Technol. 2022, 56, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738. [Google Scholar] [CrossRef]
- Cecconet, D.; Capodaglio, A.G. Sewage Sludge Biorefinery for Circular Economy. Sustainability 2022, 14, 14841. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Energy and Resources Recovery from Excess Sewage Sludge: A Holistic Analysis of Opportunities and Strategies. Resour. Conserv. Recycl. Adv. 2023, 19, 200184. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, H.; Yang, L.; You, X.; Yu, Z.; Zhang, Y.; Zhou, X. Technologies for pollutant removal and resource recovery from blackwater: A review. Front. Environ. Sci. Eng. 2023, 17, 83. [Google Scholar] [CrossRef]
- Baykal, B.B. Recycling/reusing grey water and yellow water (human urine): Motivations, perspectives and reflections into the future. Desalin. Water Treat. 2019, 172, 212–223. [Google Scholar] [CrossRef]
- Cecconet, D.; Callegari, A.; Hlavínek, P.; Capodaglio, A.G. Membrane bioreactors for sustainable, fit-for-purpose greywater treatment: A critical review. Clean. Technol. Environ. Pollut. 2019, 21, 745–762. [Google Scholar] [CrossRef]
- Pradhan, S.; Al-Ghamdi, S.G.; Mackey, H.R. Greywater treatment by ornamental plants and media for an integrated green wall system. Int. Biodeterior. Biodegrad. 2019, 145, 104792. [Google Scholar] [CrossRef]
- Oteng-Peprah, M.; Acheampong, M.A.; deVries, N.K. Greywater Characteristics, Treatment Systems, Reuse Strategies and User Perception-a Review. Water Air Soil Pollut. 2018, 229, 255. [Google Scholar] [CrossRef]
- Sijimol, M.R.; Joseph, S. Constructed wetland systems for greywater treatment and reuse: A review. Int. J. Energy Water Res. 2021, 5, 357–369. [Google Scholar] [CrossRef]
- Bolognesi, S.; Cecconet, D.; Callegari, A.; Puig, S.; Capodaglio, A.G. Tubular photo-MFC reactors as wastewater polishing treatment step with simultaneous electricity production. Bioresour. Technol. Rep. 2022, 18, 101059. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Piacentini, L.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical greywater treatment for non-potable reuse and energy recovery. Water 2021, 13, 295. [Google Scholar] [CrossRef]
- Masi, F.; Bresciani, R.; Rizzo, A.; Edathoot, A.; Patwardhan, N.; Panse, D.; Langergraber, G. Green walls for greywater treatment and recycling in dense urban areas: A case-study in Pune. J. Water Sanit. Hyg. Dev. 2016, 6, 342–347. [Google Scholar] [CrossRef]
- Kabdaslı, I.; Kusçuoglu, S.; Tünay, O.; Siciliano, A. Assessment of K-Struvite Precipitation as a Means of Nutrient Recovery from Source Separated Human Urine. Sustainability 2022, 14, 1082. [Google Scholar] [CrossRef]
- Patel, A.; Mungray, A.A.; Mungray, A.K. Technologies for the recovery of nutrients, water and energy from human urine: A review. Chemosphere 2020, 259, 127372. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Tzanakakis, V.A.; Capodaglio, A.G.; Dercas, N. A Critical Review of Water Reuse: Lessons from Prehistoric Greece for Present and Future Challenges. Water 2023, 15, 2385. [Google Scholar] [CrossRef]
- Segrè Cohen, A.; Love, N.G.; Árvai, J. Communicating the Risks and Benefits of Human Urine-Derived Fertilizer. Sustainability 2020, 12, 9973. [Google Scholar] [CrossRef]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Callegari, A.; Capodaglio, A.G. Snack-food industry effluent pre-treatment for annatto dye and yeast removal: Process improvement for effectiveness and sustainability. J. Clean. Prod. 2020, 277, 124117. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Capodaglio, A.G.; Dialynas, E.G. Wastewater Management: From Ancient Greece to Modern Times and Future. Water 2023, 15, 43. [Google Scholar] [CrossRef]
- Sevostianova, E.; Leinaue, B. Subsurface-applied tailored water: Combining nutrient benefits with efficient Turfgrass irrigation. Crop Sci. 2014, 54, 1926–1938. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1–48. [Google Scholar] [CrossRef]
- Rogers, P.D.; Grigg, N.S. Trends in dual water systems. J. Water Reuse Desalin. 2015, 5, 132–141. [Google Scholar] [CrossRef]
- Sanz, M.A. Trends in Desalination & Water Reuse. Desalination and Water Reuse Business Forum. Enhancing Climate Resilience for Cities. Singapore International Water Week. 2018. Available online: https://www.siww.com.sg/docs/default-source/default-document-library/mr-miguelsanz.pdf?sfvrsn=2 (accessed on 21 September 2023).
- Lahnsteiner, J.; van Rensburg, P.; Esterhuizen, J. Direct potable reuse—A feasible water management option. J. Water Reuse Desalin. 2018, 8, 14–28. [Google Scholar] [CrossRef]
- CPUC. What Will Be the Cost of Future Sources of Water for California? California Public Utilities Commission, Policy & Planning Division. Available online: https://www.cpuc.ca.gov/uploadedFiles/CPUC_Public_Website/Content/About_Us/Organization/Divisions/Policy_and_Planning/PPD_Work/PPD_Work_Products_(2014_forward)/PPD%20-%20Production%20costs%20for%20new%20water.pdf (accessed on 21 September 2023).
