Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Sampling and Analysis
2.3. Data Analysis
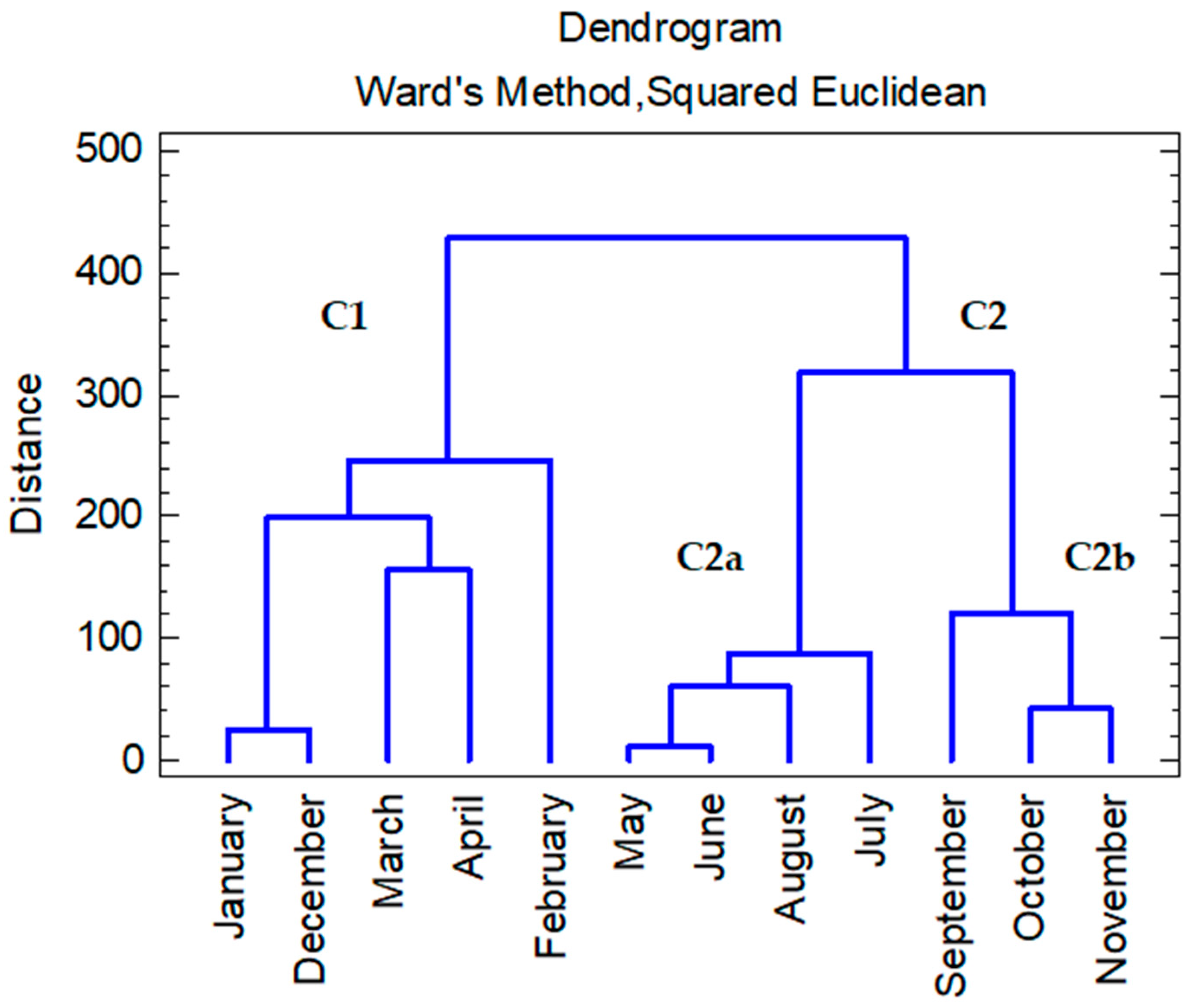
2.3.1. Cluster Analysis
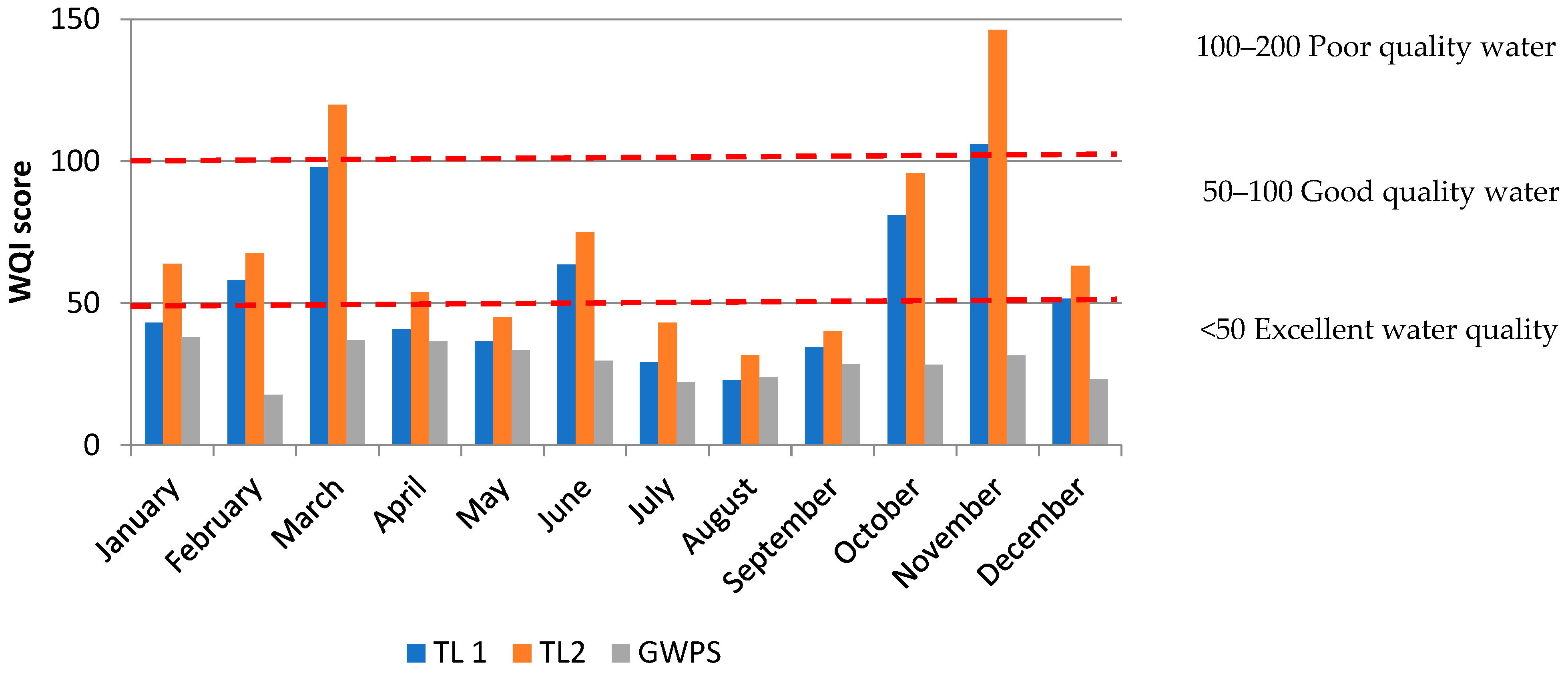
2.3.2. Water Quality Index, WQI
2.3.3. Human Health Risk Assessment
3. Results and Discussion
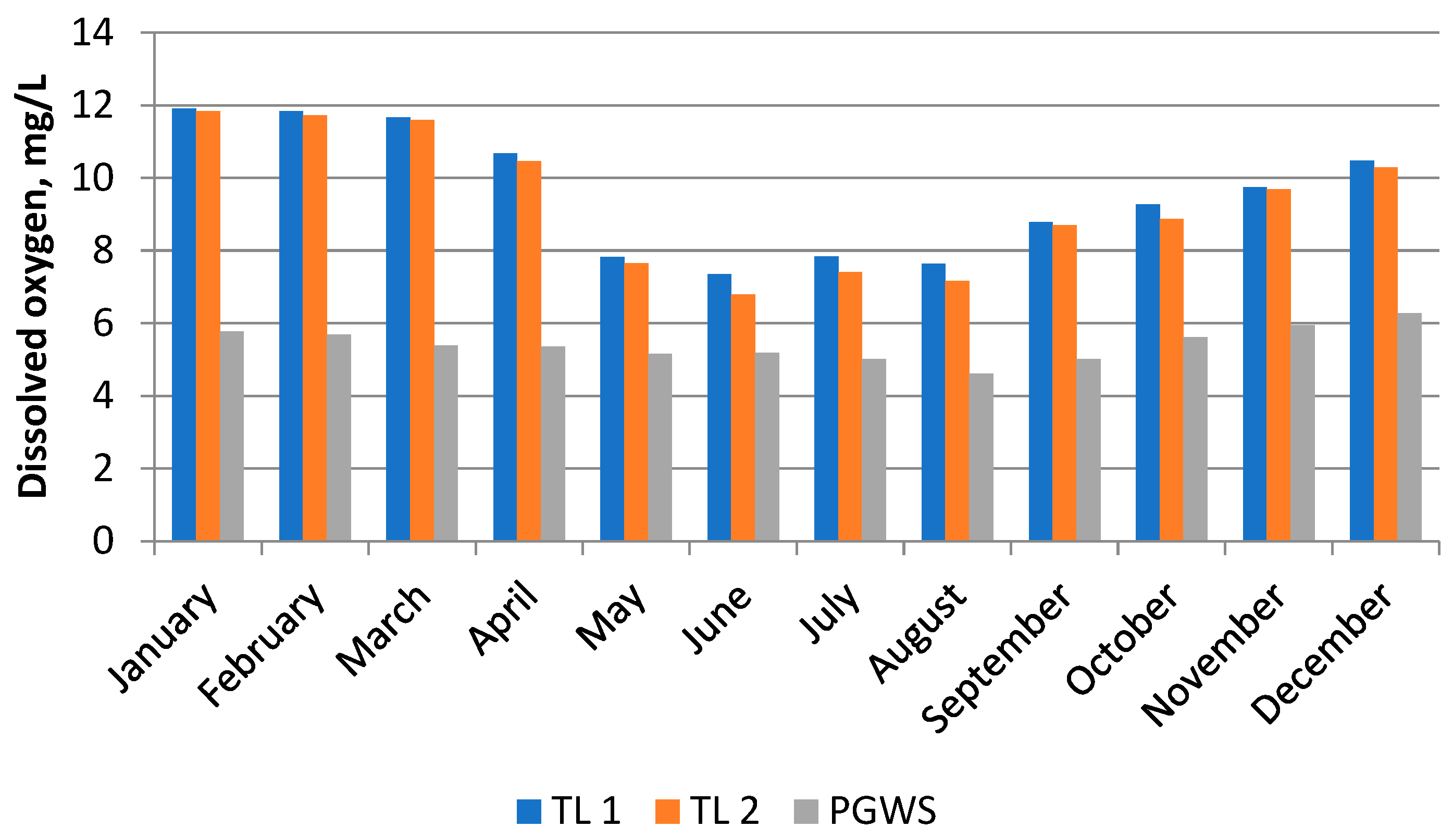
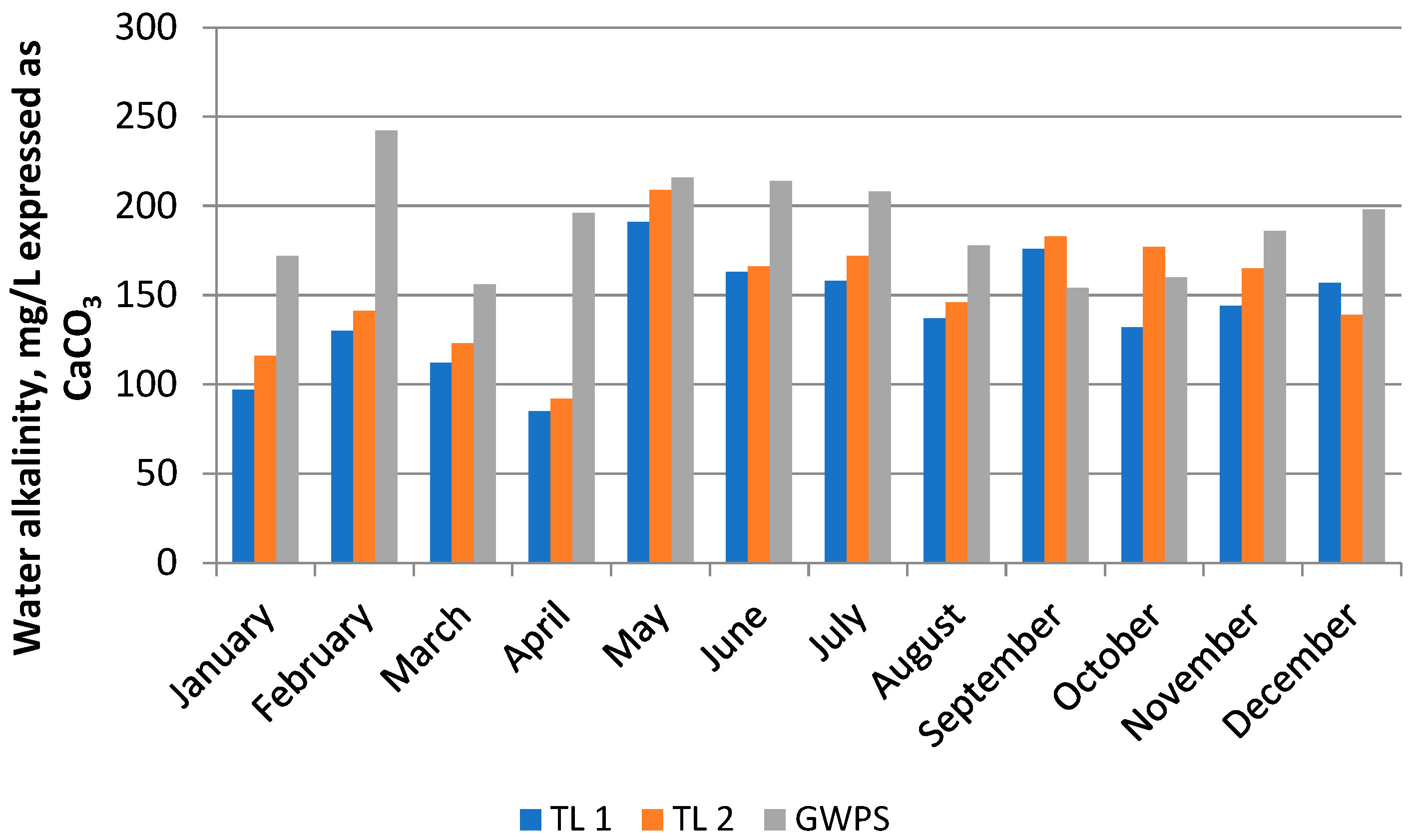
3.1. Physico-Chemical Indicators of Water Samples
3.2. Metal Composition of Water Samples
3.3. Cluster Analysis of Water Samples
3.4. Correlation Analysis
3.5. WQI Indices
3.6. Human Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duran-Encalada, J.A.; Paucar-Caceres, A.; Bandala, E.R.; Wright, G.H. The impact of global climate change on water quantity and quality: A system dynamics approach to the US–Mexican transborder region. Eur. J. Oper. Res. 2017, 256, 567–581. [Google Scholar] [CrossRef]
- Mishra, B.K.; Kumar, P.; Saraswat, C.; Chakraborty, S.; Gautam, A. Water security in a changing environment: Concept, challenges and solutions. Water 2021, 13, 490. [Google Scholar] [CrossRef]
- Georgiu, I.; Caucci, S.; Morris, J.C.; Guenther, E.; Krebs, P. Assessing the potential of water reuse uptake through a private–public partnership: A practitioner’s perspective. Circ. Econ. Sustain. 2023, 3, 199–220. [Google Scholar] [CrossRef]
- OECD. OECD Environmental Outlook to 2050: The Consequences of Inaction; OECD: Paris, France, 2012. [Google Scholar] [CrossRef]
- Tenebe, I.T.; Julian, J.P.; Emenike, P.G.C.; Dede-Bamfo, N.; Maxwell, O.; Sanni, S.E.; Babatunde, E.O.; Alves, D.D. Multi-dimensional surface water quality analyses in the Manawatu River catchment, New Zealand. Water 2023, 15, 2939. [Google Scholar] [CrossRef]
- Akhtar, N.; Ishak, M.I.S.; Bhawani, S.A.; Umar, K. Various natural and anthropogenic factors responsible for water quality degradation: A review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- UN-Habitat. SDG Indicator 6.3.1 Training Module: Safe Wastewater Treatment; United Nations Human Settlement Programme (UN-Habitat): Nairobi. Kenya, 2018. [Google Scholar]
- Baskaran, P.; Abraham, M. Evaluation of groundwater quality and heavy metal pollution index of the industrial area, Chennai. Phys. Chem. Earth 2022, 128, 103259. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłonska, J. Variability and heavy metal pollution levels in water and bottom sediments of the Liwiec and Muchawka Rivers (Poland). Water 2023, 15, 2833. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- San-Martin, M.I.; Alonso, R.M.; Ivars-Barcelo, F.; Escapa, A.; Moran, A. Complete arsenic removal from water using biocatalytic systems based on anaerobic films grown on carbon fibers. Catal. Today 2023, 423, 114269. [Google Scholar] [CrossRef]
