Quinoid Redox Mediators and Their Involvement in Environmental Pollution Treatment
Abstract
:1. Introduction
2. Quinone-Based Redox Mediators
2.1. Natural Substances
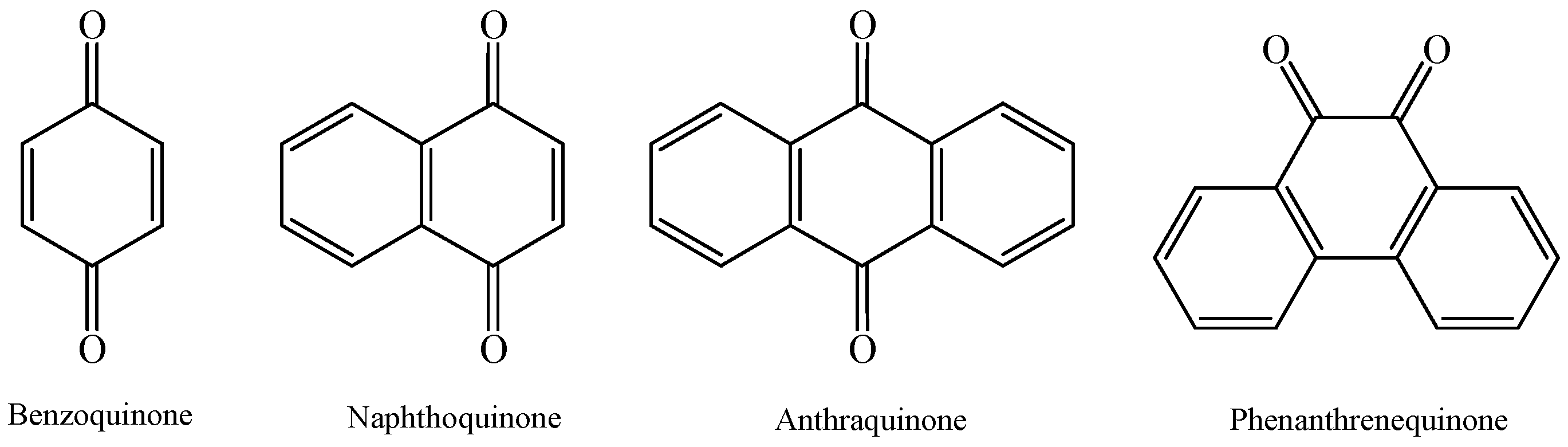
2.2. Artificially Synthesized Quinone Compounds
2.3. Microbial Secretions That Function as QRMs
2.4. Immobilized QRMs
3. Involvement of QRMs in Environmental Pollution Treatment
3.1. Biotransformation of Recalcitrant Organic Pollutants
3.2. Biotransformation of Heavy Metal Ions and Metallic Oxides
3.3. Enhancement of Microbial Fuel Cells
3.4. Activity Description of QRMs and Their Disposal after Use
4. Conclusions and Perspectives
- (i)
- More practical immobilized QRMs should be developed. Over the past few decades, diverse QRMs have been found to be capable of accelerating environmental remediation efficiency. For engineering applications, QRMs can be immobilized with many carriers. However, some immobilized mediators are not practical. Thus, we need to pay more attention to their practicality (including cost and ease of recycling and reuse).
- (ii)
- How to select the suitable QRM? In the actual reaction system, the catalytic effects of QRMs are highly affected by many reaction parameters, including redox potential, electron transfer capacity, diffusive rate, and even environmental factors (e.g., pH, temperature, and salinity). Therefore, we should carry out more studies to determine what kind of QRMs should be chosen for different reaction systems.
