Quantitative Morphometric Analysis of Morphologically Similar Species of Fragilaria (Fragilariaceae, Bacillariophyta) Allows Detection of Non-Indigenous Taxa: A Case Study from Lake Ladoga (North of European Russia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Scanning Electron and Light Microscopy
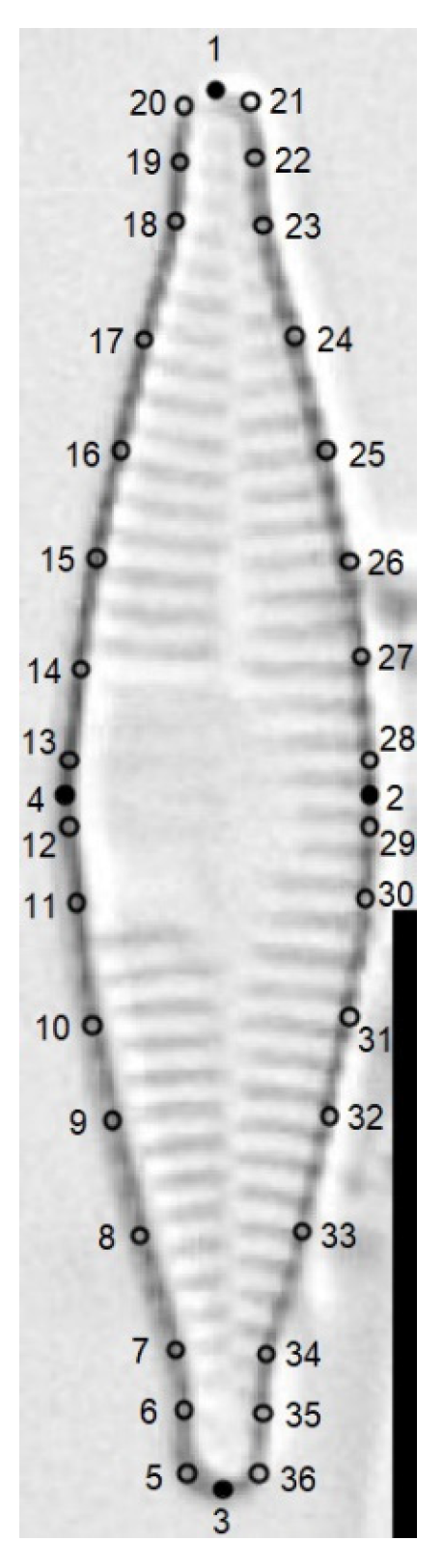
2.3. Morphological Analysis
2.4. Multivariate Statistical Analysis
2.5. Allometric Variability and Size Correction
3. Results
3.1. Microscopical Analysis
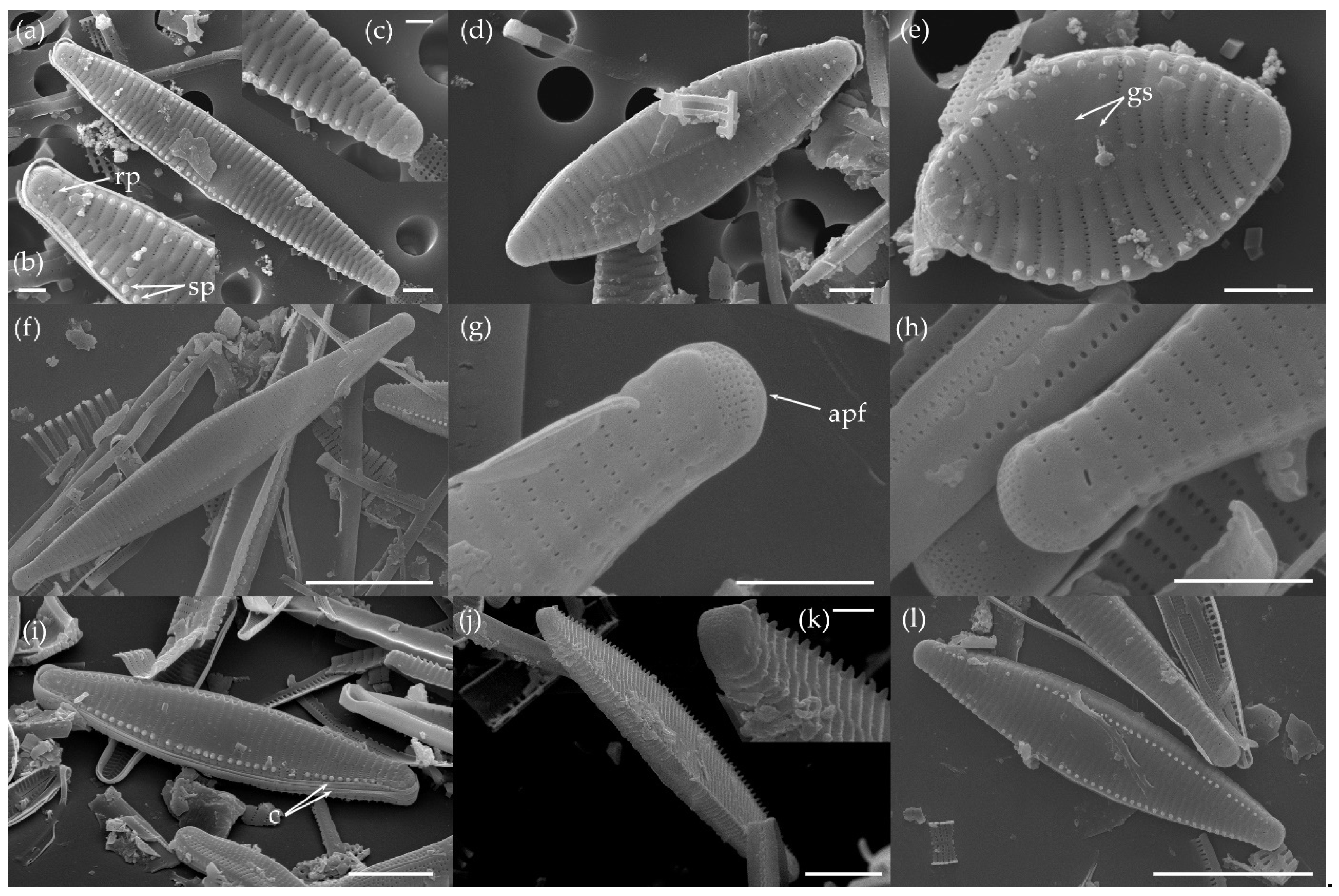
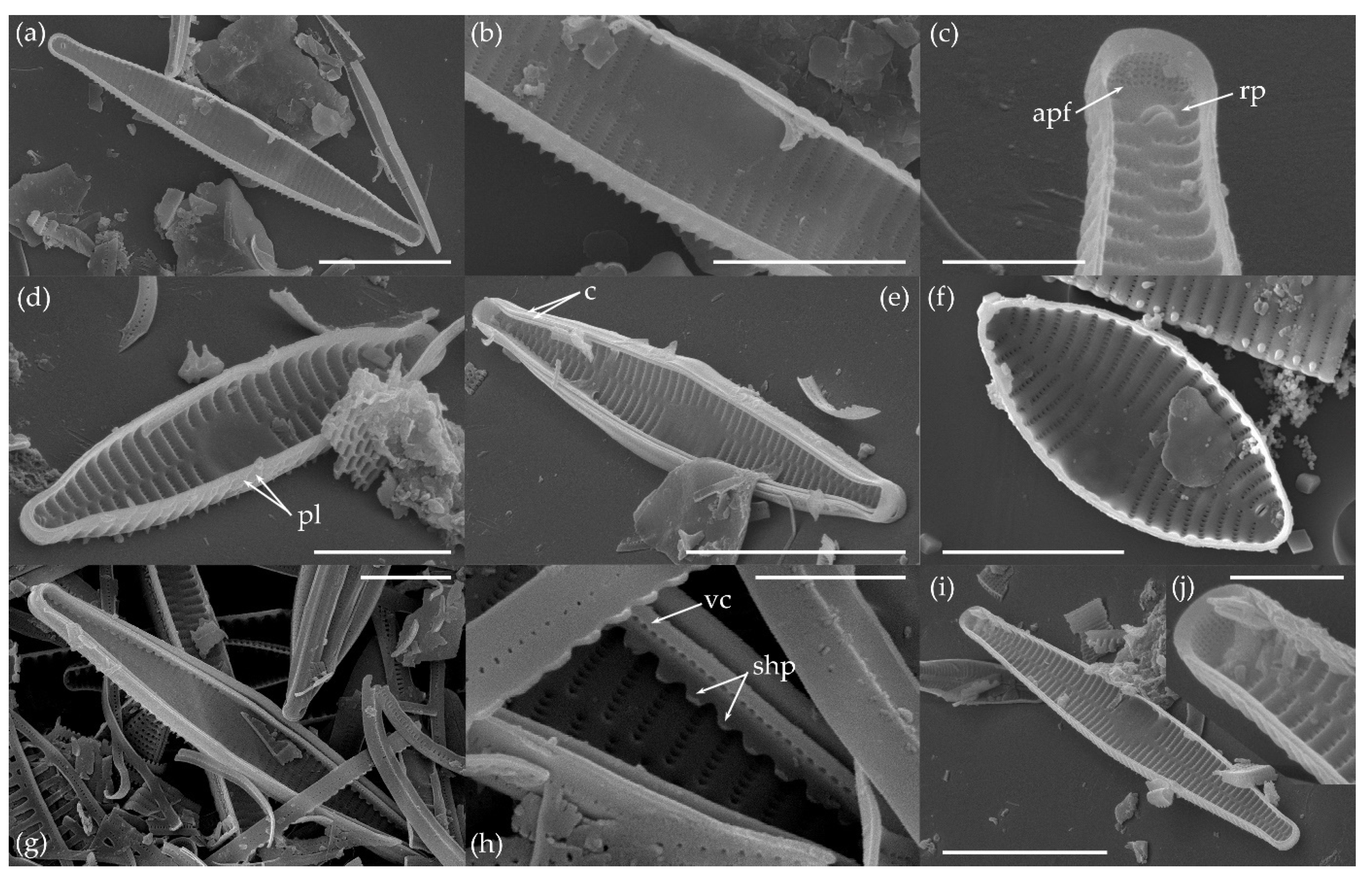
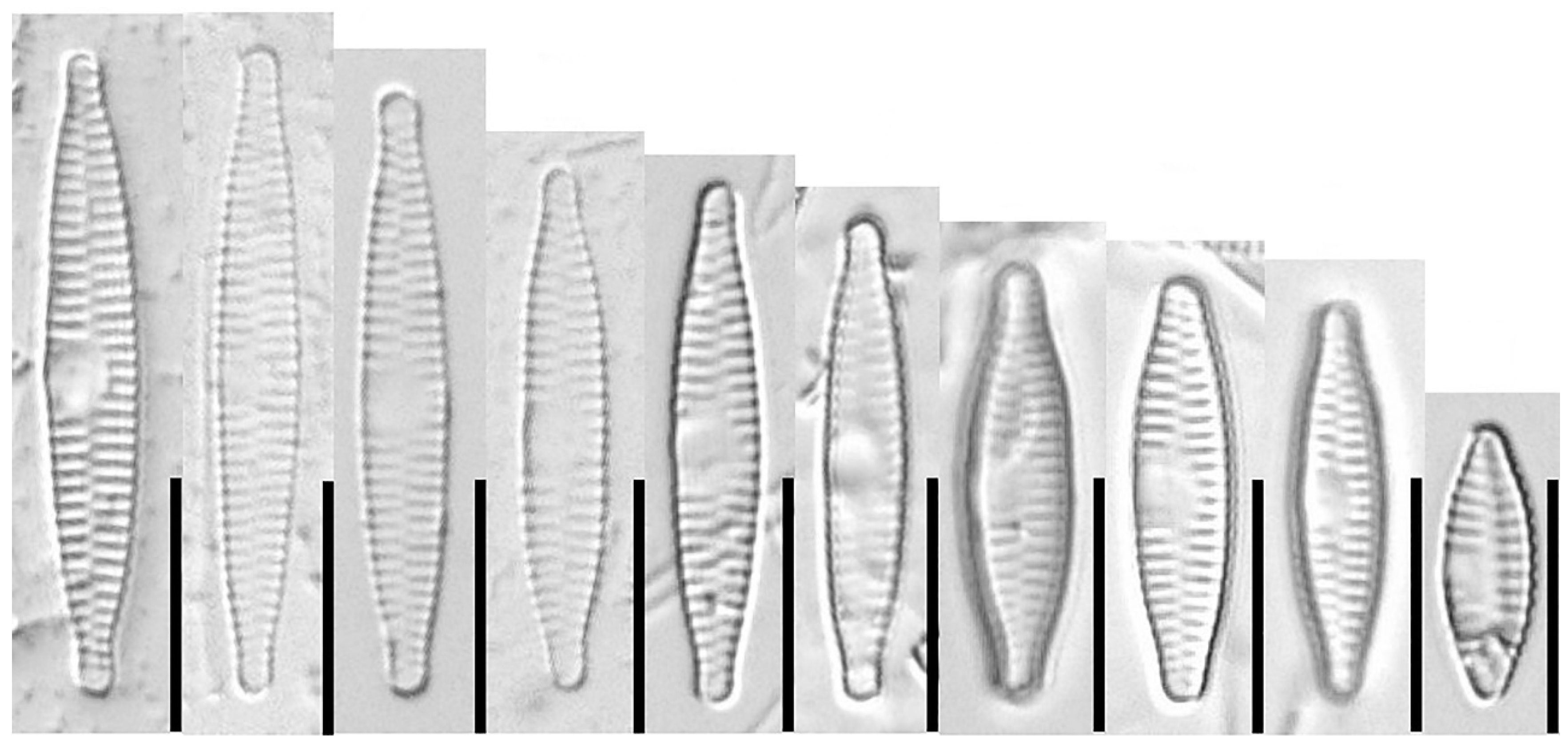
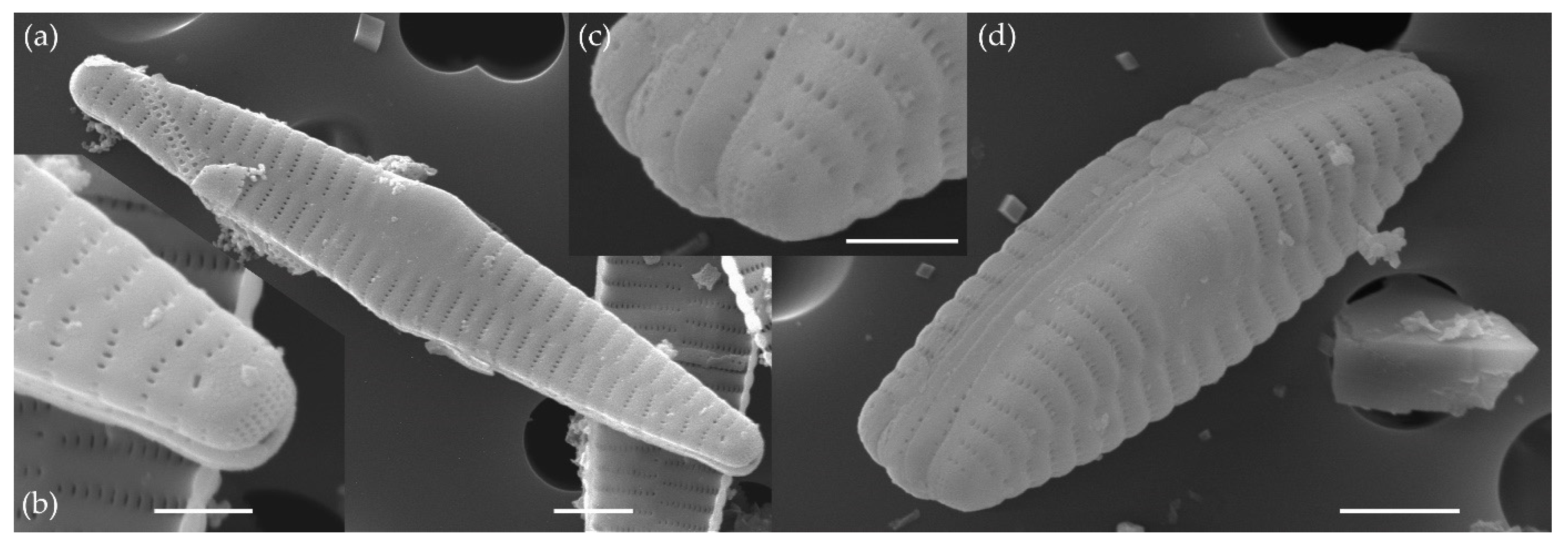
3.1.1. Fragilaria sublanceolata-baikali (Flower et D. M. Williams) Novais, C. Delgado et S. Blanco (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6)
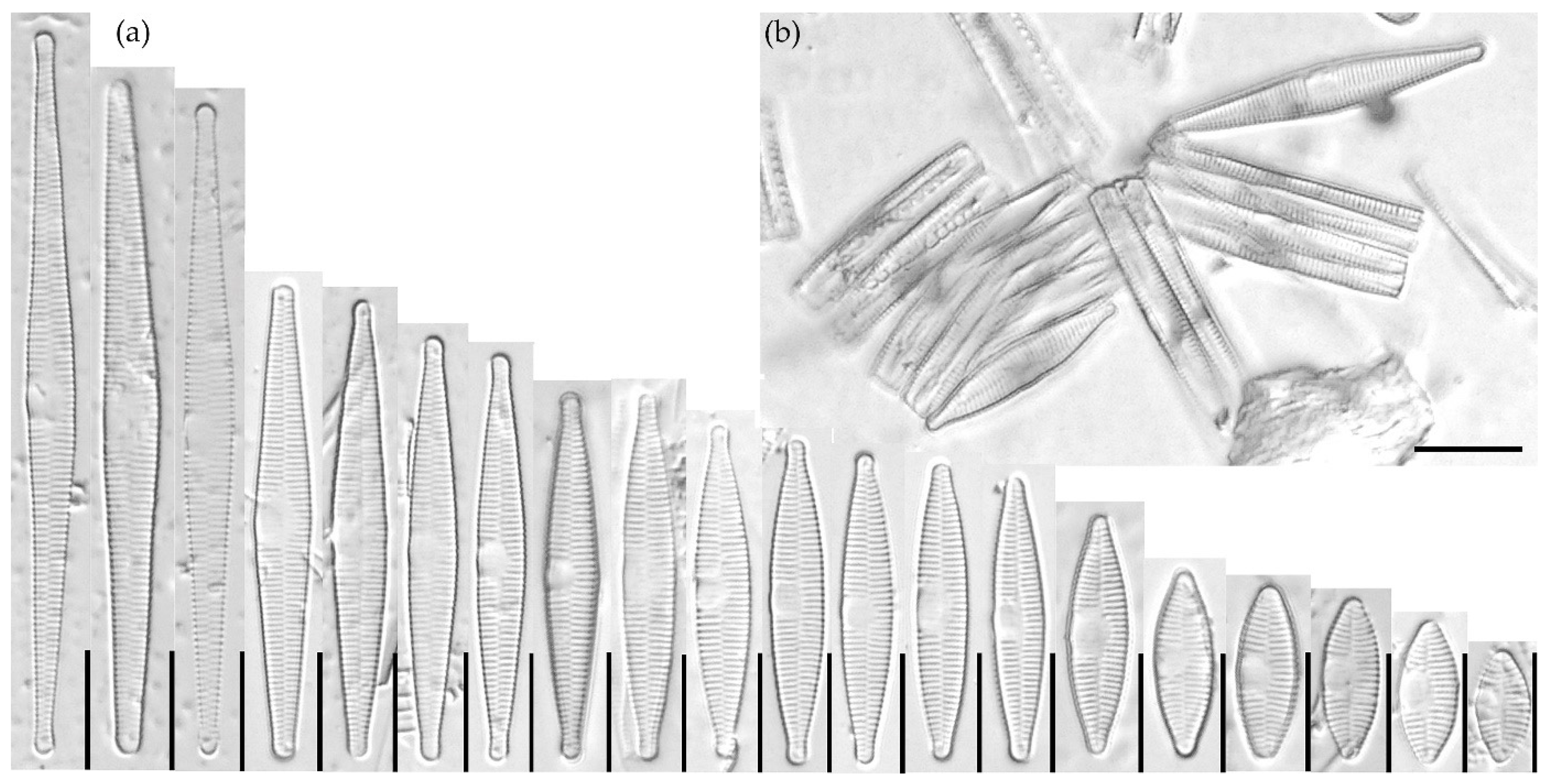
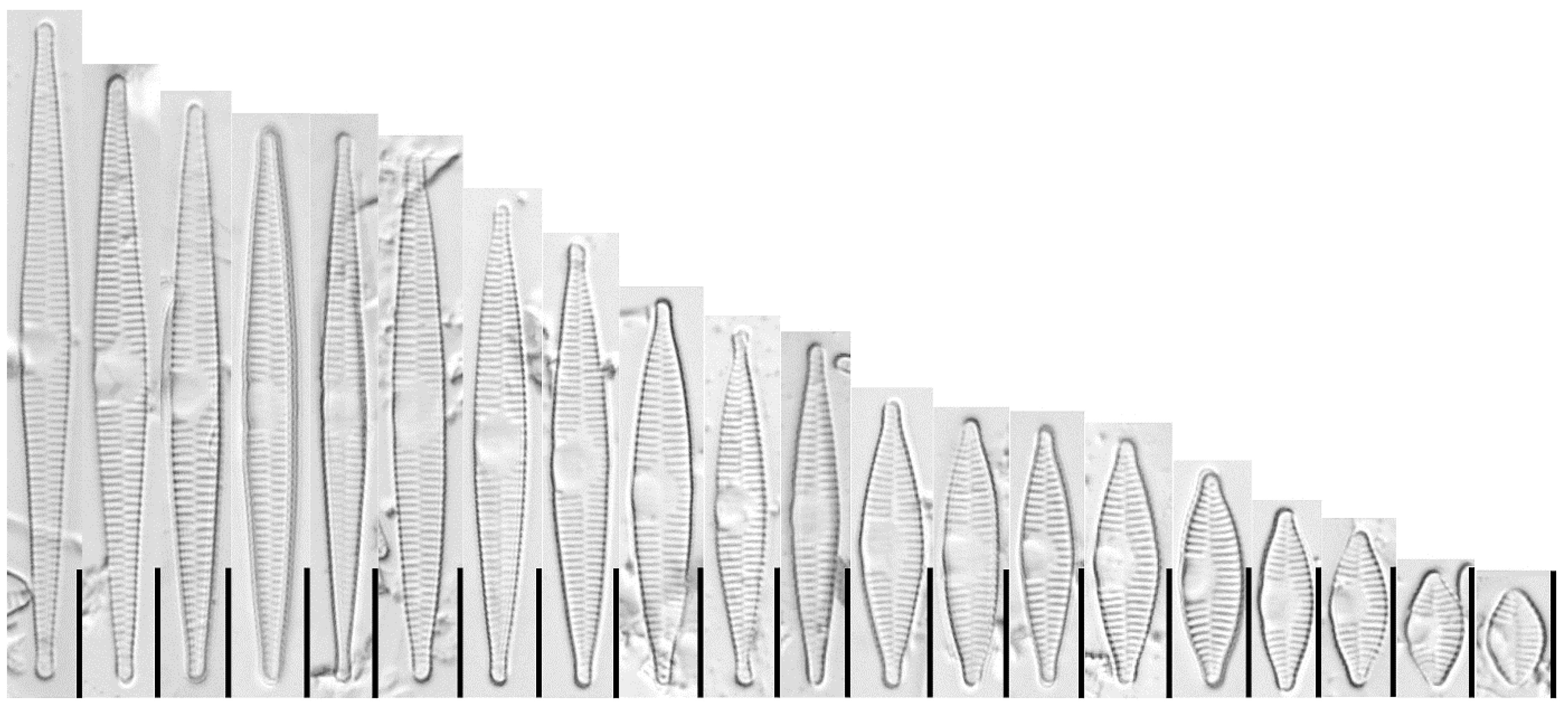
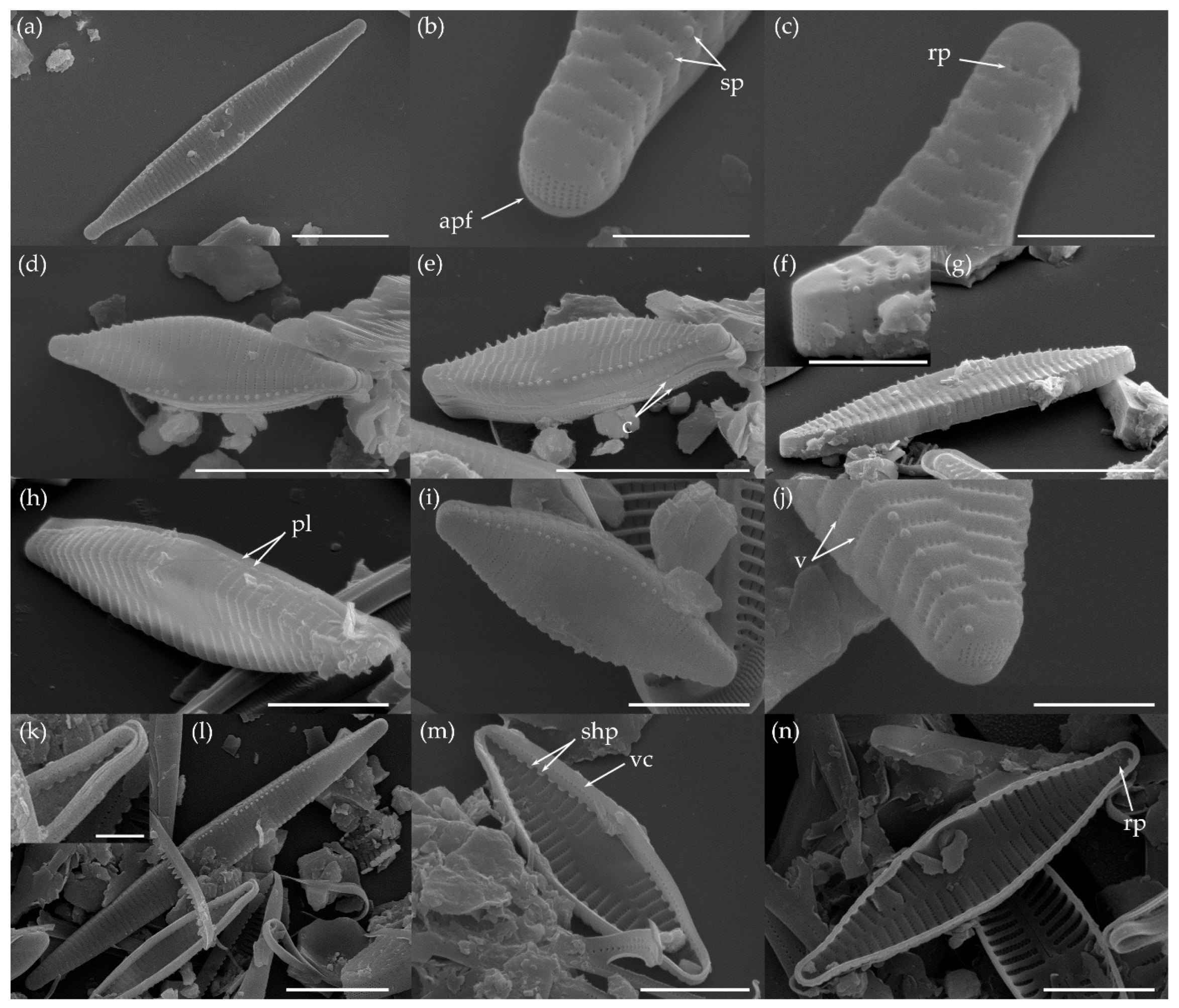
3.1.2. Fragilaria pectinalis (O. F. Müll.) Lyngb. (Figure 7 and Figure 8)
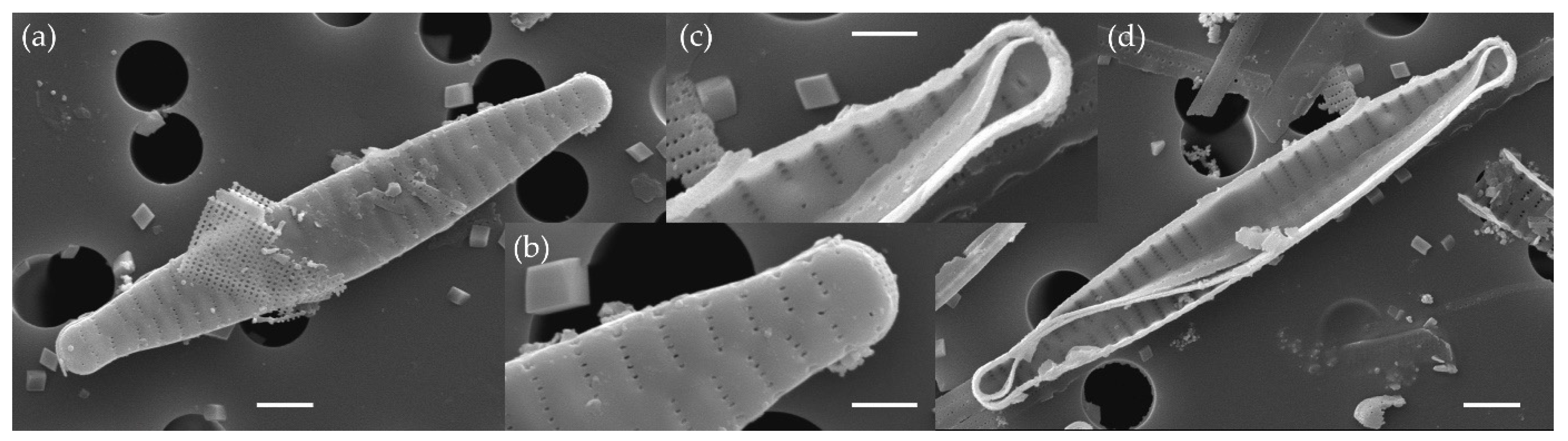
3.1.3. Fragilaria perminuta (Grunow) Lange-Bertalot (Figure 9 and Figure 10)
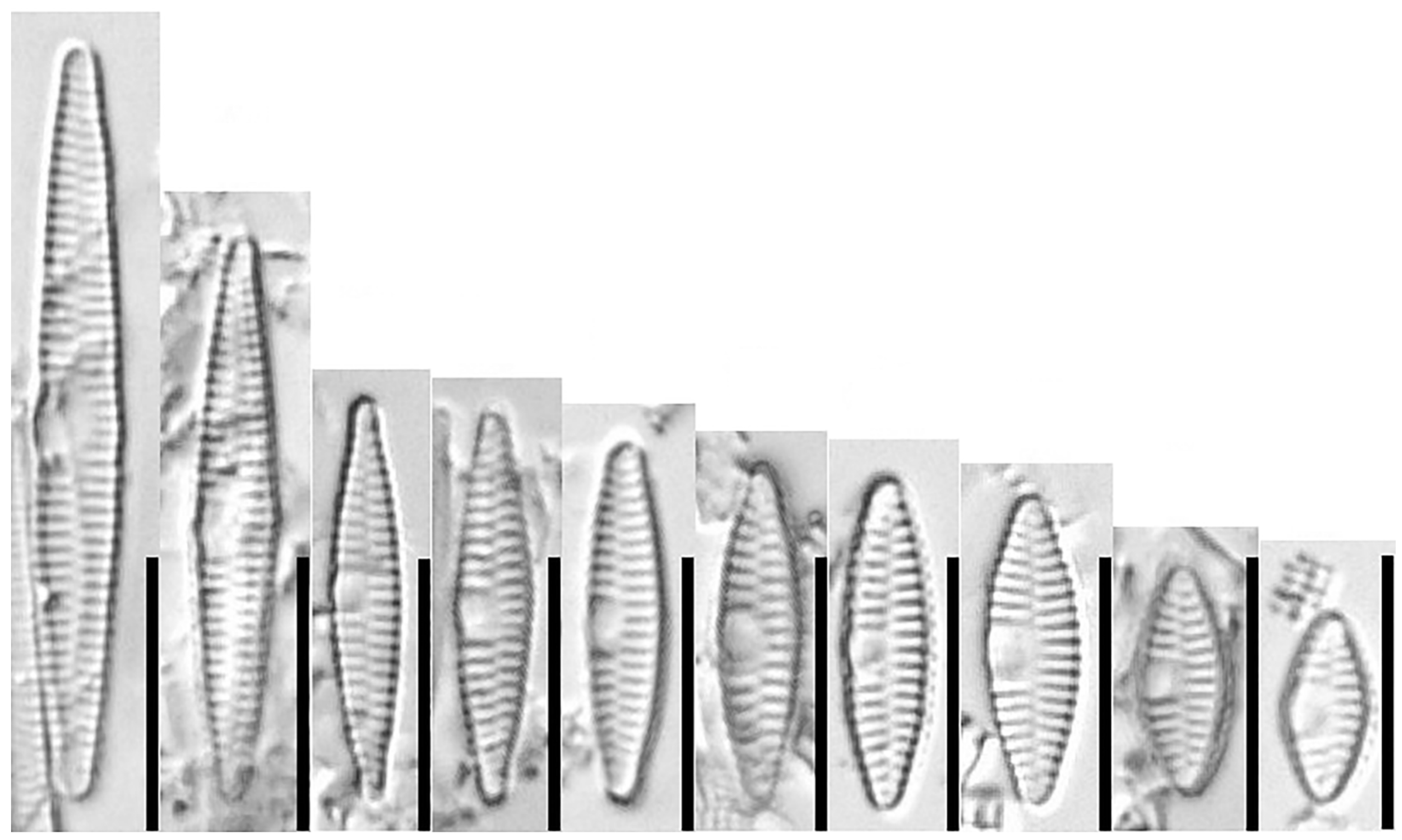
3.2. Traditional Morphometric Analysis
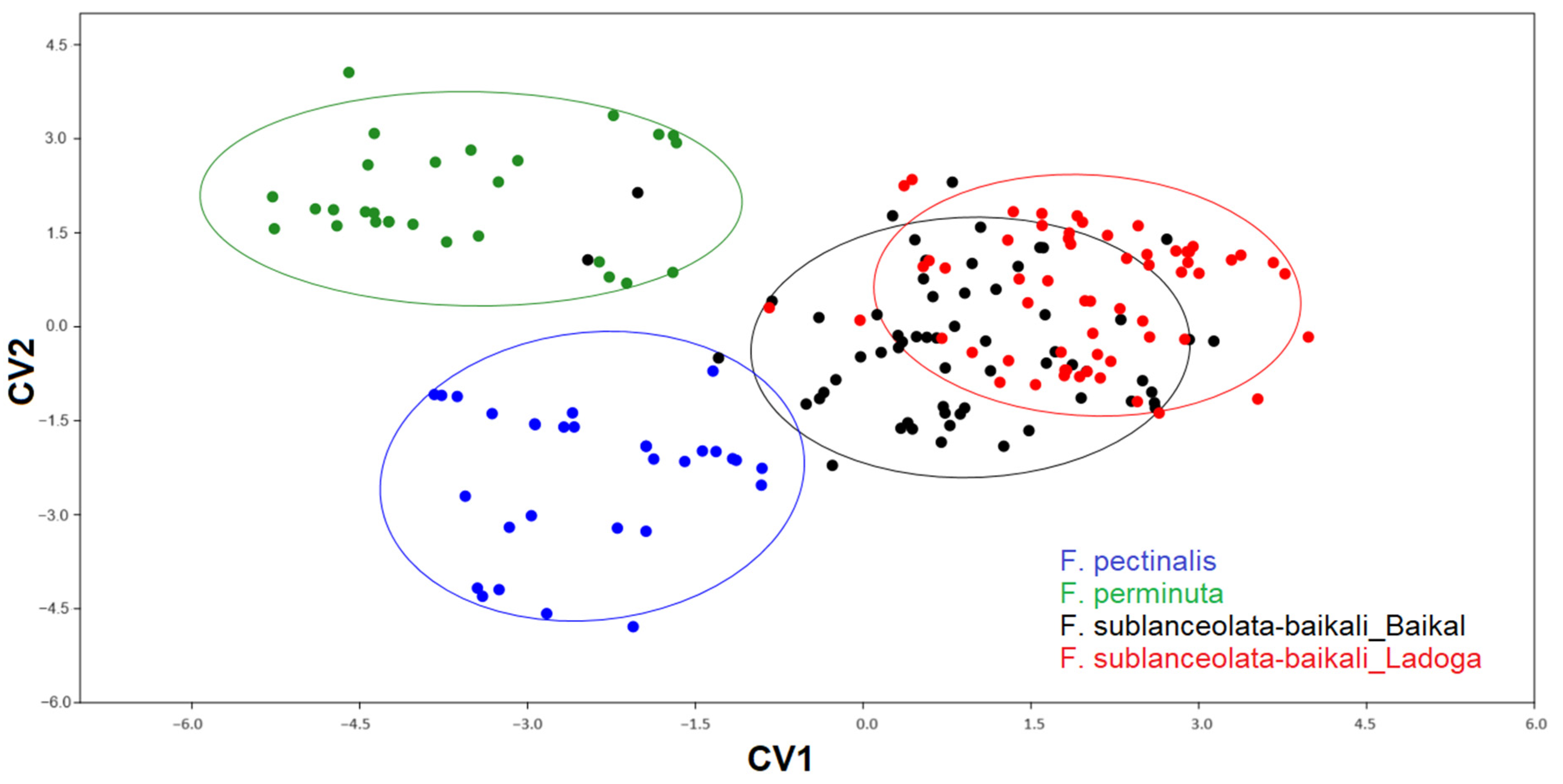
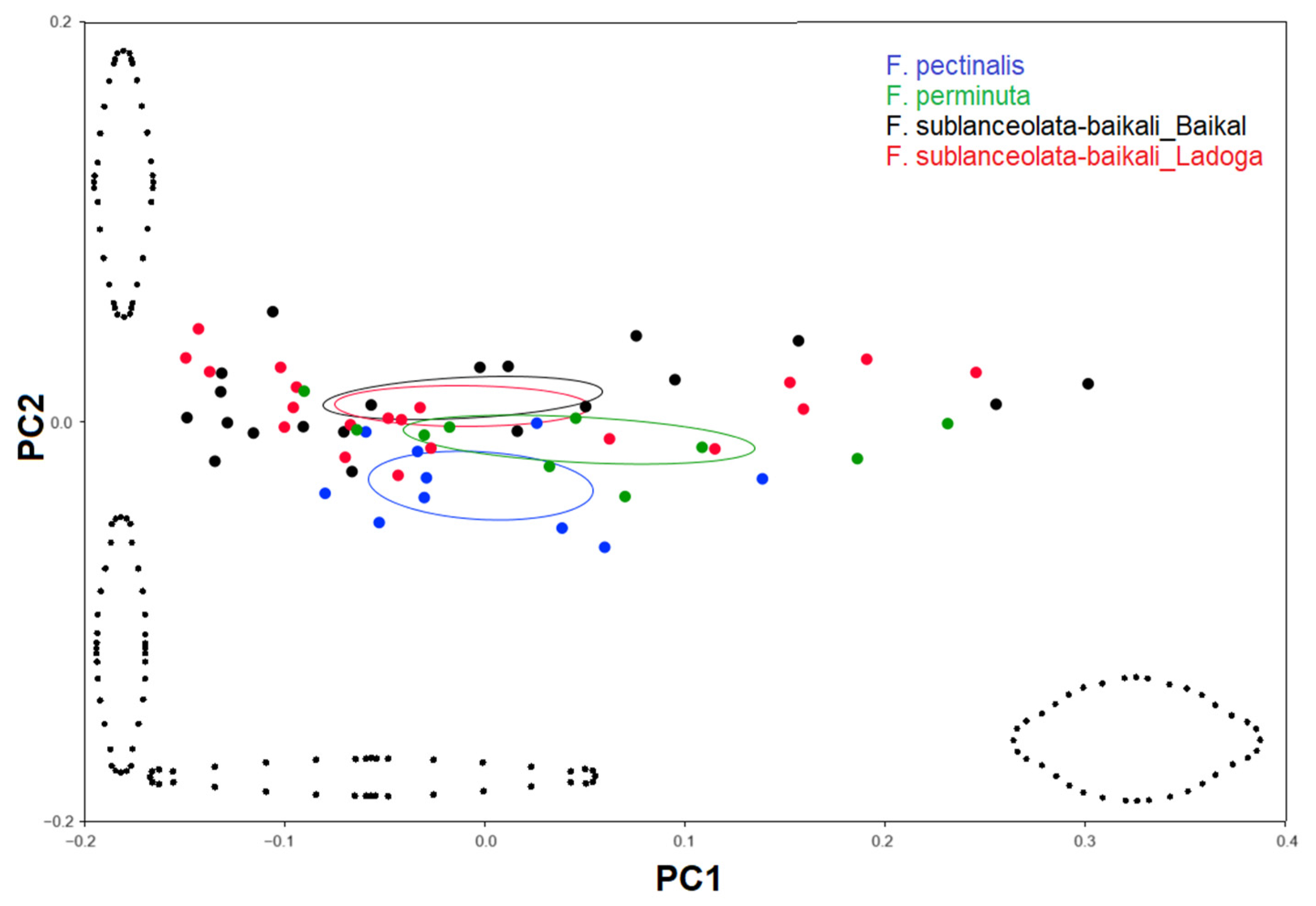
3.3. Geometric Morphometric Analysis
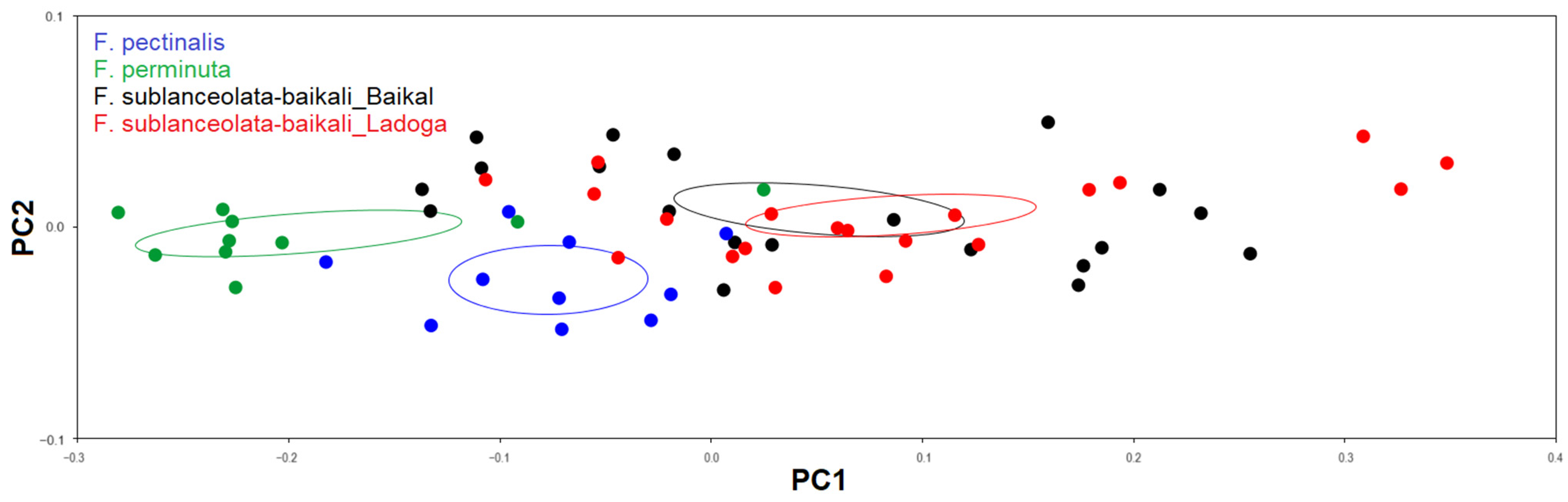
3.3.1. Testing Shape Variation in Populations
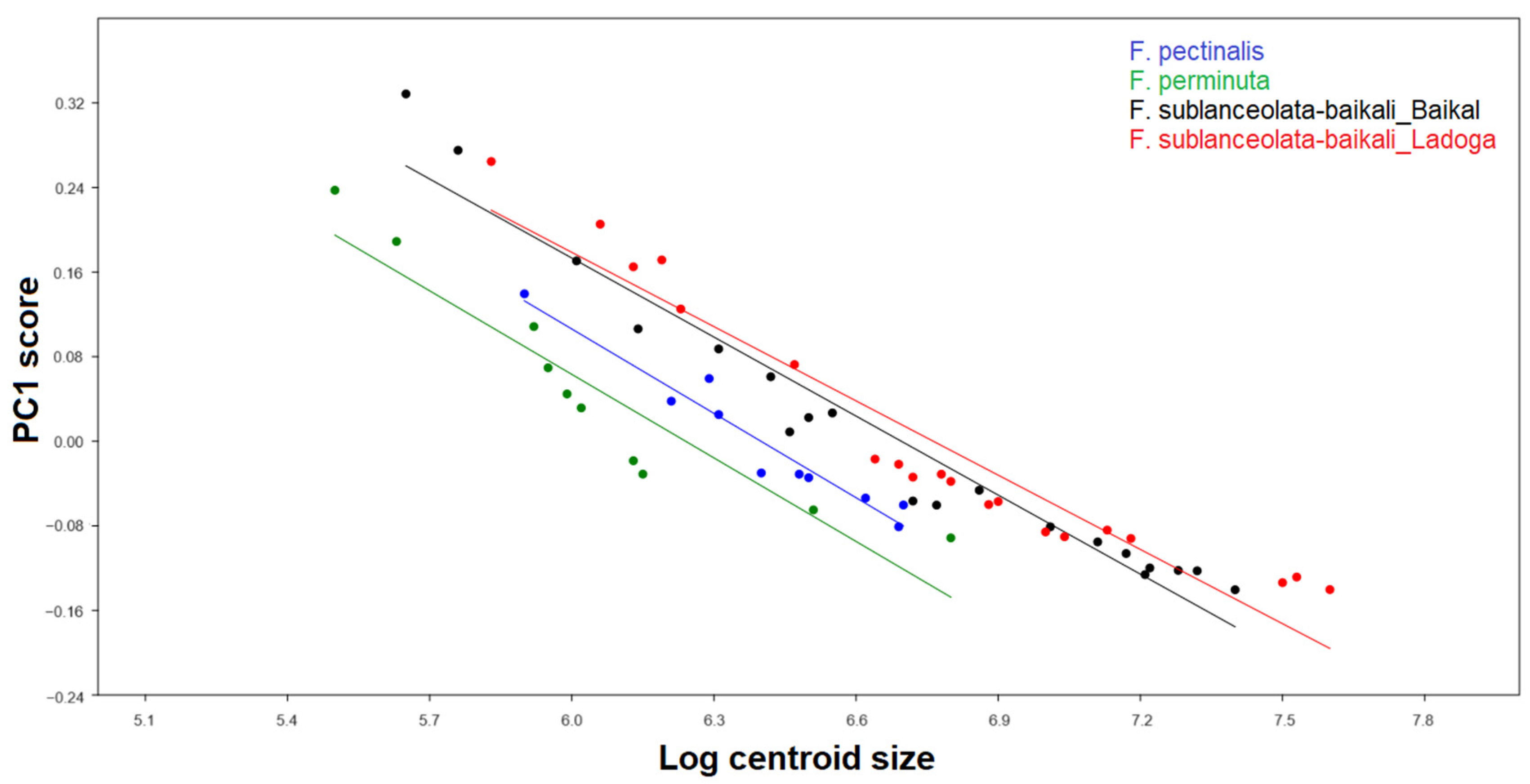
3.3.2. Testing the Effect of Size on Shape and Size Correction
4. Discussion
4.1. Microscopic Examination
4.2. Traditional and Geometric Morphometrics
4.3. Allometric Effect and Size Correction
4.4. Geographical Distribution and Ecology of F. sublanceolata-baikali
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Features | F. sublanceolata-baikali | F. battarbeeana | F. malouana | F. sandellii |
|---|---|---|---|---|
| Cells | Solitary or 2–4 cells together | Solitary | Solitary or two cells together | Solitary or maximum two cells together |
| Length, μm | 8.0–61.2 | 12–38 | 25–60 | 11–24 |
| Width, μm | 3.5–5.2 | 3.5–5 | 3–4 | 4.5–6 |