- Lin, M.-H.; Li, C.-Y.; Cheng, Y.-Y.; Guo, H.-R. Arsenic in drinking water and incidences of leukemia and lymphoma: Implication for its dual effects in carcinogenicity. Front. Public Health 2022, 10, 863882. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Hussein, T.S.; Faisal, A.A.H. Nanoparticles of (calcium/aluminium/CTAB) layered double hydroxide immobilization onto iron slag for removing of cadmium ions from aqueous environment. Arab. J. Chem. 2023, 16, 105031. [Google Scholar] [CrossRef]
- Khan, Z.I.; Ahmed, K.; Ahmed, T.; Zafar, A.; Alrefaei, A.F.; Ashfaq, A.; Akhtar, S.; Mahpara, S.; Mehmood, N.; Ugulu, I. Evaluation of nickel tixicity and potential health implications of agriculturally diversely irrigated wheat crop varieties. Arab. J. Chem. 2023, 16, 104934. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human health and environmental toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed]
- Senze, M.; Kowalska-Goralska, M.; Czyz, K. Availability of aluminum in river water supplying dam reservoirs in Lower Silesia considering the hydrochemical conditions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100535. [Google Scholar] [CrossRef]
- Mohanty, S.; Benya, A.; Hota, S.; Kumar, M.S.; Singh, S. Eco-toxicity of hexavalent chromium and its adverse impact on environment and human health in Sukinda Valley of India: A review on pollution and prevention strategies. Environ. Chem. Ecotox. 2023, 5, 46–54. [Google Scholar] [CrossRef]
- Georgaki, M.-N.; Charalambous, M.; Kazakis, N.; Talias, M.A.; Georgakis, C.; Papamitsou, T.; Mytiglaki, C. Chromium in water and carcinogenic human health risk. Environments 2023, 10, 33. [Google Scholar] [CrossRef]
- Siraj, K.; Kitte, S.A. Analysis of Copper, Zinc and Lead using Atomic Absorption Spectrophotometer in ground water of Jimma town of Southwestern Ethiopia. Int. J. Chem. Anal. Sci. 2013, 4, 201–204. [Google Scholar] [CrossRef]
- Hossain, M.; Bhattacharya, P.; Frape, S.K.; Ahmed, K.M.; Jacks, G.; Hasan, M.A.; Bromssen, M.; Shahiruzzaman, M.; Morth, C.M. A potential source of low-manganese, arsenic-safe drinking water from Intermediate Deep Aquifers (IDA), Bangladesh. Groundw. Sustain. Dev. 2023, 21, 100906. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, H.; Le, Q.V.; Peng, W.; Lam, S.S.; Yang, Y.; Li, C.; Sonne, C. Perspectives on phytoremediation of zinc pollution in air, water and soil. Sustain. Chem. Pharm. 2021, 24, 100550. [Google Scholar] [CrossRef]
- Markeb, A.A.; Moral-Vico, J.; Sanchez, A.; Font, X. Optimization of lead (II) removal from water and wastewater using a novel magnetic nanocomposite of aminopropyl triethoxysilane coated with carboxymethyl cellulose cross-linked with shitosan nanoparticles. Arab. J. Chem. 2023, 16, 105022. [Google Scholar] [CrossRef]
- Beinabaj, S.M.H.; Heydariyan, H.; Aleii, H.M.; Hosseinzadeh, A. Concentration of heavy metals in leachate, soil, and plants in Teheran’s landfill: Investigation of the effect of landfill age on the intesity of polluation. Heliyon 2023, 9, e13017. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Huang, C.W.; Li, Y.L.; Huang, T.P.; Lin, C.; Ngo, H.H.; Bui, X.T. Reduced pollution level and ecological risk of mercury-polluted sediment in a alkali-chlorine factory’s brine water storage pond after corrective actions: A case study in Southern Taiwan. Environ. Technol. Innov. 2023, 29, 103003. [Google Scholar] [CrossRef]