- Alam, S.; Borthakur, A.; Ravi, S.; Gebremichael, M.; Mohanty, S.K. Managed aquifer recharge implementation criteria to achieve water sustainability. Sci. Total Environ. 2021, 768, 144992. [Google Scholar] [CrossRef] [PubMed]
- Boguniewicz-Zabłocka, J.; Capodaglio, A.G. Analysis of Alternatives for Sustainable Stormwater Management in Small Developments of Polish Urban Catchments. Sustainability 2020, 12, 10189. [Google Scholar] [CrossRef]
- Abdel-Aal, M.; Schellart, A.; Kroll, S.; Mohamed, M.; Tait, S. Modelling the potential for multi-location in-sewer heat recovery at a city scale under different seasonal scenarios. Water Res. 2018, 145, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Iida, M.; Sakakura, Y.; Takakuwa, T. Reuse of heat energy in wastewater: Implementation examples in Japan. Water Sci. Technol. 2001, 43, 277–285. [Google Scholar] [CrossRef]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G. Sustainable management of biological solids in small treatment plants: Overview of strategies and reuse options for a solar drying facility in Poland. Environ. Sci. Pollut. Res. 2021, 28, 24680–24693. [Google Scholar] [CrossRef]
- Kretschmer, F.; Hrdy, B.; Neugebauer, G.; Stoeglehner, G. Wastewater Treatment Plants as Local Thermal Power Stations—Modifying Internal Heat Supply for Covering External Heat Demand. Processes 2021, 9, 1981. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; van Loosdrecht, M.C.M.; Jiang, H.; Liu, R. Energy recovery from wastewater: Heat over organics. Water Res. 2019, 161, 74–77. [Google Scholar] [CrossRef]
- Liu, L.; Fu, L.; Jiang, Y. Application of an exhaust heat recovery system for domestic hot water. Energy 2010, 35, 1476–1481. [Google Scholar] [CrossRef]
- Culha, O.; Gunerhan, H.; Biyik, E.; Ekren, O.; Hepbasli, A. Heat exchanger applications in wastewater source heat pumps for buildings: A key review. Energy Build. 2015, 104, 215–232. [Google Scholar] [CrossRef]
- Callegari, A.; Boguniewicz-Zablocka, J.; Capodaglio, A.G. Energy recovery and efficiency improvement for an activated sludge, agro-food WWTP upgrade. Water Pract. Technol. 2018, 13, 909–921. [Google Scholar] [CrossRef]
- Tian, Z.; Lee, S.; Chen, G. Heat transfer in thermoelectric materials and devices. J. Heat Transf. 2013, 135, 15. [Google Scholar] [CrossRef]
- Zou, S.; Kanimba, E.; Diller, T.E.; Tian, Z.; He, Z. Modeling assisted evaluation of direct electricity generation from waste heat of wastewater via a thermoelectric generator. Sci. Total Environ. 2018, 635, 1215–1224. [Google Scholar] [CrossRef]
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European union (EU). Rev. Ambiente Agua 2015, 10, 9–21. [Google Scholar] [CrossRef]
- Fernández-Arévalo, T.; Lizarralde, I.; Fdz-Polanco, F.; Pérez-Elvira, S.I.; Garrido, J.M.; Puig, S.; Poch, M.; Grau, P.; Ayesa, E. Quantitative assessment of energy and resource recovery in wastewater treatment plants based on plant-wide simulations. Water Res. 2017, 118, 272–288. [Google Scholar] [CrossRef]
- Pan, J.; Maa, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Yin, C.K.; Shen, Y.; Yuan, R.; Zhua, N.; Yuan, H.; Lou, Z. Sludge-based biochar-assisted thermophilic anaerobic digestion of waste-activated sludge in microbialelectrolysis cell for methane production. Bioresour. Technol. 2019, 284, 315–324. [Google Scholar] [CrossRef]
- Zeeman, G.; Kujawa, K.; de Mes, T.; Hernandez, L.; de Graaff, M.; Abu-Ghunmi, L.; Mels, A.; Meulman, B.; Temmink, H.; Buisman, C.; et al. Anaerobic treatment as a core technology for energy, nutrients and water recovery from source-separated domestic waste(water). Water Sci. Technol. 2008, 57, 1207–1212. [Google Scholar] [CrossRef]
- Lettinga, G.; Hulshoff Pol, L.W. UASB-process design for various types of wastewaters. Water Sci. Technol. 1991, 24, 87–107. [Google Scholar] [CrossRef]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. UASB performance and perspectives in urban wastewater treatment at sub-mesothropic operating temperature. Water 2022, 14, 115. [Google Scholar] [CrossRef]
- Serrano León, E.; Perales Vargas-Machuca, J.A.; Lara Corona, E.; Arbib, A.; Rogalla, F.; Fernández Boizán, M. Anaerobic digestion of municipal sewage under psychrophilic conditions. J. Clean. Prod. 2018, 198, 931–939. [Google Scholar] [CrossRef]
- Cecconet, D.; Mainardis, M.; Callegari, A.; Capodaglio, A.G. Psychrophilic treatment of municipal wastewater with a combined UASB/ASD system, and perspectives for improving urban WWTP sustainability. Chemosphere 2022, 297, 134228. [Google Scholar] [CrossRef] [PubMed]
- van Lier, J.B.; van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, L.; Guo, B.; Zhang, Y.; Liu, Y. Enhancing biomethane recovery from source-diverted blackwater through hydrogenotrophic methanogenesis dominant pathway. Chem. Eng. J. 2019, 378, 122258. [Google Scholar] [CrossRef]
- Mostafa, A.; Elsamadony, M.; El-Dissouky, A.; Elhusseiny, A.; Tawfik, A. Biological H2 potential harvested from complex gelatinaceous wastewater via attached versus suspended growth culture anaerobes. Bioresour. Technol. 2017, 231, 9–18. [Google Scholar] [CrossRef]