| Valve outline | Narrowly lanceolate in larger specimens to elliptic-lanceolate in smaller specimens | Lanceolate, with weakly to clearly convex margins (in smaller specimens) | Linear to very narrowly linear–lanceolate, with almost-parallel to weakly convex margin | Elliptic-lanceolate to lanceolate in longer valves with distinctly convex margins |
| Apices | Protracted, subcapitate (in larger specimens) to rostrate apices (in smaller specimens) | Protracted, rostrate, subcapitate to even capitate | Slightly protracted, rostrate to acutely rounded | Slightly protracted, subrostrate to acutely rounded |
| Sternum (axial area) | Narrow, linear, not widening towards the central area | Very narrow, up to 1/8 of total valve width, linear, occasionally weakly widening only at central area | Very narrow, up to 1/8 of total valve width, linear, only at central area weakly widening | Very narrow, maximum 1/10 of the total valve width, linear, gradually widening towards central area |
| Central area | Asymmetrical, formed by unilateral hyaline fascia, clearly swollen on one side with shortened or almost not-shortened striae | Clearly asymmetrical, unilaterally expanded, forming a distinct hyaline zone on one side with almost not-shortened striae on the other | Clearly asymmetrical, forming a distinct unilaterally hyaline zone with almost not-shortened striae on the other side | Large unilateral hyaline zone with weakly shortened striae on the other side |
| Ghost striae | Only rarely observed | Only rarely observed | Only rarely observed | Occasionally visible |
| Striae per 10 μm | 16–20 | 18–19 | 17–19 | 18–19 |
| Stria pattern | Not interrupted in the valve face/mantle junction, uniseriate, parallel to very weakly radiate throughout, becoming slightly more radiate near the apices | Not interrupted in the valve face/mantle junction, uniseriate, parallel to very weakly radiate, only becoming more radiate near apices | Not interrupted in the valve face/mantle junction, uniseriate, parallel to weakly radiate throughout valve | Not interrupted in the valve face/mantle junction, uniseriate, weakly radiate throughout valve |