- Muhammad, S.; Shah, M.T.; Khan, S. Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 2011, 98, 334–343. [Google Scholar] [CrossRef]
- Biswas, A.K.; Tortajada, C. Water crisis and water wars: Myths and realities. Int. J. Water Resour. Dev. 2019, 35, 727–731. [Google Scholar] [CrossRef]
- Mihali, C.; Dippong, T. Water quality assessment of Remeti watercourse, Maramures, Romania, located in a Natura 2000 protected area subjected to anthropic pressure. J. Contam. Hydrol. 2023, 257, 104216. [Google Scholar] [CrossRef]
- Dippong, T.; Mihali, C.; Mare Rosca, O.; Marian, M.; Payer, M.M.; Țîbîrnac, M.; Bud, S.; Coman, G.; Kovacs, E.; Hoaghia, M.-A. Physico-chemical characterization of water wells from Remeti, Maramures. Stud. Univ. Babes-Bolyai Chem. 2021, 66, 197–211. [Google Scholar] [CrossRef]
- Varol, M.; Tokatli, C. Evaluation of the water quality of a highly polluted stream with water quality indices and health risk assessment methods. Chemosphere 2023, 311, 137096. [Google Scholar] [CrossRef]
- Dippong, T.; Mihali, C.; Cical, E. Metode de Determinare a Proprietăților Fizico-Chimice ale Alimentelor; Risoprint: Cluj-Napoca, Romania, 2016; ISBN 978-973-53-1773-7. (In Romanian) [Google Scholar]
- SR EN ISO 5667-3:2018; Calitatea Apei. Prelevare. Partea 3: Conservarea şi Manevrarea Probelor de Apă. ISO: Geneva, Switzerland, 2018. (In Romanian)
- ISO 7888:1985; Water Quality—Determination of Electrical Conductivity. ISO: Geneva, Switzerland, 1985.
- SR EN ISO 10523:2012; Calitatea Apei. Determinarea pH-ului. ISO: Geneva, Switzerland, 2012. (In Romanian)
- SR EN ISO 8467:2001; Calitatea Apei. Determinarea Indicelui de Permanganat. ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR EN ISO 5814:2013; Calitatea Apei. Determinarea Conţinutului de Oxigen Dizolvat. Metoda Electrochimică cu Sonda. ISO: Geneva, Switzerland, 2013. (In Romanian)
- SR EN ISO 9963-1:2002; Calitatea Apei. Determinarea Alcalinităţii. Partea 1: Determinarea Alcalinităţii Totale şi Permanent. ISO: Geneva, Switzerland, 2002. (In Romanian)
- SR ISO 9297:2001; Calitatea Apei. Determinarea Conţinutului de Cloruri. Titrare cu Azotat de Argint Utilizând Cromatul ca Indicator (Metoda Mohr). ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR ISO 6058:2008; Calitatea Apei. Determinarea Calciului. Metoda Iitrimetrică cu EDTA. ISO: Geneva, Switzerland, 2008. (In Romanian)
- SR ISO 10566:2001; Calitatea Apei. Determinarea Conţinutului de Aluminiu. Metoda Spectrometrică cu Violet de Pirocatechol. ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR ISO 7150-1:2001; Calitatea Apei. Determinarea Conţinutului de Amoniu. Partea 1: Metoda Spectrometrică Manual. ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR ISO 7890-3:2000; Calitatea Apei. Determinarea Conţinutului de Azotaţi. Partea 3: Metoda Spectrometrică cu Acid Sulfosalicylic. ISO: Geneva, Switzerland, 2000. (In Romanian)