- Dionisi, D.; Silva, I.M.O. Production of ethanol, organic acids and hydrogen: An opportunity for mixed culture biotechnology? Rev. Environ. Sci. Bio/Technol. 2016, 15, 213–242. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Peixoto, G.; Albrecht, F.R.; Saavedra del Aguila, N.K.; Zaiat, M. Potential to produce biohydrogen from various wastewaters. Energy Sustain. Dev. 2010, 14, 143–148. [Google Scholar] [CrossRef]
- Paudel, S.; Kang, Y.; Yoo, Y.S.; Seo, G.T. Hydrogen production in the anaerobic treatment of domestic-grade synthetic wastewater. Sustainability 2015, 7, 16260–16272. [Google Scholar] [CrossRef]
- Mu, Y.; Yu, H.Q. Biological hydrogen production in a UASB reactor with granules. I: Physicochemical characteristics of hydrogen-producing granules. Biotechnol. Bioeng. 2006, 94, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Ali, M.; Danial, A.; Zhao, S.; Meng, F.; Nasr, M. 2-biofuels (H2 and CH4) production from anaerobic digestion of biscuits wastewater: Experimental study and techno-economic analysis. J. Water Proc. Eng. 2021, 39, 101736. [Google Scholar] [CrossRef]
- Qyyum, M.A.; Ihsanullah, I.; Ahmad, R.; Ismail, S.; Khan, A.; Nizami, A.S.; Tawfik, A. Biohydrogen production from real industrial wastewater: Potential bioreactors, challenges in commercialization and future directions. Int. J. Hydrogen Energy 2021, 47, 37154–37170. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.-E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef]
- Molognoni, D.; Chiarolla, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Industrial wastewater treatment with a bioelectrochemical process: Assessment of depuration efficiency and energy production. Water Sci. Technol. 2018, 77, 134–144. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Sustainable and efficient biohydrogen production via electrohydrogenesis. Proc. Nat. Acad. Sci. USA 2007, 104, 18871–18873. [Google Scholar] [CrossRef]
- Islam, A.K.M.K.; Dunlop, P.S.M.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-Hydrogen Production from Wastewater: A Comparative Study of Low Energy Intensive Production Processes. Clean. Technol. 2021, 3, 156–182. [Google Scholar] [CrossRef]
- Cruz, H.; Law, Y.; Guest, J.S.; Rabaey, K.; Batstone, D.J.; Laycock, B.; Verstraete, W.; Pikaar, I. Mainstream ammonium recovery to advance sustainable urban wastewater management. Environ. Sci. Technol. 2019, 53, 11066–11079. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A perspective on the use of ammonia as a clean fuel: Challenges and solutions. Int. J. Energy Res. 2020, 45, 4827–4834. [Google Scholar] [CrossRef]
- EurEau. Europe’s Water in Figures. An Overview of the European Drinking Water and Waste Water Sectors, 2021 ed.; The European Federation of National Associations of Water Services: Brussels, Belgium, 2021. [Google Scholar]
- Davey, C.J.; Luqmani, B.; Thomas, N.; McAdam, E.J. Transforming wastewater ammonia to carbon free energy: Integrating fuel cell technology with ammonia stripping for direct power production. Separation. Purif. Technol. 2022, 289, 120755. [Google Scholar] [CrossRef]
- Sari, M.A.; Badruzzaman, M.; Cherchi, C.; Swindle, M.; Ajami, N.; Jacangelo, J.G. Recent innovations and trends in in-conduit hydropower technologies and their applications in water distribution systems. J. Environ. Manag. 2018, 228, 416–428. [Google Scholar]
- Uchiyama, T.; Honda, S.; Okayama, T.; Degawa, T. A Feasibility Study of Power Generation from Sewage Using a Hollowed Pico-Hydraulic Turbine. Engineering 2016, 2, 510–517. [Google Scholar] [CrossRef]
- Sarkar, P.; Sharma, B.; Malik, U. Energy generation from grey water in high raised buildings: The case of India. Ren. Energy 2014, 69, 284–289. [Google Scholar] [CrossRef]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Daneshgar, S.; Cecconet, D.; Capsoni, D.; Capodaglio, A.G. Side-Stream Phosphorus Recovery in Activated Sludge Processes. Water 2022, 14, 1861. [Google Scholar] [CrossRef]
- Fang, L.; Li, J.S.; Guo, M.Z.; Cheeseman, C.R.; Tsang, D.C.W.; Donatello, S.; Poon, C.S. Phosphorus recovery and leaching of trace elements from incinerated sewage sludge ash (ISSA). Chemosphere 2018, 193, 278–287. [Google Scholar] [CrossRef]
- Reza, A.; Chen, L. Optimization and modeling of ammonia nitrogen removal from anaerobically digested liquid dairy manure using vacuum thermal stripping process. Sci. Total Environ. 2022, 851 Pt 2, 158321. [Google Scholar] [CrossRef]
- Song, Y.C.; Woo, J.H.; Oh, G.G.; Kim, D.H.; Lee, C.Y.; Kim, H.W. External electric field promotes ammonia stripping from wastewater. Water Res. 2021, 203, 117518. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct concentration of municipal sewage by forward osmosis and membrane fouling behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef]
- Mondor, M.; Masse, L.; Ippersiel, D.; Lamarche, F.; Masse, D. Use of electrodialysis and reverse osmosis for the recovery and concentration of ammonia from swine manure. Bioresour. Technol. 2008, 99, 7363–7368. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.; Posmanik, R.; Hatch, L.E.; Tester, J.W.; Walker, S.L.; Barsanti, K.C.; Jassby, D. Coupling hydrothermal liquefaction and membrane distillation to treat anaerobic digestate from food and dairy farm waste. Bioresour. Technol. 2018, 267, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Wang, Y.; Du, Y.; Feng, H.; Xu, T. Simultaneous recovery of ammonium and phosphorus via the integration of electrodialysis with struvite reactor. J. Membr. Sci. 2015, 490, 65–71. [Google Scholar] [CrossRef]