| Areolae per 1 μm | 7 | nd | nd | nd |
| Areola shape | Rounded to apically elongated | Rounded | Rounded | Rounded |
| Spines | Thick, conical spines located on the virgae; spines occasionally absent (independent from valve length); linking spines absent | Thick, conical, located on the virgae (occasionally two spines per virga) | Small, thick, acute, located on the virgae * | Small, narrow, acute, located on the virgae ** |
| Apical pore field | Composed of up to 6 regular rows of pores | Large, composed of up to 7 parallel rows of small pores | Large, composed of up to 7 parallel rows of small pores | Relatively large, well-defined, composed of 5–8 parallel rows of small pores |
| Rimo-portula per valve | 1 | 1 | 1 | 1 |
| Mantle plaques | Only rarely observed | nd | Large, visible at mantle edge | Clearly visible at mantle edge |
| Girdle | Composed of several copulae; girdle bands with one row of small unoccluded perforations, located at mid-line; valvocopula open, closely attached to mantle interior, with sawtooth-shaped projections attached to the valve | nd | nd | Composed of several open perforated copulae |
| Refe-rences | This study | [65] | [65] | [64,65] |
References
- Williamson, M. Biological Invasions; Chapman and Hall: London, UK, 1996; p. 244. [Google Scholar]
- Ricciardi, A.; Rasmussen, J.B. Predicting the identity and impact of future biological invaders: A priority for aquatic resource management. Can. J. Fish. Aquat. Sci. 1998, 55, 1759–1765. [Google Scholar] [CrossRef]
- Buczkó, K.; Trábert, Z.; Stenger-Kovács, C.; Tapolczai, K.; Bíró, T.; Duleba, M.; Földi, A.; Korponai, J.; Vadkerti, E.; Végvári, Z.; et al. Rapid expansion of an aquatic invasive species (AIS) in Central-European surface waters; a case study of Achnanthidium delmontii. Ecol. Indic. 2022, 135, 108547. [Google Scholar] [CrossRef]
- Duleba, M.; Ector, L.; Horváth, Z.; Kiss, K.T.; Molnár, L.F.; Pohner, Z.; Szilágyi, Z.; Tóth, B.; Vad, C.F.; Várbíró, G.; et al. Biogeography and phylogenetic position of a warm-stenotherm centric diatom, Skeletonema potamos (C.I. Weber) Hasle and its long-term dynamics in the River Danube. Protist 2014, 165, 715–729. [Google Scholar] [CrossRef]