- SR EN 26777:2002; Calitatea Apei. Determinarea Conţinutului de Nitriţi. Metoda Prin Spectrometrie de Absorbţie Moleculară. ISO: Geneva, Switzerland, 2002. (In Romanian)
- SR EN ISO 6878:2005; Calitatea Apei. Determinarea Fosforului. Metoda Spectrofotometrică cu Molibdat de Amoniu. ISO: Geneva, Switzerland, 2005. (In Romanian)
- SR ISO 6332:1996; Calitatea Apei. Determinarea Conţinutului de Fier. Metoda Spectrometrică cu 1,10—fenantrolină. ISO: Geneva, Switzerland, 1996. (In Romanian)
- SR EN ISO 7027:2001Calitatea Apei. Determinarea Turbidităţii. ISO: Geneva, Switzerland,, 2001. (In Romanian)
- Dippong, T.; Mihali, C.; Hoaghia, A.M.; Cical, E.; Cosma, A. Chemical modeling of groundwater quality in the aquifer of Seini town-Somes Plain, Northwestern Romania. Ecotoxicol. Environ. Saf. 2019, 168, 88–101. [Google Scholar] [CrossRef]
- Mare Roșca, O.; Dippong, T.; Marian, M.; Mihali, C.; Mihalescu, L.; Hoaghia, M.A.; Jelea, M. Impact of anthropogenic activities on water quality parameters of glacial lakes from Rodnei mountains, Romania. Environ. Res. 2020, 182, 109136. [Google Scholar] [CrossRef]
- Uddin, M.G.; Nash, S.; Agnieszka, I.; Olbert, A.I. A review of water quality index models and their use for assessing surface water quality. Ecol. Indic. 2021, 122, 107218. [Google Scholar] [CrossRef]
- Varol, S.; Davraz, A. Evaluation of the groundwater quality with WQI (Water Quality Index) and multivariate analysis: A case study of the Tefenni plain (Burdur/Turkey). Environ. Earth Sci. 2014, 73, 1725–1744. [Google Scholar] [CrossRef]
- Napo, G.; Akpataku, K.V.; Seyf-Laye, A.S.M.; Gnazou, M.D.T.; Bawa, L.M.; Djaneye-Boundjou, G. Assessment of Shallow Groundwater Quality Using Water Quality Index and Human Risk Assessment in the Vogan-Attitogon Plateau, Southeastern (Togo). J. Environ. Poll. Hum. Health 2021, 9, 50–63. [Google Scholar] [CrossRef]
- Council Directive. 98/83/EC—On the Quality of Water Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31998L0083 (accessed on 9 October 2023).
- Chatanga, P.; Ntuli, V.; Mugomeri, E.; Keketsi, T.; Chikowore, T.V.N. Situational analysis of physico-chemical, biochemical, and microbiological quality of water along Mohokare River, Lesotho. Egypt. J. Aquat. Res. 2019, 45, 45–51. [Google Scholar] [CrossRef]
- Rupias, O.J.B.; Pereira, S.Y.; Silva de Abreu, A.E. Hydrogeochemistry and groundwater quality assessment using the water quality index and heavy-metal pollution index in the alluvial plain of Atibaia river-Campinas/SP, Brazil. Groundw. Sustain. Dev. 2021, 15, 100661. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Average Height and Weight by Country. Available online: https://www.worlddata.info/average-bodyheight.php (accessed on 2 November 2023).