- Ward, A.J.; Arola, K.; Thompson Brewster, E.; Mehta, C.M.; Batstone, D.J. Nutrient recovery from wastewater through pilot scale electrodialysis. Water Res. 2018, 135, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Wu, G.; Luo, J.; Wang, S. Development of a selective electrodialysis for nutrient recovery and desalination during secondary effluent treatment. Chem. Eng. J. 2017, 322, 224–233. [Google Scholar] [CrossRef]
- Kedwell, K.C.; Jørgensen, M.K.; Quist-Jensen, C.A.; Pham, T.D.; Van der Bruggen, B.; Lykkegaard Christensen, M. Selective electrodialysis for simultaneous but separate phosphate and ammonium recovery. Environ. Technol. 2019, 42, 2177–2186. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy. Biomass Convers. Biorefinery 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies. Water 2023, 15, 1195. [Google Scholar] [CrossRef]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef]
- Mihelcic, J.R.; Fry, L.M.; Shaw, R. Global potential of phosphorus recovery from human urine and feces. Chemosphere 2011, 84, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Baur, R.; Benisch, M.; Clark, D.; Sprick, R.G. Struvite Control—A Common and Nuisance. WEFTEC Proc. Water Environ. Fed. 2002, 14, 480–495. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation, control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An overview of technologies to recover phosphorus as struvite from wastewater: Advantages and shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, S.; Vanrolleghem, P.A.; Vaneeckhaute, C.; Buttafava, A.; Capodaglio, A.G. Optimization of P compounds recovery from aerobic sludge by chemical modeling and response surface methodology combination. Sci. Total Environ. 2019, 668, 668–677. [Google Scholar] [CrossRef]
- Dockhorn, T. About the economy of phosphorus recovery. In International Conference on Nutrient Recovery from Wastewater Streams, Vancouver, Canada; IWA Publishing: London, UK, 2009; pp. 145–158. ISBN 9781843392323. [Google Scholar]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.P. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 2014, 170, 221–229. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yuan, B.; Li, X.; Ren, Y. Impacts of sludge retention time on sludge characteristics and membrane fouling in a submerged osmotic membrane bioreactor. Bioresour. Technol. 2014, 161, 340–347. [Google Scholar] [CrossRef]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining nutrients (N, K, P) from urban source-separated urine by forward osmosis dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef]
- Zhi, W.T.; Yue, C.; Ong, Y.K.; Chung, T.S. Molecular design of nanofiltration membranes for the recovery of phosphorus from sewage sludge. ACS Sustain. Chem. Eng. 2016, 4, 5570–5577. [Google Scholar]
- Bolognesi, S.; Bañeras, L.; Perona-Vico, E.; Balaguer, M.D.; Puig, S. Carbon dioxide to bio-oil in a bioelectrochemical system-assisted microalgae biorefinery process. Sust. Energy Fuels 2022, 6, 150–161. [Google Scholar] [CrossRef]
- Scarcelli, P.G.; Ruas, G.; Lopez-Serna, R.; Leite Serejo, M.; Blanco, S.; Boncz, M.A.; Muñoz, R. Integration of algae-based sewage treatment with anaerobic digestion of the bacterial-algal biomass and biogas upgrading. Bioresour. Technol. 2021, 340, 125552. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Liu, R.; Li, Y.; Zhang, M.; Hao, X.; Liu, J. Review on the fate and recovery of cellulose in wastewater treatment. Resour. Conserv. Recycl. 2022, 184, 106354. [Google Scholar] [CrossRef]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; van Loosdrecht, M.C.M. Sieving wastewater-cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 43–48. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.; Liu, G. Degradation kinetics of toilet paper fiber during wastewater treatment: Effects of solid retention time and microbial community. Chemosphere 2019, 225, 915–926. [Google Scholar] [CrossRef]
- Franchi, A.; Williams, K.; Lyng, T.O.; Lem, W.; Santoro, D. Rotating Belt Filters as Enabling Technology for Energy-Neutral Wastewater Treatment Plants: Current Status and Applications. WEFTEC 2015 Proc. Water Environ. Fed. 2015, 13, 1743–1749. [Google Scholar] [CrossRef]
- Harremoes, P.; Capodaglio, A.G.; Hellstrom, B.G.; Henze, M.; Jensen, K.N.; Lynggaard-Jensen, A.; Otterpohl, R.; Soeberg, H. Wastewater treatment plants under transient loading- Performance, modelling and control. Water Sci. Technol. 1993, 27, 71–115. [Google Scholar] [CrossRef]
- Glińska, K.; Aqlan, M.; Giralt, J.; Torrens, E.; Fortuny, A.; Montané, D.; Stüber, F.; Fabregat, A.; Font, J.; Olkiewicz, M.; et al. Separation of cellulose from industrial paper mill wastewater dried sludge using a commercial and cheap ionic liquid. Water Sci. Technol. 2019, 79, 1897–1904. [Google Scholar] [CrossRef]
- Global Recycling. Recovery of Cellulose from Waste Water. Available online: https://global-recycling.info/archives/1665 (accessed on 18 July 2023).