- (iii)
- What are the molecular mechanism of the QRMs-catalyzed biotransformation process? Although the electron transfer mechanism of electrochemically active bacteria, especially for the genera Geobacter and Shewanella, has been studied widely, the interaction between cells and QRMs, and the related molecular mechanism, remain to be further understood. Moreover, QRM-catalyzed long-distance electron transfer is still unclear at the molecular level. Thus, the molecular mechanisms of QRM-catalyzed biotransformation processes should be studied further.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roxon, J.J.; Ryan, A.J.; Wright, S.E. Enzymatic reduction of tartrazine by Proteus vulgaris from rats. Food Cosmet. Toxicol. 1967, 5, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.; Husain, Q. Applications of Redox Mediators in the Treatment of Organic Pollutants by Using Oxidoreductive Enzymes: A Review. Crit. Rev. Environ. Sci. Technol. 2008, 38, 1–42. [Google Scholar] [CrossRef]
- Lovley, D.R.; Bluntharris, E.L.; Ejp, P.; Woodward, J.C.; Coates, J.D. Humic substances as electron acceptors for microbial respiration. Nature 1996, 382, 445–448. [Google Scholar] [CrossRef]
- Lovley, D.R.; Fraga, J.L.; Coates, J.D.; Blunt-Harris, E.L. Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1999, 1, 89–98. [Google Scholar] [CrossRef]
- Van der Zee, F.P.; Cervantes, F.J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Liu, W.; Chen, Y. New applications of quinone redox mediators: Modifying nature-derived materials for anaerobic biotransformation process. Sci. Total Environ. 2020, 744, 140652. [Google Scholar] [CrossRef]
- Rau, J.; Knackmuss, H.J.; Stolz, A. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria. Environ. Sci. Technol. 2002, 36, 1497–1504. [Google Scholar] [CrossRef]
- van der Zee, F.P.; Bouwman, R.H.; Strik, D.P.; Lettinga, G.; Field, J.A. Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnol. Bioeng. 2001, 75, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Zhou, J.; Yang, F.; Jin, C.; Qu, Y.; Li, A.; Zhang, L. Enhancement of nitroaromatic compounds anaerobic biotransformation using a novel immobilized redox mediator prepared by electropolymerization. Bioresour. Technol. 2008, 99, 6908–6916. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.S.; Barkovskii, A.L.; Adriaens, P. Reductive transformation of dioxins: An assessment of the contribution of dissolved organic matter to dechlorination reactions. Environ. Sci. Technol. 1999, 33, 3837–3842. [Google Scholar] [CrossRef]
- Perlinger, J.A.; Angst, W.; Schwarzenbach, R.P. Kinetics of the reduction of hexachloroethane by juglone in solutions containing hydrogen sulfide. Environ. Sci. Technol. 1996, 30, 3408–3417. [Google Scholar] [CrossRef]
- Wolf, M.; Kappler, A.; Jiang, J.; Meckenstock, R.U. Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ. Sci. Technol. 2009, 43, 5679–5685. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.; Wang, J.; Jin, R.; Zhou, J.; Lv, H. Enhanced chromate reduction by resting Escherichia coli cells in the presence of quinone redox mediators. Bioresour. Technol. 2010, 101, 8127–8131. [Google Scholar] [CrossRef]
- Wang, X.; Liu, G.; Zhou, J.; Wang, J.; Jin, R.; Lv, H. Quinone-mediated reduction of selenite and tellurite by Escherichia coli. Bioresour. Technol. 2011, 102, 3268–3271. [Google Scholar] [CrossRef]