- Rimet, F.; Couté, A.; Piuz, A.; Berthon, V.; Druart, J.-C. Achnanthidium druartii sp. nov. (Achnanthales, Bacillariophyta), a new species invading European rivers. Vie Milieu Environ. 2010, 60, 185–195. [Google Scholar]
- Blanco, S.; Ector, L. Distribution, ecology and nuisance effects of the freshwater invasive diatom Didymosphenia geminata (Lyngbye) M. Schmidt: A literature review. Nova Hedwig. 2009, 88, 347–422. [Google Scholar] [CrossRef]
- Spaulding, S.A.; Kilroy, C.; Edlund, M.B. Diatoms as non-native species. In The Diatoms: Applications for the Environmental and Earth Sciences, 2nd ed.; Smol, J.P., Stoermer, E.F., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 560–569. [Google Scholar]
- Hohn, M.H. Qualitative and quantitative analyses of plankton diatoms, Bass Island area, Lake Erie, 1938–1965. Bull. Ohio Biol. Surv. 1969, 3, 1–211. [Google Scholar]
- Rusanov, A.G.; Duleba, M.; Kiss, K.T.; Ács, É. Morphological characteristics of selected species of Fragilaria capucina/vaucheriae species complex in Lake Ladoga. In Proceedings of the XVII International Scientific Conference “Diatom Algae: Morphology, Biology, Systematics, Floristics, Ecology, Paleogeography, Biostratigraphy”, Kolorgrad, Minsk, Belarus, 23–28 August 2021. (In Russian with English Summary). [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 2/3. Teil: Centrales, Fragilariaceae, Eunotiaceae. Süsswasserflora von Mitteleuropa, 2nd ed.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2000; p. 599. [Google Scholar]
- Flower, R.J.; Pomazkina, G.V.; Rodionova, E.; Williams, D.M. Local and meso-scale diversity patterns of benthic diatoms in Lake Baikal. In Proceedings of the 17th International Diatom Symposium, Ottawa, ON, Canada, 25–31 August 2002. [Google Scholar]