- Zhou, Y.; Stanchev, P.; Katsou, E.; Awad, S.; Fan, M. A circular economy use of recovered sludge cellulose in wood plastic composite production: Recycling and eco-efficiency assessment. Waste Manag. 2019, 99, 42–48. [Google Scholar] [CrossRef]
- Marami, H.; He, L.; Rafiee, S.; Khoshnevisan, B.; Tsapekos, P.; Mobli, H.; Elyasi, S.N.; Liu, H.; Angelidaki, I. Bridging to circular bioeconomy through a novel biorefinery platform on a wastewater treatment plant. Ren. Sustain. Energy Rev. 2022, 154, 111895. [Google Scholar] [CrossRef]
- Callegari, A.; Hlavinek, P.; Capodaglio, A.G. Production of energy (biodiesel) and recovery of materials (biochar) from pyrolysis of urban waste sludge. Rev. Ambiente Agua 2018, 13, e2128. [Google Scholar] [CrossRef]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Marsalek, J.; Viklander, M. The pollution conveyed by urban runoff: A review of sources. Sci. Total Environ. 2020, 709, 136125. [Google Scholar] [CrossRef]
- Karvelas, M.; Katsoyiannis, A.; Samara, C. Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere 2003, 53, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, A.; Kimura, R.; Arai, S. Rare earth elements and other trace elements in wastewater treatment sludges. Soil Sci. Plant Nutr. 1998, 44, 433–441. [Google Scholar] [CrossRef]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kovač Kralj, A.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef]
- Westerhoff, P.; Lee, S.; Yang, Y.; Gordon, G.W.; Hristovski, K.; Halden, R.U.; Herckes, P. Characterization, recovery opportunities, and valuation of metals in municipal sludges from U.S. wastewater treatment plants nationwide. Environ. Sci. Technol. 2015, 49, 9479–9488. [Google Scholar] [CrossRef]
- Yesil, H.; Molaey, R.; Calli, B.; Tugtas, A.E. Removal and recovery of heavy metals from sewage sludge via three-stage integrated process. Chemosphere 2021, 280, 130650. [Google Scholar] [CrossRef]
- Carbonbrief. Climate Change Made 2022’s Northern-Hemisphere Droughts ‘at Least 20 Times’ More Likely. Available online: https://www.carbonbrief.org/climate-change-made-2022s-northern-hemisphere-droughts-at-least-20-times-more-likely/#:~:text=The%20summer%20of%202022%20saw,compound%20already%20high%20food%20prices (accessed on 18 December 2022).
- Bixio, D.; De Heyder, B.; Cikurel, H.; Muston, M.; Miska, V.; Joksimovic, D.; Schäfer, A.I.; Ravazzini, A.; Aharoni, A.; Savic, D.; et al. Municipal wastewater reclamation: Where do we stand? An overview of treatment technology and management practice. Water Sci. Technol. Water Supply 2005, 5, 77–85. [Google Scholar] [CrossRef]
- Zhu, Z.; Dou, J. Current status of reclaimed water in China: An overview. J. Water Reuse Desalin. 2018, 8, 293–307. [Google Scholar] [CrossRef]
- Kumar, A.; Goyal, K. Water reuse in India: Current perspective and future potential. Adv. Chem. Pollut. Environ. Manag. Prot. 2020, 6, 33–63. [Google Scholar]