- Aranda-Tamaura, C.; Estrada-Alvarado, M.I.; Texier, A.C.; Cuervo, F.; Gómez, J.; Cervantes, F.J. Effects of different quinoid redox mediators on the removal of sulphide and nitrate via denitrification. Chemosphere 2007, 69, 1722–1727. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R.; Cortinas, I.; Feijoo, G.; Moreira, M.T.; Kopplin, M.; Gandolfi, A.J. Facile reduction of arsenate in methanogenic sludge. Biodegradation 2004, 15, 185–196. [Google Scholar] [CrossRef]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P. Reduction of polymeric azo and nitro dyes by intestinal bacteria. Appl. Environ. Microbiol. 1981, 41, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Stolz, A. Oxygen-insensitive nitroreductases NfsA and NfsB of Escherichia coli function under anaerobic conditions as lawsone-dependent azo reductases. Appl. Environ. Microbiol. 2003, 69, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.D.; Bisschops, I.A.; Cervantes, F.J.; van Lier, J.B. Effect of different redox mediators during thermophilic azo dye reduction by anaerobic granular sludge and comparative study between mesophilic (30 °C) and thermophilic (55 °C) treatments for decolourisation of textile wastewaters. Chemosphere 2004, 55, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, L.; Gu, J.-D. Microbial electrocatalysis: Redox mediators responsible for extracellular electron transfer. Biotechnol. Adv. 2018, 36, 1815–1827. [Google Scholar] [CrossRef]
- Smith, J.A.; Nevin, K.P.; Lovley, D.R. Syntrophic growth via quinone-mediated interspecies electron transfer. Front. Microbiol. 2015, 6, 121. [Google Scholar] [CrossRef]
- Ratasuk, N.; Nanny, M.A. Characterization and quantification of reversible redox sites in humic substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef] [PubMed]
- Kaden, J.; Galushko, A.S.; Schink, B. Cysteine-mediated electron transfer in syntrophic acetate oxidation by cocultures of Geobacter sulfurreducens and Wolinella succinogenes. Arch. Microbiol. 2002, 178, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.G.; Guo, J.; Xu, Z.-C.; Xu, M.-Y.; Sun, G.-P. Humic substances act as electron acceptor and redox mediator for microbial dissimilatory azoreduction by Shewanella decolorationis S12. J. Microbiol. Biotechnol. 2007, 17, 428–437. [Google Scholar] [PubMed]
- Zhou, G.W.; Yang, X.-R.; Li, H.; Marshall, C.W.; Zheng, B.-X.; Yan, Y.; Su, J.-Q.; Zhu, Y.G. Electron shuttles enhance anaerobic ammonium oxidation coupled to iron(III) reduction. Environ. Sci. Technol. 2016, 50, 9298–9307. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Li, P.; Lu, H.; Jin, R.; Liu, G. Effects of redox mediators on anaerobic degradation of phenol by Shewanella sp. XB. Appl. Biochem. Biotechnol. 2015, 175, 3162–3172. [Google Scholar] [CrossRef] [PubMed]
- Bosire, E.M.; Rosenbaum, M.A. Electrochemical potential influences phenazine production, electron transfer and consequently electric current generation by Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Light, S.H.; Su, L.; Rivera-Lugo, R.; Cornejo, J.A.; Louie, A.; Iavarone, A.T.; Ajo-Franklin, C.M.; Portnoy, D.A. A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 2018, 562, 140–144. [Google Scholar] [CrossRef]
- Shi, Z.; Zachara, J.M.; Shi, L.; Wang, Z.; Moore, D.A.; Kennedy, D.W.; Fredrickson, J.K. Redox reactions of reduced flavin mononucleotide (FMN), riboflavin (RBF), and anthraquinone-2, 6-disulfonate (AQDS) with ferrihydrite and lepidocrocite. Environ. Sci. Technol. 2012, 46, 11644–11652. [Google Scholar] [CrossRef]
- Wang, Y.; Newman, D.K. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol. 2008, 42, 2380–2386. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Z.; Shi, L.; White, G.F.; Richardson, D.J.; Clarke, T.A.; Fredrickson, J.K.; Zachara, J.M. Effects of soluble flavin on heterogeneous electron transfer between surface-exposed bacterial cytochromes and iron oxides. Geochim. Cosmochim. Acta 2015, 163, 299–310. [Google Scholar] [CrossRef]