- Novais, M.H.; Almeida, S.F.P.; Blanco, S.; Delgado, C. Morphology and ecology of Fragilaria misarelensis sp. nov. (Bacillariophyta), a new diatom species from southwest of Europe. Phycologia 2019, 58, 128–144. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Hürimann, J.; Williams, D.M.; Wetzel, C.E.; Ector, L. Type analysis of Fragilaria capucina f. lanceolata-baikali and Fragilaria capucina f. sublanceolata-baikali (Bacillariophyta, Fragilariaceae). Not. Algarum 2021, 181, 1–5. [Google Scholar]
- Tuji, A.; Williams, D.M. Typification of Conferva pectinalis O. F. Müll. (Bacillariophyceae) and the identity of the type of an alleged synonym, Fragilaria capucina Desm. Taxon 2006, 55, 193–199. [Google Scholar] [CrossRef]
- Tuji, A.; Williams, D.M. Examination of types in the Fragilaria pectinalis-capitellata species complex. In Proceedings of the 19th International Diatom Symposium, Irkutsk, Russia, 28 August–3 September 2006. [Google Scholar]
- Wetzel, C.E.; Ector, L. Taxonomy and ecology of Fragilaria microvaucheriae sp. nov. and comparison with the type materials of F. uliginosa and F. vaucheriae. Cryptogam. Algol. 2015, 36, 271–289. [Google Scholar] [CrossRef]
- Delgado, C.; Novais, M.H.; Blanco, S.; Almeida, S.F.P. Examination and comparison of Fragilaria candidagilae sp. nov. with type material of Fragilaria recapitellata, F. capucina, F. perminuta, F. intermedia and F. neointermedia (Fragilariales, Bacillariophyceae). Phytotaxa 2015, 231, 1–18. [Google Scholar] [CrossRef]
- Van de Vijver, B.; Ector, L. Analysis of the type material of Synedra perminuta (Bacillariophyceae) with the description of two new Fragilaria species from Sweden. Phytotaxa 2020, 468, 89–100. [Google Scholar] [CrossRef]
- Delgado, C.; Novais, M.H.; Blanco, S.; Almeida, S.F.P. Fragilaria rinoi sp. nov. (Fragilariales, Fragilariophyceae) from periphytic river samples in Central Portugal. Eur. J. Taxon. 2016, 248, 1–16. [Google Scholar] [CrossRef]
- Rusanov, A.G.; Ector, L.; Morales, E.A.; Kiss, K.T.; Ács, É. Morphometric analyses of Staurosira inflata comb. nov. (Bacillariophyceae) and the morphologically related Staurosira tabellaria from north-western Russia. Eur. J. Phycol. 2018, 53, 336–349. [Google Scholar] [CrossRef]