- EU. Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Official Journal of the European Union, 5 June 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32020R0741 (accessed on 21 September 2023).
- US EPA. 2012 Guidelines for Water Reuse. EPA/600/R-12/618; Office of Wastewater Management, Office of Water: Washington, DC, USA, 2012.
- Tzanakakis, V.A.; Capodaglio, A.G.; Angelakis, A.N. Insights into Global Water Reuse Opportunities. Sustainability 2023, 15, 13007. [Google Scholar] [CrossRef]
- US EPA. 2017 Potable Reuse Compendium. (EPA/810/R-17/002); US Environmental Protection Agency, Office of Ground Water and Drinking Water, Office of Water: Washington, DC, USA, 2017.
- Luthy, R.G.; Wolfand, J.M.; Bradshaw, J.L. Urban Water Revolution: Sustainable Water Futures for California Cities. J. Environ. Eng. 2020, 146, 7. [Google Scholar] [CrossRef]
- Saliba, R.; Callieris, R.; D’Agostino, D.; Roma, R.; Scardigno, A. Stakeholders’ attitude towards the reuse of treated wastewater for irrigation in Mediterranean agriculture. Agric. Water Manag. 2018, 204, 60–68. [Google Scholar] [CrossRef]
- Katakojwala, R.; Mohan, S.V. A critical view on the environmental sustainability of biorefinery systems. Curr. Opin. Green Sustain. Chem. 2021, 27, 100392. [Google Scholar] [CrossRef]
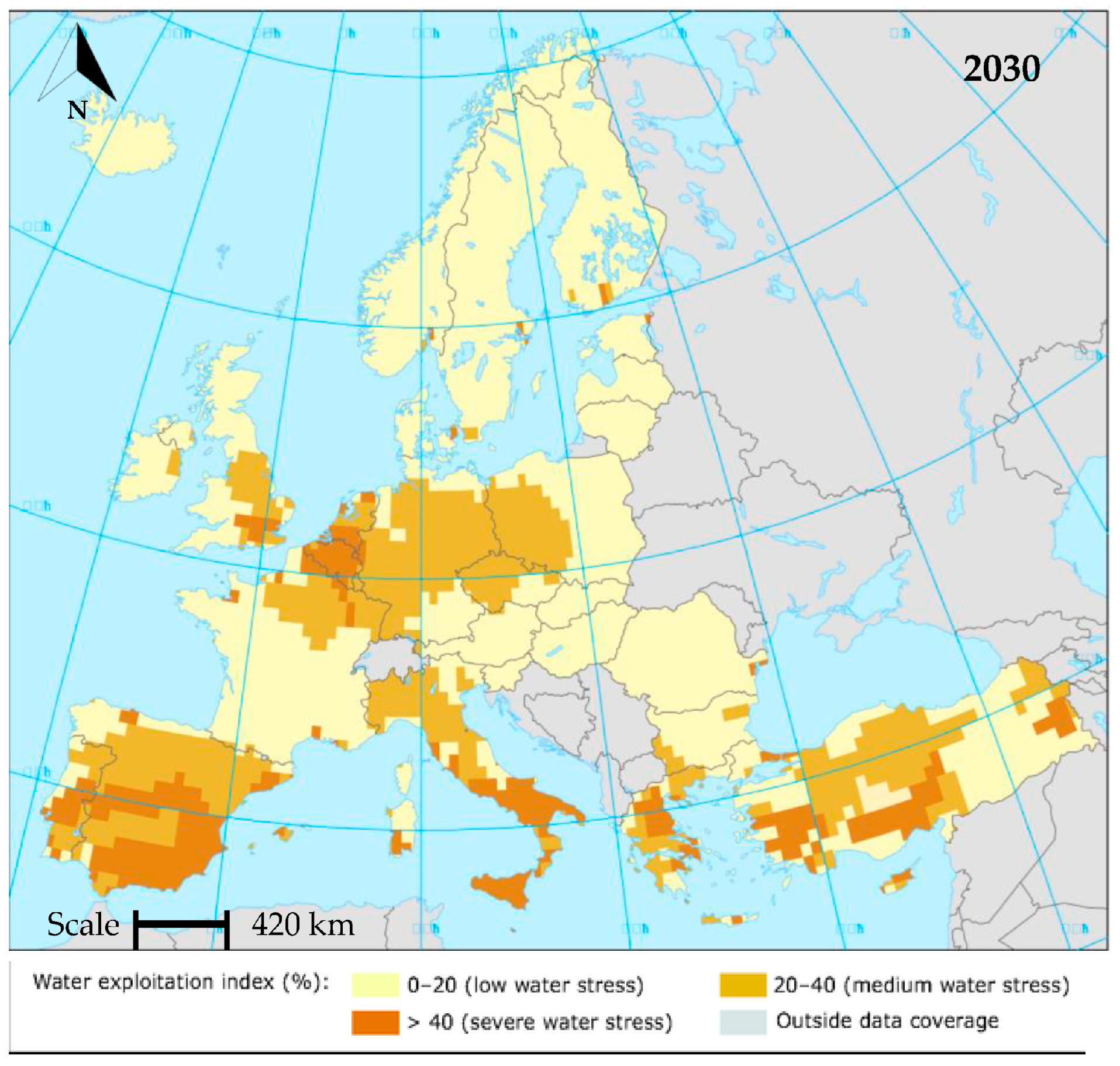
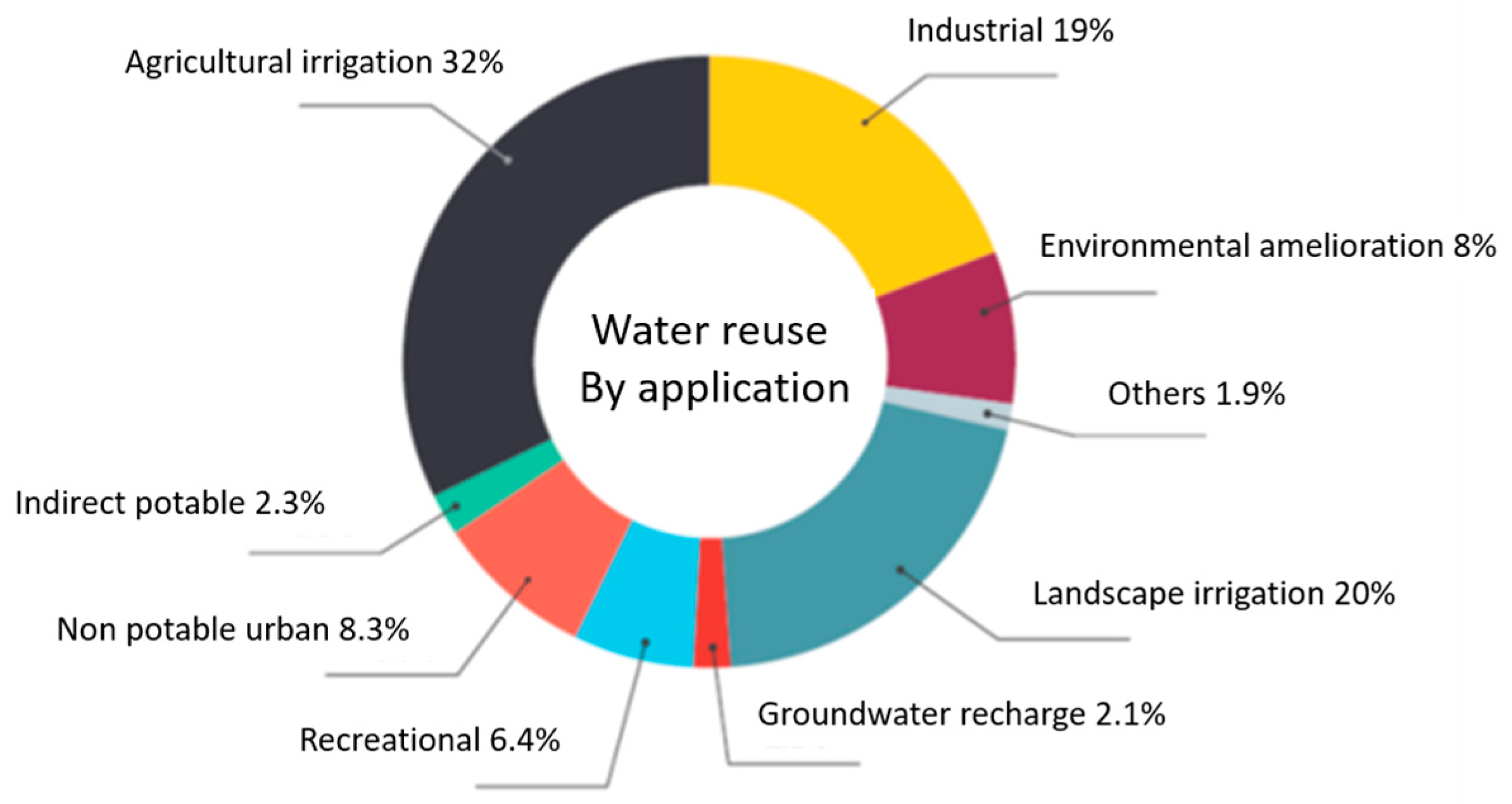
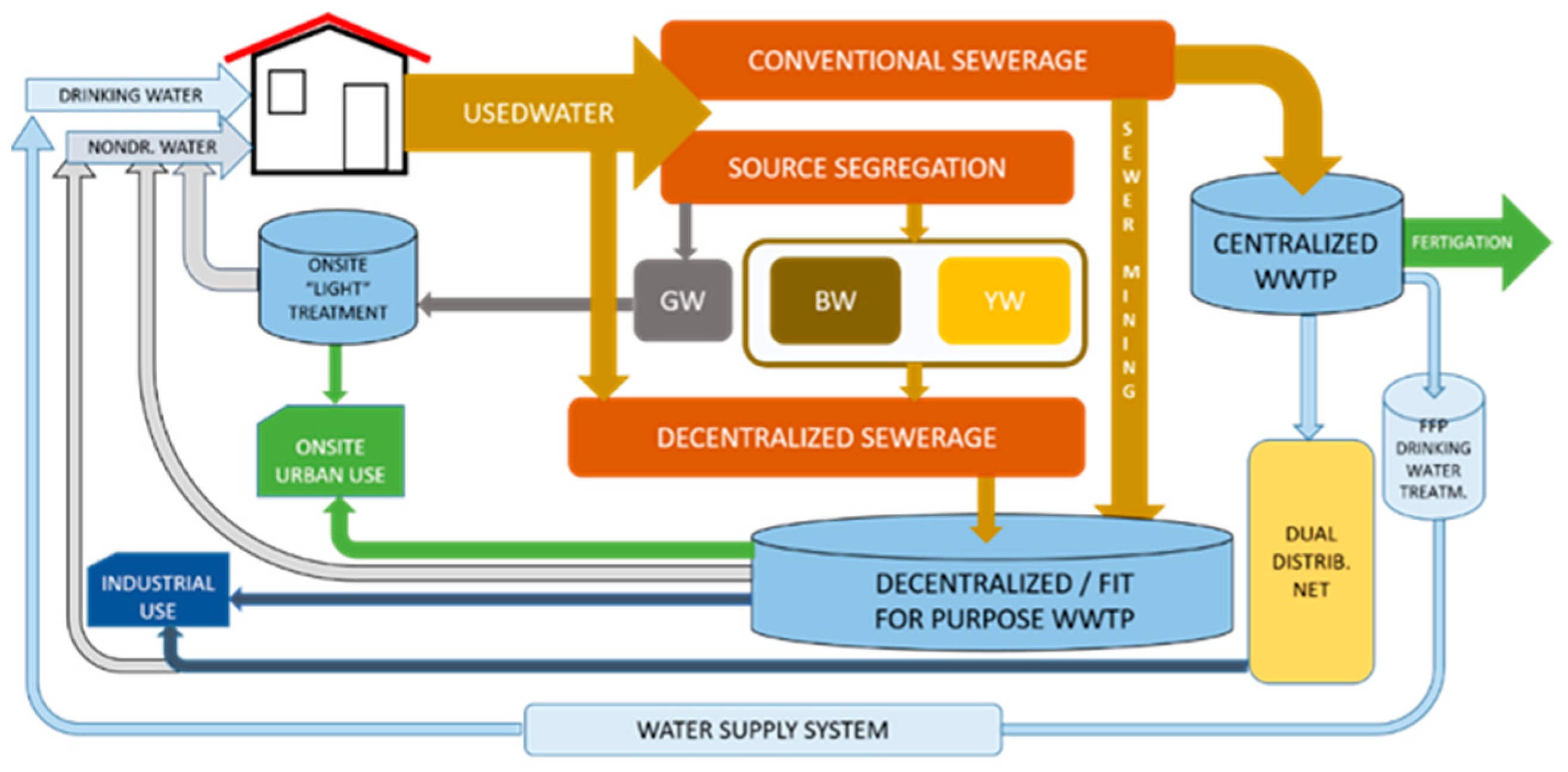



| Black Water (BW) | Grey Water (GW) | Yellow Water (YW) | |
|---|---|---|---|
| COD (mg/L) | 5000–93,000 | 200–500 | 4000–11,000 |
| N (mg/L) | 1500–16,000 | 6–25 | 4000–11,000 |
| P (mg/L) | 500–3000 | 0.4–8 | 200–4000 |
| Solids | High | Low | N/A |
| Pathogens | High | Low | High |
| Micropollutants | High | High | Low * |
| Type of Reuse | Pros | Cons | Comments | Ref. |
|---|---|---|---|---|
| Irrigation | Treated water reused for irrigation can reduce freshwater consumption. Irrigation with treated effluents can contribute N and P, necessary for crops. | Possibility of crop contamination by various contaminants, including emerging substances and pathogens. | Fertigation contributes water and nutrients to crops. Soil and crop types should be compatible with effluent characteristics. Stakeholders’ (farmers, consumers) perception and acceptance of this practice are essential. | [13,18,72,73] |
| Urban and industrial uses | Treated effluents are suitable for several types of non-potable reuse, allowing the use of high-quality freshwater for potable use. | Infrastructure is usually absent for dual water distribution. | Dual-distribution networks present in water-scarce areas. Fit-for-purpose treatment can provide reused water at competitive costs. Industrial, non-contact uses are usually well accepted. | [8,14,17,27,74,75,76,80] |
| Domestic uses | Onsite treated used water could provide about 2/3 of current domestic uses that do not require drinking water quality. | Dual piping in households required. | Acceptance of domestic reuse practices depends on water availability situations (scarcity conditions and cost of drinking water). Proper public communication is highly important. | [34,59,60,61,63,65,66] |