- Tian, T.; Fan, X.; Feng, M.; Su, L.; Zhang, W.; Chi, H.; Fu, D. Flavin-mediated extracellular electron transfer in Gram-positive bacteria Bacillus cereus DIF1 and Rhodococcus ruber DIF2. RSC Adv. 2019, 9, 40903–40909. [Google Scholar] [CrossRef]
- Ackerley, D.; Gonzalez, C.; Keyhan, M.; Blake, R.; Matin, A. Mechanism of chromate reduction by the Escherichia coli protein, NfsA, and the role of different chromate reductases in minimizing oxidative stress during chromate reduction. Environ. Microbiol. 2004, 6, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, J.; Jin, R.; Zhou, M.; Wang, J.; Lu, H.; Qu, Y. Enhancing survival of Escherichia coli by expression of azoreductase AZR possessing quinone reductase activity. Appl. Microbiol. Biotechnol. 2008, 80, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhou, J.; Lv, H.; Xiang, X.; Wang, J.; Zhou, M.; Qv, Y. Azoreductase from Rhodobacter sphaeroides AS1. 1737 is a flavodoxin that also functions as nitroreductase and flavin mononucleotide reductase. Appl. Microbiol. Biotechnol. 2007, 76, 1271–1279. [Google Scholar] [CrossRef]
- Morokutti, A.; Lyskowski, A.; Sollner, S.; Pointner, E.; Fitzpatrick, T.B.; Kratky, C.; Gruber, K.; Macheroux, P. Structure and function of YcnD from Bacillus subtilis, a flavin-containing oxidoreductase. Biochemistry 2005, 44, 13724–13733. [Google Scholar] [CrossRef]
- Park, C.; Keyhan, M.; Wielinga, B.; Fendorf, S.; Matin, A. Purification to homogeneity and characterization of a novel Pseudomonas putida chromate reductase. Appl. Environ. Microbiol. 2000, 66, 1788–1795. [Google Scholar] [CrossRef]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial chromate reductase, a potential enzyme for bioremediation of hexavalent chromium: A review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef]
- Vorontsov, I.I.; Minasov, G.; Brunzelle, J.S.; Shuvalova, L.; Kiryukhina, O.; Collart, F.R.; Anderson, W.F. Crystal structure of an apo form of Shigella flexneri ArsH protein with an NADPH-dependent FMN reductase activity. Protein Sci. 2007, 16, 2483–2490. [Google Scholar] [CrossRef]
- Gu, B.; Yan, H.; Zhou, P.; Watson, D.B.; Park, M.; Istok, J. Natural humics impact uranium bioreduction and oxidation. Environ. Sci. Technol. 2005, 39, 5268–5275. [Google Scholar] [CrossRef]
- Luan, F.; Burgos, W.D.; Xie, L.; Zhou, Q. Bioreduction of nitrobenzene; natural organic matter, and hematite by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2010, 44, 184–190. [Google Scholar] [CrossRef]
- O’Loughlin, E.J. Effects of electron transfer mediators on the bioreduction of lepidocrocite (γ-FeOOH) by Shewanella putrefaciens CN32. Environ. Sci. Technol. 2008, 42, 6876–6882. [Google Scholar] [CrossRef]
- Alvarez, L.; Perez-Cruz, M.; Rangel-Mendez, J.; Cervantes, F. Immobilized redox mediator on metal-oxides nanoparticles and its catalytic effect in a reductive decolorization process. J. Hazard. Mater. 2010, 184, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.J.; Garcia-Espinosa, A.; Moreno-Reynosa, M.A.; Rangel-Mendez, J.R. Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes. Environ. Sci. Technol. 2010, 44, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, J.; Wang, J.; Yang, F.; Jin, C.; Zhang, G. Anaerobic biotransformation of azo dye using polypyrrole/anthraquinonedisulphonate modified active carbon felt as a novel immobilized redox mediator. Sep. Purif. Technol. 2009, 66, 375–382. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, H.; Wang, J.; Zhou, J.; Zhou, Y. A novel quinone/reduced graphene oxide composite as a solid-phase redox mediator for chemical and biological Acid Yellow 36 reduction. RSC Adv. 2014, 4, 47297–47303. [Google Scholar] [CrossRef]