- Pal, M.; Samal, N.R.; Roy, P.K.; Roy, M.B. Electrical conductivity of lake waters as environmental monitoring—A case study of Rudrasagar Lake. J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 2319–2399. [Google Scholar] [CrossRef]
- Asadi, E.; Isazadeh, M.; Samadianfard, S.; Ramli, M.F.; Mosavi, A.; Nabipour, N.; Shamshriband, S.; Hajnal, E.; Chau, K.-W. Groundwater quality assessment for sustainable drinking and irrigation. Sustainability 2020, 12, 177. [Google Scholar] [CrossRef]
- Adesakin, T.A.; Oyewale, A.T.; Bayero, U.; Mohammed, A.N.; Aduwo, I.A.; Ahmed, P.Z.; Abubakar, N.D.; Barje, I.B. Asses ment of bacteriological quality and physicochemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon 2020, 6, e04773. [Google Scholar] [CrossRef]
- Spyra, A. Acidic, neutral and alkaline forest ponds and lanscape element affecting the biodiversity of freshwater snails. Sci. Nat. 2017, 104, 73. [Google Scholar] [CrossRef]
- Du, J.; Shen, J. Decoupling the influence of biological and physical processes on the dissolved oxygen in the Chesapeake Bay. J. Geophys. Res. Oceans 2015, 120, 78–93. [Google Scholar] [CrossRef]
- Borzog-Haddad, O.; Delpasand, M.; Loaiciga, H.A. Chapter 10: Water quality, hygiene and health. In Economical, Political, and Social Issues in Water Resources; Borzog-Haddad, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 217–257. [Google Scholar] [CrossRef]
- Order No. 1146/2002 for the Approval of the Normative on the Reference Objectives for the Classification of Surface Water Quality Issued by the Ministry of Water and Environmental Protection—MAPM. Available online: https://lege5.ro/gratuit/hezteobt/clasificarea-calitatii-apei-de-suprafata-si-a-sedimentelor-normativ?dp=giztcmjzgiyta (accessed on 15 September 2023). (in Romanian).
- Zdorovennova, G.; Palshin, N.; Golosov, S.; Efremova, T.; Belashev, B.; Bogdanov, S.; Fedorova, I.; Zverev, I.; Zdorovennov, R.; Terzhevik, A. Dissolved Oxygen in a Shallow Ice-Covered Lake in Winter: Effect of Changes in Light, Thermal and Ice Regimes. Water 2021, 13, 2435. [Google Scholar] [CrossRef]
- Constantin, S.; Doxaran, D.; Constantinescu, Ș. Estimation of water turbidity and analysis of its spatio-temporal variability in the Danube River plume (Black Sea) using MODIS satellite data. Cont. Shelf Res. 2016, 112, 14–30. [Google Scholar] [CrossRef]
- Mann, A.G.; Tam, C.C.; Higgins, C.D.; Rodrigues, L.C. The association between drinking water turbidity and gastrointestinal illness: A systematic review. BMC Public Health 2007, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Horvat, M.; Horvat, Z. Long term sediment transport simulation of the Danube, Sava and Tisa rivers. Int. J. Sediment Res. 2020, 35, 550–561. [Google Scholar] [CrossRef]
- Fan, B.; Li, J.; Wang, X.; Chen, J.; Gao, X.; Li, W.; Ai, S.; Cui, L.; Gao, S.; Liu, Z. Ammonia spatiotemporal distribution and risk assessment for freshwater species in aquatic ecosystem in China. Ecotoxicol. Environ. Saf. 2021, 207, 111541. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-Z.; Xue, M.-Y.; Yang, S.-B.; Zha, J.-W.; Wang, G.-X.; Ling, F. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish. Immunol. 2017, 70, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, O.J.; Mothiba, M. Physico-chemical characteristics and pollution levels of heavy metals in the rivers in Thohoyandou, South Africa. J. Hydrol. 2005, 308, 122–127. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, Q.; Wang, H.; Wang, Y. Identification of Sources and Transformations of Nitrate in the Intense Human Activity Region of North China Using a Multi-Isotope and Bayesian Mode. Int. J. Environ. Res. Public Health 2021, 18, 8642. [Google Scholar] [CrossRef]
- Sebilo, M.; Mayer, B.; Nicolardot, B.; Pinay, G. Long-term fate of nitrate fertilizer in agricultural soils. Proc. Natl. Acad. Sci. USA 2013, 110, 18185–18189. [Google Scholar] [CrossRef]
- Singh, A.K.; Mondal, G.C.; Kumar, S.; Singh, T.B.; Tewary, B.K.; Sinha, A. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ. Geol. 2008, 54, 745–758. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus management in Europe in a changing world. AMBIO 2015, 44, S180–S192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lan, T.; Shang, G.; Li, J.; Geng, J. Adsorption performance of phosphate in water by mixed precursor base geopolymers. J. Contam. Hydrol. 2023, 255, 104166. [Google Scholar] [CrossRef] [PubMed]
- Proorocu, M.; Coste, C.; Mihaescu, T.; Varban, D.; Aschilean, I. The quality of the surface water in 6 North—West Region. ProEnvironment 2008, 1, 29–31. [Google Scholar]
- Zhang, H.; Chen, J.; Douglas, G.; Haffner, D.G. Plateau Lake Water Quality and Eutrophication: Status and Challenges. Water 2023, 15, 337. [Google Scholar] [CrossRef]
- Adeyemi, A.A.; Ojekunle, Z.O. Concentration and health risk assessment of industrial heavy metals pollution in Ogun state, Nigeria. Sci. Afr. 2021, 11, e00666. [Google Scholar] [CrossRef]
- Ibrahim, K.O.; Gomo, M.; Oke, S.A. Groundwater quality assessment of shallow aquifer hand dug wells in rural localities of Ilorin northcentral Nigeria: Implications for domestic and irrigation uses. Groundw. Sustain. Develop. 2019, 9, 100226. [Google Scholar] [CrossRef]
- Kaushal, S.S. Increased salinization decreases safe drinking water. Environ. Sci. Technol. 2016, 50, 2765–2766. [Google Scholar] [CrossRef]
- Chapra, S.C.; Camacho, L.A.; McBride, G.B. Impact of Global Warming on Dissolved Oxygen and BOD Assimilative Capacity of the World’s Rivers: Modeling Analysis. Water 2021, 13, 2408. [Google Scholar] [CrossRef]