| Aquifer recharge and Indirect potable reuse | Replenishment of aquifers increases future availability of water and supply resilience. Low-cost practice. Impurities are naturally filtered by subsoil formations. | Hydrogeological conditions should be verified to avoid preferential contaminant transport. Excessive recharge may affect underground infrastructure. | Urbanization reduces aquifer recharge. MAR and similar approaches can restore hydrological groundwater balance. Aquifer recharge is part of many indirect potable reuse schemes. IPR schemes are often accepted as they “blend” with the natural water cycle. | [8,13,14,15,16,74,79,80] |
| Direct Potable reuse | Can provide drinking water to areas with critical water scarcity. This practice is a consolidated technology with many application examples globally. | Treatment can be energy-intensive. Citizens’ acceptance may initially be low. | Treatment technology can provide drinking water directly from WWTP effluents. DPR acceptance increases with water shortage and can be boosted by proper communication strategies. | [13,14,74,77,78] |
| Element/ Technology | Pros | Cons | Comments | Ref. |
|---|---|---|---|---|
| Ammonia/ stripping | Easy implementation. Various process forms exist with different recovery yields. | pH buffering and high temperatures may be required. | Vacuum thermal stripping reduces the required process temperature. | [124,125,126] |
| Ammonia/ adsorption | Zeolite adsorption easy to implement. High recovery (˃98%) even at low initial concentrations. | High chemical demand for regeneration. Filter suffers from fouling problems. | New polymer-based adsorbents may enhance recovery and cost-effectiveness. | [114,136] |
| Ammonia/ concentration | Membrane-based processes can recover pure ammonia (no metal, pathogen contamination). | Relatively energy intensive. | Forward and reverse osmosis, membrane distillation, and electrodialysis, alone or in combination, available at intermediate TRLs. | [127,128,129,130,131,132,133,134] |
| Ammonia/ others | Bioelectrochemical systems can achieve 100% ammonia recovery with a positive energy balance. | Technology more complex and costly than stripping/ adsorption. Low TRL (pilot scale only). | Material issues hinder BESs’ industrial development. Promising technology for the future. | [131,133] |
| Phosphorous/ precipitation | P precipitation occurs naturally in WWTPs. Controlled precipitation can avoid serious scaling problems. High TRL. | Efficient precipitation requires costly chemical addition and controlled conditions. A P-concentrated solution is needed. | Can be implemented from the liquid or solids line. Struvite or other fertilizer-value minerals can be obtained. Many proprietary commercial processes are available. Can be combined with P pre-concentration by membranes or electrochemical technologies. | [129,137,139,140,141,142,143,144,145,146,147] |