- Zhang, H.K.; Hu, X. Catalytic reduction of NACs by nano Fe3O4/quinone composites in the presence of a novel marine exoelectrogenic bacterium under hypersaline conditions. RSC Adv. 2017, 7, 11852–11861. [Google Scholar] [CrossRef]
- Zhang, H.K.; Lu, H.; Zhang, S.; Liu, G.; Li, G.; Zhou, J.; Wang, J. A novel modification of poly (ethylene terephthalate) fiber using anthraquinone-2-sulfonate for accelerating azo dyes and nitroaromatics removal. Sep. Purif. Technol. 2014, 132, 323–329. [Google Scholar] [CrossRef]
- Zhang, H.K.; Lu, H.; Wang, J.; Zhou, J.T.; Sui, M. Cr(VI) reduction and Cr(III) immobilization by Acinetobacter sp. HK-1 with the assistance of a novel quinone/graphene oxide composite. Environ. Sci. Technol. 2014, 48, 12876–12885. [Google Scholar] [CrossRef] [PubMed]
- Ju, K.-S.; Parales, R.E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Nitroaromatic compounds: Environmental toxicity, carcinogenicity, mutagenicity, therapy and mechanism. J. Appl. Toxicol. 2014, 34, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Cui, D.; Zhang, M.; Wang, J.; Wang, H.; Zhao, M. Effect of quinoid redox mediators during azo dye decolorization by anaerobic sludge: Considering the catalyzing mechanism and the methane production. Ecotoxicol. Environ. Saf. 2020, 202, 110859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, K.; Hu, X. Enhanced bioreduction of 2, 5-dichlorobenzene by an AHQ/RGO binary nanocomposite through a synergistic effect with outer membrane proteins of Shewanella oneidensis MR-1. Chem. Eng. J. 2020, 389, 124464. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Kostandarithes, H.M.; Li, S.; Plymale, A.E.; Daly, M. Reduction of Fe(III), Cr(VI), U(VI), and Tc(VII) by Deinococcus radiodurans R1, Applied and environmental microbiology. Appl. Environ. Microbiol. 2000, 66, 2006–2011. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J. Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef]
- He, Q.; Sanford, R.A. Characterization of Fe (III) reduction by chlororespiring Anaeromxyobacter dehalogenans. Appl. Environ. Microbiol. 2003, 69, 2712–2718. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, I.S.; Gadd, G.M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, T.; Li, X.; Li, F. Exogenous electron shuttle-mediated extracellular electron transfer of Shewanella putrefaciens 200: Electrochemical parameters and thermodynamics. Environ. Sci. Technol. 2014, 48, 9306–9314. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, J.; Song, Y.; Chen, Z.; Zhao, R. Acceleration mechanism of bioavailable Fe(III) on Te(IV) bioreduction of Shewanella oneidensis MR-1: Promotion of electron generation, electron transfer and energy level. J. Hazard. Mater. 2020, 403, 123728. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Cheng, M.; Li, Y.; Chen, W. Quinoid Redox Mediators and Their Involvement in Environmental Pollution Treatment. Water 2023, 15, 3981. https://doi.org/10.3390/w15223981
Zhang H, Cheng M, Li Y, Chen W. Quinoid Redox Mediators and Their Involvement in Environmental Pollution Treatment. Water. 2023; 15(22):3981. https://doi.org/10.3390/w15223981
Chicago/Turabian StyleZhang, Haikun, Manman Cheng, Yan Li, and Wenhao Chen. 2023. "Quinoid Redox Mediators and Their Involvement in Environmental Pollution Treatment" Water 15, no. 22: 3981. https://doi.org/10.3390/w15223981
APA StyleZhang, H., Cheng, M., Li, Y., & Chen, W. (2023). Quinoid Redox Mediators and Their Involvement in Environmental Pollution Treatment. Water, 15(22), 3981. https://doi.org/10.3390/w15223981