- Hu, X.; Shi, X.; Su, R.; Jin, Y.; Ren, S.; Li, X. Spatiotemporal patterns and influencing factors of dissolved heavy metals off the Yangtze River Estuary, East China Sea. Mar. Pollut. Bull. 2022, 182, 112975. [Google Scholar] [CrossRef]
- Du Laing, G.; De Vos, R.; Vandecasteele, B.; Lesage, E.; Tack, F.M.G.; Verloo, M.G. Effect of salinity on heavy metal mobility and availability in intertidal sediments of the Scheldt estuary. Estuar. Coast. Shelf Sci. 2008, 77, 589–602. [Google Scholar] [CrossRef]
- Moya, S.M.; Botella, N.B. Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in water treatment by magnetism and electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Tenan, M.R.; Nicolle, A.; Moralli, D.; Verbouwe, E.; Jankowska, J.D.; Durin, M.-A.; Green, C.M.; Mandriota, S.J.; Sappino, A.P. Aluminum Enters Mammalian Cells and Destabilizes Chromosome Structure and Number. Int. J. Molec. Sci. 2021, 22, 9515. [Google Scholar] [CrossRef] [PubMed]
- Krupinska, I. Aluminium drinking water treatment residuals and their toxic impact on human health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.O.; Almeida, C.A.; Calderon, M.; Mallea, M.A.; Gonzalez, P. Assessment of the water self-purification capacity on a river affected by organic pollution: Application of chemometrics in spatial and temporal variations. Environ. Sci. Pollut. Res. 2014, 21, 10583–10593. [Google Scholar] [CrossRef]
- Weisner, M.L.; Harris, M.S.; Mitsova, D.; Liu, W. Drinking water disparities and aluminum concentrations: Assessing socio-spatial dimensions across an urban landscape. Soc. Sci. Humanit. Open 2023, 8, 100536. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Hosseini, M.R.; Dehbandi, R.; Khodadadi, N.; Keshtkar, M.; Shahvasani, E.; Elshall, A.S.; Azhdarpoor, A. Probabilistic human health risk assessment and Sobol sensitivity reveal the major health risk parameters of aluminum in drinking water in Shiraz, Iran. Environ. Geochem. Health 2023, 45, 7665–7677. [Google Scholar] [CrossRef]
- Rondeau, V.; Commenges, D.; Jacqmin-Gadda, H.; Dartigues, J.-F. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: An 8-year follow-up study. Am. J. Epidemiol. 2000, 152, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Rosseland, B.O.; Eldhuset, T.D.; Staurnes, M. Environmental effects of aluminium. Environ. Geochem. Health 1990, 12, 17–27. [Google Scholar] [CrossRef]
- Verbruggen, E.M.J.; Smit, C.E.; van Vlaardingen, P.L.A. Environmental Quality Standards for Barium in Surface Water: Proposal for an Update According to the Methodology of the Water Framework Directive; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2020; pp. 1–111. [CrossRef]
- Kaur, A.; Parkash, C. Barium: Presence in environment and health effects. Eco. Environ. Cons. 2022, 28, 2210–2213. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011; pp. 1–564. [Google Scholar]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RCS Adv. 2022, 12, 9139. [Google Scholar] [CrossRef]
- World Health Organization. Nickel in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2021; pp. 1–36. [Google Scholar]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef]
- Algül, F.; Beyhan, M. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci. Rep. 2020, 10, 11782. [Google Scholar] [CrossRef] [PubMed]
- Krok, B.; Mohammadian, S.; Noll, H.M.; Surau, C.; Markwort, S.; Fritzsche, A.; Nachev, M.; Sures, B.; Meckenstock, R.U. Remediation of zinc-contaminated groundwater by iron oxide in situ adsorption barriers—From lab to the field. Sci. Total Environ. 2022, 897, 151066. [Google Scholar] [CrossRef] [PubMed]
- Eslami, H.; Esmaeili, A.; Razaeian, M.; Salari, M.; Hosseini, A.N.; Mobini, M.; Barani, A. Potentially toxic metal concentration, spatial distribution, and health risk assessment in drinking groundwater resources of southeast Iran. Geosci. Front. 2022, 13, 101276. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef]
- van Wendel de Joode, B.; Barbeau, B.; Bouchard, M.F.; Mora, A.M.; Skytt, A.; Cordoba, L.; Quesada, R.; Lundh, T.; Lindh, C.H.; Mergler, D. Manganese concentrations in drinking water from villages near banana plantations with aerial mancozeb spraying in Costa Rica: Results from the Infants’ Environmental Health Study. Environ. Pollut. 2016, 215, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Dumitru, M.; Vrinceanu, N.; Eftene, A.; Anghel, A.; Vrinceanu, A.; Ignat, P.; Dumitru, S.; Mocanu, V. Soil heavy metal status from Maramures county, Romania. In Proceedings of the GLOREP 2108 Conference, Timisoara, Romania, 15–17 November 2018. [Google Scholar]
- Strungaru, S.-A.; Nicoara, M.; Teodosiu, C.; Baltag, E.; Ciobanu, C.; Plavan, G. Patterns of toxic metals bioaccumulation in a cross-border freshwater reservoir. Chemosphere 2018, 207, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Soroaga, L.V.; Amarandei, C.; Negru, A.G.; Olariu, R.I.; Arsene, C. Assessment of the Anthropogenic Impact and Distribution of Potentially Toxic and Rare Earth Elements in Lake Sediments from North-Eastern Romania. Toxics 2022, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Commission, Directorate-General for Environment, The Environmental Implementation Review—Romania; Publications Office of the European Union: Luxembourg, 2019; pp. 1–35. Available online: https://environment.ec.europa.eu (accessed on 13 November 2023).
- European Commission. EU Environmental Implementation Review Country Report—Romania. 2017. Available online: http://ec.europa.eu/environment/eir/pdf/report_ro_en.pdf (accessed on 13 November 2023).