| Phosphorous/ leaching | Incinerated sludge waste is an available P source. Chemical extraction technologies available. | Chemical extraction may cause heavy metal leaching, in addition to P. This could create subsequent reuse problems. | Pre-treatment agents (e.g., EDTA) can achieve high-purity P leaching from ISSA. | [123] |
| Material/ Chemical | Pros | Cons | Comments | Ref. |
|---|---|---|---|---|
| Cellulose | Valuable raw industrial material. Easily recovered through physical means. Removal of cellulose from WWTP influent could decrease aeration requirements by up to 30%. | Requires additional equipment. Some recovery processes may require substantial energy and chemical input. | Cellulose can be valorized as raw polymeric material in industrial applications or as substrate for AD, the production of chemicals, etc. | [152,153,154,155,156,157,158,159] |
| Protein | Protein-rich biomass has an amino acid profile comparable to standard animal protein and could be a substitute for soybean meal. Has less of an environmental impact than conventional animal protein sources. | Requires dedicated processes. | Methane-oxidizing bacteria are MP-rich microorganisms that use methane as carbon and energy sources to assimilate nitrogen into proteins. Integrated WWTP/microbial protein systems have been demonstrated. | [160] |
| Biorefinery-derived Products (PHAs, EPSs, VFAs, biopesticides, enzymes, etc.) | Molecules, more industrially valuable than biogas, are produced through microbial metabolism and immobilized in biomass during wastewater treatment. | Bioferinery processes are generally complex, and TRL is generally too low for commercial adoption. | Appropriate sequencing of biorefinery processes could maximize recovery of value-added products and facilitate subsequent biosolid processing steps | [56,57,135,160,161] |
| Metals, REEs | WWTPs concentrate metals and REEs from wastewater into biosolids. Metal content in wastewater could be highly valuable. | Low concentrations limit the quantities of recoverable materials from the liquid phase. Some recovery methods from the sludge phase may generate hazardous residues, increasing waste treatment and disposal costs. | Integration of metal recovery into the sludge processing end of wastewater treatment could contribute to improving its CE footprint. | [21,162,163,164,165,166,167,168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capodaglio, A.G. Urban Wastewater Mining for Circular Resource Recovery: Approaches and Technology Analysis. Water 2023, 15, 3967. https://doi.org/10.3390/w15223967
Capodaglio AG. Urban Wastewater Mining for Circular Resource Recovery: Approaches and Technology Analysis. Water. 2023; 15(22):3967. https://doi.org/10.3390/w15223967
Chicago/Turabian StyleCapodaglio, Andrea G. 2023. "Urban Wastewater Mining for Circular Resource Recovery: Approaches and Technology Analysis" Water 15, no. 22: 3967. https://doi.org/10.3390/w15223967