- Beszteri, B.; Ács, É.; Medlin, L. Conventional and geometric morphometric studies of valve ultrastructural variation in two closely related Cyclotella species (Bacillariophyta). Eur. J. Phycol. 2005, 40, 89–103. [Google Scholar] [CrossRef][Green Version]
- Potapova, M.; Hamilton, P.B. Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. J. Phycol. 2007, 43, 561–575. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991; p. 435. [Google Scholar]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the ‘revolution’. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Heterochrony and allometry: The analysis of evolutionary change in ontogeny. Biol. Rev. 1998, 73, 79–123. [Google Scholar] [CrossRef]
- Klingenberg, C.P. Size, shape, and form: Concepts of allometry in geometric morphometrics. Dev. Genes Evol. 2016, 226, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Geitler, L. Der Formwechsel der pennaten Diatomeen (Kieselalgen). Arch. Protistenk. 1932, 78, 1–226. [Google Scholar]
- Mann, D.G. The origins of shape and form in diatoms: The interplay between morphogenetic studies and systematics. In Shape and Form in Plants and Fungi; Ingra, D.S., Hudson, A.J., Eds.; Academic Press: London, UK, 1994; pp. 17–38. [Google Scholar]
- English, J.D.; Potapova, M.G. Ontogenetic and interspecific valve shape variation in the Pinnatae group of the genus Surirella and the description of S. lacrimula sp. nov. Diatom Res. 2012, 27, 9–27. [Google Scholar] [CrossRef]
- Edgar, R.K.; Saleh, A.I.; Edgar, S.M. A morphometric diagnosis using continuous characters of Pinnunavis edkuensis, sp. nov. (Bacillariophyta: Bacillariophyceae), a brackish-marine species from Egypt. Phytotaxa 2015, 212, 1–56. [Google Scholar] [CrossRef]
- Jungers, W.L.; Falsetti, A.B.; Wall, C.E. Shape, relative size, and size-adjustments in morphometrics. Am. J. Phys. Anthropol. 1995, 38, 137–161. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P.; Bernhard, M.; Schaefer, K.; Bookstein, F.L. Comparison of cranial ontogenetic trajectories among great apes and humans. J. Hum. Evol. 2004, 46, 679–697. [Google Scholar] [CrossRef]