- Khokhar, L.A.K.; Khuhawar, M.Y.; Khuhawar, T.M.J.; Lanjwani, M.F.; Arain, G.M.; Khokhar, F.M.; Khaskheli, M.I. Spatial variability and hydrochemical quality of groundwater of Hyderabad Rural, Sindh, Pakistan. Sustain. Water Res. Manag. 2023, 9, 164. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, A.; Yadav, S.; Singhal, M.K. Assessment of heavy metal contamination in water of Kali River using principle component and cluster analysis, India. Sustain. Water Resour. Manag. 2018, 4, 573–581. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, A.; Shukla, P. Study of water quality in Hindon River using pollution index and environmetrics, India. Desalination Water Treat. 2016, 57, 19121–19130. [Google Scholar] [CrossRef]
- Babcsanyi, I.; Tamas, M.; Szatmari, J.; Hambek-Olah, B.; Farsang, A. Assessing the impacts of the main river and anthropogenic use on the degree of metal contamination of oxbow lake sediments (Tisza River Valley, Hungary). J. Soils Sediments 2020, 20, 1662–1675. [Google Scholar] [CrossRef]
- Sakan, S.; Grzetic, I.; Dordevic, I. Distribution and Fractionation of Heavy Metals in the Tisa (Tisza) River Sediments. Environ. Sci. Pollut. Res. 2007, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Sakan, S.M.; Dordevic, D.S.; Dragan, D.M.; Predrag, P.S. Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J. Environ. Manag. 2009, 90, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Sur, I.M.; Moldovan, A.; Micle, V.; Polyak, E.T. Assessment of surface water quality in the Baia Mare area, Romania. Water 2022, 14, 3118. [Google Scholar] [CrossRef]
- Ewaid, S.H.; Abed, S.A. Water quality index for Al-Gharraf River, southern Iraq. Egypt. J. Aquat. Res. 2017, 43, 117–122. [Google Scholar] [CrossRef]
- Goher, M.E.; Hassan, A.M.; Abdel-Moniem, I.A.; Fahmy, A.H.; Seliem, M.; El-Sayed, S.M. Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt. J. Aquat. Res. 2014, 40, 225–233. [Google Scholar] [CrossRef]
- Mohamed, A.; Saiful, I.; Towfiqul, I.; Bhuyan, S.; Shafiuddin, A.; Zillur, R.; Mostafizur, R. Toxic metal pollution and ecological risk assessment in water and sediment at ship breaking sites in the bay of Bengal coast, Bangladesh. Mar. Pollut. Bull. 2021, 175, 113274. [Google Scholar] [CrossRef]












| Physico-Chemical Parameter | Parametric Value, pv * | Weight, Wi | Relative Weight, wi | Variation of Q ** |
|---|---|---|---|---|
| EC (μS/cm) | 2500 | 4 × 10−4 | 5.69 × 10−5 | 9.84–99.52 |
| pH | 9.5 | 0.11 | 0.0156 | 2–40 |
| DO (mg/L) | 5 | 0.2 | 0.0284 | 0.1–11.91 |
| T (NTU) ** | 5 | 0.2 | 0.0284 | 43.2–186 |
| NH4+ (mg/L) | 0.5 | 2 | 0.2843 | 17.4–382 |
| NO3− (mg/L) | 50 | 0.02 | 0.0028 | 1.34–42 |
| NO2− (mg/L) | 0.5 | 2 | 0.2843 | 0–0.016 |
| Cl− (mg/L) | 250 | 4 × 10−3 | 5.69 × 10−4 | 7–74.72 |
| PO43− (mg/L) | 0.4 | 2.5 | 0.3554 | 12.5–130 |
| Parameter | Teceu Lake 1 (TL1) | Teceu Lake 2 (TL2) | Potable Groundwater Source (PGWS) | Mean | SD | CV, % | Stnd. Skewness | Stnd. Kurtosis | MAC * |
|---|---|---|---|---|---|---|---|---|---|
| EC (μS/cm) | 330 ± 81 | 346 ± 85 | 1917.5 ± 290.9 | 864.7 | 775 | 89.6 | 2.13 | −1.23 | 2500 |
| pH | 7.74 ± 0.18 | 7.49 ± 0.23 | 7.10 ± 0.17 | 7.44 | 0.33 | 4.5 | −0.25 | −1.23 | 6.5–9.5 |
| DO (mg/L) | 9.59 ± 1.71 | 9.35 ± 1.85 | 5.42 ± 0.46 | 8.12 | 2.41 | 29.72 | 0.50 | −1.63 | |
| OS (%) | 93.82 ± 6.97 | 91.67 ± 8.34 | 54.75 ± 8.27 | 80.05 | 19.7 | 24.63 | −1.62 | −1.27 | |
| T (NTU) ** | 4.45 ± 2.07 | 4.32 ± 2.01 | 4.14 ± 1.20 | 4.09 | 1.27 | 31.04 | 0.02 | −1.65 | 5 |
| NH4+ (mg/L) | 0.54 ± 0.39 | 0.68 ± 0.52 | 0.28 ± 0.19 | 0.50 | 0.42 | 83.44 | 4.11 | 3.47 | 0.5 |
| NO3− (mg/L) | 2.38 ± 2.99 | 3.7 ± 4.21 | 5.38 ± 5.41 | 3.82 | 4.37 | 114.5 | 5.93 | 8.20 | 50 |
| NO2− (mg/L) | 0.0008 ± 0.0006 | 0.0011 ± 0.0022 | 0.0005 ± 0.0006 | 8.6 × 10−4 | 0.0013 | 166.0 | 10.17 | 26.04 | 0.5 |
| ht (og) | 7.53 ± 1.70 | 8.50 ± 2.32 | 21.72 ± 4.45 | 12.82 | 7.08 | 55.23 | 2.50 | 0.38 | Min 5 |
| A (mg/L CaCO3) | 140.16 ± 31.45 | 152.42 ± 32.55 | 190.02 ± 27.33 | 160.86 | 36.62 | 22.77 | −0.22 | −0.26 | |
| Cl− (mg/L) | 39.46 ± 16.26 | 42.25 ± 16.55 | 234.05 ± 112.80 | 86.65 | 68.66 | 79.24 | 1.89 | −1.32 | 250 |
| PO43− (mg/L) | 0.23 ± 0.13 | 0.31 ± 0.14 | 0.18 ± 0.10 | 0.22 | 0.14 | 66.16 | 0.69 | −0.89 | 0.4 |
| Temp (°C) | 13.2 ± 8.8 | 13.4 ± 8.9 | 13.10 ± 7.7 | 13.08 | 8.14 | 62.22 | 0.94 | −1.75 |
| Parameter | Teceu Lake TL1 | Teceu Lake TL2 | Groundwater Potable Source | Mean | SD | CV, % | Stnd. Skewness | MAC | MAC WHO |
|---|---|---|---|---|---|---|---|---|---|