- Sidlauskas, B.L.; Mol, J.H.; Vari, R.P. Dealing with allometry in linear and geometric morphometrics: A taxonomic case study in the Leporinus cylindriformis group (Characiformes: Anostomidae) with description of a new species from Suriname. Zool. J. Linn. Soc. 2011, 162, 103–130. [Google Scholar] [CrossRef]
- Viscosi, V.; Antonecchia, G.; Lepais, O.; Fortini, P.; Gerber, S.; Loy, A. Leaf shape and size differentiation in white oaks: Assessment of allometric relationships among three sympatric species and their hybrids. Int. J. Plant Sci. 2012, 173, 875–884. [Google Scholar] [CrossRef]
- Davydova, N.N. The composition and conditions of formation diatom complexes on the surface layer of bottom deposits of Ladoga Lake. In Plant Resources of Lake Ladoga; Nauka: Leningrad, Russia, 1968; pp. 131–174. (In Russian) [Google Scholar]
- Balonov, I.M. Preparation of diatoms and golden algae for electron microscopy. In Study Methods of Biogeocenoses of Inland Waterbodies; Mordukhai-Boltovskoi, F.D., Ed.; Nauka: Moscow, Russia, 1975; pp. 87–90. (In Russian) [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms: Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; p. 747. [Google Scholar]
- Gogorev, R.M.; Chudaev, D.A.; Stepanova, V.A.; Kulikovskiy, M.S. Russian and English terminological glossary on morphology of diatoms. Nov. Sist. Nizs. Rast. 2018, 52, 265–309. [Google Scholar] [CrossRef]
- Gololobova, M.A.; Gogorev, R.M.; Lyakh, A.M.; Dorofeyuk, N.I. The main valve shapes of diatoms: Terminology. I. Valve shapes symmetrical to the apical axis and valve shapes with radial symmetry. Nov. Sist. Nizs. Rast. 2022, 56, 29–54. [Google Scholar] [CrossRef]
- Bookstein, F.L. Landmark methods for forms without landmarks: Morphometrics of group differences in outline shape. Med. Image Anal. 1997, 1, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. The tps series of software. Hystrix 2015, 26, 9–12. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Gunz, P. Advances in geometric morphometrics. Evol. Biol. 2009, 36, 235–247. [Google Scholar] [CrossRef]
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef]
- AlgaeBase. Available online: https://www.algaebase.org (accessed on 19 September 2023).
- Van de Vijver, B.; Mertens, A.; Ector, L. Analysis of the type material of Synedra deformis W. Sm. and Synedra vaucheriae var. deformis Grunow (Fragilariaceae, Bacillariophyta). Cryptogam. Algologie 2020, 41, 137–149. [Google Scholar] [CrossRef]
- Morales, E.A. Morphological studies in selected fragilarioid diatoms (Bacillariophyceae) from Connecticut waters (U.S.A.). Proc. Acad. Nat. Sci. Philadelphia 2001, 151, 105–120. [Google Scholar] [CrossRef]
- Williams, D.M.; Round, F.E. Revision of the genus Fragilaria. Diatom Res. 1987, 2, 267–288. [Google Scholar] [CrossRef]
- Williams, D.M. Spines and homologues in ‘araphid’ diatoms. Plant Ecol. Evol. 2019, 152, 150–162. [Google Scholar] [CrossRef]
- Popovskaya, G.I.; Likhoshway, Y.V.; Genkal, S.I.; Firsova, A.D. The role of endemic diatom algae in the phytoplankton of Lake Baikal. Hydrobiologia 2006, 568, 87–94. [Google Scholar] [CrossRef]
- Popovskaya, G.I.; Genkal, S.I.; Likhoshway, Y.V. Diatoms of the Plankton of Lake Baikal: Atlas and Key; Nauka: Novosibirsk, Russia, 2016; p. 180. (In Russian) [Google Scholar]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Metzeltin, D.; Witkowski, A. Lake Baikal: Hotspot of endemic diatoms I. Iconogr. Diatomol. 2012, 23, 1–861. [Google Scholar]
- Kulikovskiy, M.S.; Lange-Bertalot, H.; Kuznetsova, I.V. Lake Baikal: Hotspot of endemic diatoms II. Iconogr. Diatomol. 2015, 26, 1–657. [Google Scholar]
- Bukhtiyarova, L.N.; Pomazkina, G.V. Bacillariophyta of Lake Baikal: Baikalia, Slavia, Navigeia, Placogeia, Grachevia, Goldfishia, Nadiya, Cymbelgeia; M.G. Kholodny Institute of Botany: Lviv, Ukraine, 2013; p. 184. (In Russian) [Google Scholar]
- Pomazkina, G.V.; Rodionova, E.V.; Sherbakova, T.A. Benthic Diatom Agae of the Family Naviculaceae of Lake Baikal: Atlas and Key; Nauka: Novosibirsk, Russia, 2018; p. 313. (In Russian) [Google Scholar]
- Kudersky, L.A. Fish acclimatization in water bodies of Russia: State and course of development. Probl. Fish. 2001, 2, 6–85. (In Russian) [Google Scholar]
- Kurashov, E.A.; Barbashova, M.A.; Barkov, D.V.; Rusanov, A.G.; Lavrova, M.S. Invasive amphipods as a factor of transformation of Lake Ladoga ecosystems. Russ. J. Biol. Invasions 2012, 3, 202–212. [Google Scholar] [CrossRef]
- Panov, V.E. Establishment of the Baikalian endemic amphipod Gmelinoides fasciatus Stebb. in Lake Ladoga. Hydrobiologia 1996, 322, 187–192. [Google Scholar] [CrossRef]
- Genkal, S.I.; Trifonova, I.S. Centric diatoms (Centrophyceae, Bacillariophyta) in plankton of Lake Ladoga and it tributaries. Int. J. Algae 2003, 5, 56–67. [Google Scholar] [CrossRef]
- Genkal, S.I.; Trifonova, I.S. Diatom Algae of the Plankton of Lake Ladoga and Water-Bodies of Its Basin; Rybinsk Printing House: Rybinsk, Russia, 2009; p. 72. (In Russian) [Google Scholar]
- Van de Vijver, B.; Jarlman, A.; De Haan, M.; Ector, L. New and interesting diatom species (Bacillariophyceae) from Swedish rivers. Nova Hedwig. 2012, 141, 237–253. [Google Scholar]
- Van de Vijver, B.; Williams, D.M.; Kelly, M.; Jarlman, A.; Wetzel, C.E.; Ector, L. Analysis of some species resembling Fragilaria capucina (Fragilariaceae, Bacillariophyta). Fottea 2021, 21, 128–151. [Google Scholar] [CrossRef]
| Characters | Fragilaria sublanceolata-baikali | Fragilaria pectinalis | Fragilaria perminuta | ||
|---|---|---|---|---|---|
| Ladoga | Baikal | Baikal * | Ladoga | Ladoga | |
| (n = 60) | (n = 60) | (n = 30) | (n = 30) | ||
| Valve length, μm | 9.6–61.2 | 8.0–51.3 | 10–45 | 11.3–25.8 | 6.9–28.1 |
| Valve width, μm | 3.8–5.2 | 3.5–5.2 | 4.0–5.5 | 3.2–4.2 | 2.8–3.6 |
| Length-to-width ratio | 2.0–14.6 | 1.7–12.8 | nd | 3.0–7.8 | 2.2–8.3 |
| Valve outline | Narrowly lanceolate (larger specimens) to elliptic-lanceolate (smaller specimens) | Linear lanceolate (larger specimens) to lanceolate (smaller specimens) | Narrowly rhombic-lanceolate (larger specimens) to broadly lanceolate (smaller specimens) | ||
| Apices | Protracted, subcapitate (larger specimens) to rostrate (smaller specimens) | Protracted, subcapitate (larger specimens) to approximately rostrate (smaller specimens) | Slightly protracted, approximately rostrate (larger specimens) to not protracted, cuneately rounded (smaller specimens) | ||
| Axial area | Narrow, linear, not widening towards the central area | ||||
| Central area | Unilateral hyaline fascia, clearly swollen on one side; opposite side with several shortened or almost not shortened striae | Unilateral hyaline fascia; opposite side with several shortened striae | Unilateral hyaline fascia, often swollen on one side; opposite side with several shortened striae | ||
| Striae in 10 μm | 17–20 | 16–20 | 17–20 | 14–17 | 18–20 |
| Stria pattern | Alternating, not interrupted in the valve face/mantle junction, uniseriate, parallel to very weakly radiate throughout, becoming slightly more radiate near the apices | Alternating, not interrupted in the valve face/mantle junction, uniseriate, parallel to very weakly radiate near the apices | |||
| Areolae in 1 μm | 7 | 7 | nd | 5–6 | 7 |
| Areola shape | Small, rounded to apically elongated | ||||
| Spines | Marginal spines (spinules) located on the virgae usually present, conical, never in the shape of linking spines; spinules occasionally absent (independent from valve length) | Absent | Absent | ||
| Apical pore field | Present on both apices, composed of up to 6 rows of porelli | Present on both apices; composed of several regular rows of porelli | |||
| Rimoportula | Present at 1 apex per valve | ||||
| Morphological Characteristics | CV1 | CV2 |
|---|---|---|
| Valve length | 115.8 | −19.0 |
| Valve width | −85.9 | 6.1 |
| Length-to-width ratio | −102.3 | 18.6 |
| Striae density | 14.6 | 46.2 |
| Fragilaria Populations | Slope | Intercept | R2 | p |
|---|---|---|---|---|
| F. sublanceolata-baikali from Lake Ladoga | −0.530 ± 0.031 | 0.757 ± 0.045 | 0.942 | <0.0001 |
| F. sublanceolata-baikali from Lake Baikal | −0.551 ± 0.025 | 0.771 ± 0.035 | 0.965 | <0.0001 |
| F. pectinalis | −0.586 ± 0.058 | 0.730 ± 0.074 | 0.928 | <0.0001 |
| F. perminuta | −0.585 ± 0.064 | 0.707 ± 0.073 | 0.912 | <0.0001 |
| Fragilaria Populations | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 0.110 | <0.0001 | <0.0001 | |
| 2 | 0.605 | <0.0001 | <0.0001 | |
| 3 | 0.525 | 0.649 | <0.01 | |
| 4 | 0.424 | 0.578 | 0.994 |
| Fragilaria Populations | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1 | 0.420 | <0.01 | <0.0001 | |
| 2 | 0.66 | <0.001 | <0.0001 | |
| 3 | 8.94 | 14.04 | <0.01 | |
| 4 | 27.64 | 36.09 | 11.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanov, A.G.; Gololobova, M.A.; Kolobov, M.Y.; Duleba, M.; Georgiev, A.A.; Grigorszky, I.; Kiss, K.T.; Ács, É.; Somlyai, I. Quantitative Morphometric Analysis of Morphologically Similar Species of Fragilaria (Fragilariaceae, Bacillariophyta) Allows Detection of Non-Indigenous Taxa: A Case Study from Lake Ladoga (North of European Russia). Water 2023, 15, 3994. https://doi.org/10.3390/w15223994
Rusanov AG, Gololobova MA, Kolobov MY, Duleba M, Georgiev AA, Grigorszky I, Kiss KT, Ács É, Somlyai I. Quantitative Morphometric Analysis of Morphologically Similar Species of Fragilaria (Fragilariaceae, Bacillariophyta) Allows Detection of Non-Indigenous Taxa: A Case Study from Lake Ladoga (North of European Russia). Water. 2023; 15(22):3994. https://doi.org/10.3390/w15223994
Chicago/Turabian StyleRusanov, Alexander G., Maria A. Gololobova, Mikhail Y. Kolobov, Mónika Duleba, Anton A. Georgiev, István Grigorszky, Keve T. Kiss, Éva Ács, and Imre Somlyai. 2023. "Quantitative Morphometric Analysis of Morphologically Similar Species of Fragilaria (Fragilariaceae, Bacillariophyta) Allows Detection of Non-Indigenous Taxa: A Case Study from Lake Ladoga (North of European Russia)" Water 15, no. 22: 3994. https://doi.org/10.3390/w15223994
APA StyleRusanov, A. G., Gololobova, M. A., Kolobov, M. Y., Duleba, M., Georgiev, A. A., Grigorszky, I., Kiss, K. T., Ács, É., & Somlyai, I. (2023). Quantitative Morphometric Analysis of Morphologically Similar Species of Fragilaria (Fragilariaceae, Bacillariophyta) Allows Detection of Non-Indigenous Taxa: A Case Study from Lake Ladoga (North of European Russia). Water, 15(22), 3994. https://doi.org/10.3390/w15223994