| Al (μg/L) | 50.30 ± 2.1 * | 26.03 ± 1.6 | 117.8 ± 3.5 | 64.71 | 47.55 | 73.48 | 0.87 | 200 | 100–200 |
| Ba (μg/L) | 64.74 ± 2.9 | 39.80 ± 1.8 | 88.98 ± 3.4 | 64.51 | 24.59 | 38.12 | −0.03 | 700 | 700–2000 |
| Cu (μg/L) | 6.44 ± 0.46 | 4.17 ± 0.29 | 8.01 ± 0.58 | 6.21 | 1.93 | 31.11 | −0.38 | 2000 | 2000 |
| Ga (μg/L) | 24.39 ± 1.37 | 14.67 ± 0.84 | 35.99 ± 1.63 | 25.02 | 1.93 | 42.67 | 0.19 | - | - |
| Li (μg/L) | 3.84 ± 0.38 | 2.83 ± 0.23 | 10.22 ± 0.86 | 5.63 | 4.01 | 71.17 | 1.14 | 30 | 0–130 |
| Mn (μg/L) | 93.21 ± 3.66 | 94.13 ± 3.71 | 92.65 ± 3.55 | 93.33 | 0.75 | 0.80 | 0.49 | 50 | 50 |
| Ni (μg/L) | 2.74 ± 0.14 | 4.70 ± 0.23 | 3.22 ± 0.17 | 3.55 | 1.02 | 28.75 | 0.93 | 20 | 7.9–16.6 |
| Rb (μg/L) | 0.75 ± 0.04 | 0.55 ± 0.02 | 0.28 ± 0.01 | 0.53 | 0.24 | 44.79 | −0.31 | - | - |
| Zn (μg/L) | 57.1 ± 2.86 | 18.07 ± 0.93 | 92.23 ± 4.61 | 55.8 | 37.1 | 66.48 | −0.11 | 5000 | 5000 |
| Ti (μg/L) | 22.7 ± 1.14 | 18.83 ± 0.94 | 42.13 ± 2.11 | 27.88 | 12.49 | 44.47 | 1.09 | - | - |
| Mo (μg/L) | 2.60 ± 0.12 | 1.85 ± 0.09 | 0.86 ± 0.03 | 1.77 | 0.87 | 49.31 | −0.29 | - | - |
| K (mg/L) | 1.98 ± 0.09 | 0.95 ± 0.04 | 7.40 ± 0.26 | 3.44 | 3.47 | 100.63 | 1.1 | 10 | 10 |
| Na (mg/L) | 9.41 ± 0.46 | 9.16 ± 0.42 | 31.26 ± 1.46 | 16.61 | 12.68 | 76.38 | 1.22 | 200 | 200 |
| Fe (mg/L) | 0.095 ± 0.005 | 0.128 ± 0.006 | 0.39 ± 0.021 | 0.20 | 0.16 | 79.10 | 1.17 | 0.2 | 0.200 |
| Mg (mg/L) | 18.36 ± 0.12 | 27.41 ± 0.84 | 38.76 ± 1.27 | 28.16 | 10.25 | 36.41 | 1.08 | 50 | 50 |
| Ca (mg/L) | 48.43 ± 0.33 | 40.02 ± 0.67 | 73.97 ± 1.49 | 54.14 | 17.67 | 32.65 | −0.86 | 100 | 100 |
| Sr (μg/L) | 0.220 ± 0.009 | 0.100 ± 0.005 | 0.890 ± 0.043 | 0.40 | 0.43 | 105.55 | 1.12 | 7000 | 50 |
| Al | Ba | Cu | Ga | Li | Mn | Ni | Rb | Zn | Ti | Mo | K | Na | Fe | Mg | Ca | Sr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1 | ||||||||||||||||
| Ba | 0.96 | 1 | |||||||||||||||
| Cu | 0.93 | 0.99 | 1 | ||||||||||||||
| Ga | 0.97 | 0.99 * | 0.98 | 1 | |||||||||||||
| Li | 0.99 | 0.91 | 0.87 | 0.94 | 1 | ||||||||||||
| Mn | −0.91 | −0.99 | −0.99 * | −0.98 | −0.85 | 1 | |||||||||||
| Ni | −0.51 | −0.73 | −0.79 | −0.68 | −0.40 | 0.81 | 1 | ||||||||||
| Rb | −0.76 | −0.56 | −0.48 | −0.61 | −0.84 | 0.45 | −0.15 | 1 | |||||||||
| Zn | 0.95 | 0.99 * | 0.99 * | 0.99 | 0.91 | −0.99 | −0.74 | −0.54 | 1 | ||||||||
| Ti | 0.99 | 0.93 | 0.89 | 0.95 | 0.99 * | −0.87 | −0.43 | −0.83 | 0.92 | 1 | |||||||
| Mo | −0.76 | −0.56 | −0.47 | −0.60 | −0.84 | 0.45 | −0.16 | 1 ** | −0.54 | −0.83 | 1 | ||||||
| K | 0.99 | 0.92 | 0.88 | 0.95 | 0.99 | −0.87 | −0.42 | −0.83 | 0.91 | 1 ** | −0.83 | 1 | |||||
| Na | −0.97 | 0.87 | 0.81 | 0.89 | 0.99 | −0.79 | −0.29 | −0.90 | 0.85 | 0.98 | −0.89 | 0.99 | 1 | ||||
| Fe | 0.93 | 0.80 | 0.74 | 0.83 | 0.97 | −0.72 | −0.18 | −0.94 | 0.79 | 0.96 | −0.94 | 0.97 | 0.99 | 1 | |||
| Mg | 0.91 | 0.76 | 0.70 | 0.80 | 0.96 | −0.67 | −0.12 | −0.96 | 0.75 | 0.94 | −0.96 | 0.95 | 0.89 | 0.99 * | 1 | ||
| Ca | 0.51 | 0.25 | 0.16 | 0.31 | 0.61 | −0.12 | 0.48 | −0.94 | 0.23 | 0.59 | −0.94 | 0.59 | 0.97 | 0.77 | 0.81 | 1 | |
| Sr | 0.99 | 0.92 | 0.88 | 0.95 | 0.99 ** | −0.87 | −0.41 | −0.83 | 0.91 | 0.99 ** | −0.83 | 1 ** | 0.99 | 0.97 | 0.95 | 0.60 | 1 |
| EC | 0.96 | 0.82 | 0.80 | 0.89 | 0.99 | −0.79 | −0.28 | −0.91 | 0.84 | 0.98 | −0.90 | 0.98 | 0.99 ** | 0.99 | 0.89 | 0.97 | 0.98 |
| pH | −0.83 | −0.65 | −0.57 | −0.69 | −0.89 | 0.54 | −0.05 | 0.99 | −0.63 | −0.88 | 0.99 | −0.88 | −0.94 | −0.97 | −0.99 | −0.84 | −0.88 |
| DO | −0.94 | −0.83 | −0.77 | −0.86 | −0.98 | 0.75 | 0.22 | 0.93 | −0.81 | −0.98 | 0.93 | −0.97 | −0.99 * | −0.99 * | −0.92 | −0.95 | −0.98 |
| T | −0.95 | −0.83 | −0.78 | −0.87 | −0.99 | 0.76 | 0.24 | 0.92 | −0.82 | −0.97 | 0.92 | −0.98 | −0.99 * | −0.99 * | −0.91 | −0.95 | −0.98 |
| NH4+ | −0.99 * | −0.95 | −0.92 | −0.97 | −0.99 | 0.90 | 0.48 | 0.79 | −0.94 | −0.99 * | 0.79 | −0.99 * | −0.97 | −0.95 | −0.78 | −0.99 * | −0.99 * |
| NO3− | 0.94 | 0.82 | 0.70 | 0.85 | 0.98 | −0.74 | −0.20 | −0.94 | 0.81 | −0.97 | −0.93 | 0.97 | 0.99 | 0.99 * | 0.93 | 0.95 | 0.98 |
| ht | 0.94 | 0.80 | 0.74 | 0.84 | 0.97 | −0.72 | −0.14 | −0.95 | 0.79 | 0.96 | −0.94 | 0.97 | 0.99 | 1 * | 0.94 | 0.94 | 0.97 |
| A | 0.52 | 0.26 | 0.17 | 0.32 | 0.62 | −0.14 | 0.47 | −0.95 | 0.84 | 0.59 | −0.95 | 0.60 | 0.71 | 0.78 | 0.95 | 0.53 | 0.61 |
| Cl− | 0.97 | 0.86 | 0.80 | 0.89 | 0.99 | −0.78 | −0.27 | −0.91 | 0.85 | 0.98 | −0.90 | 0.98 | 0.99 * | 0.99 | 0.90 | 0.97 | 0.98 |
| PO43− | 0.82 | 0.63 | 0.55 | 0.67 | 0.88 | −0.53 | 0.07 | −0.99 | 0.61 | 0.87 | −0.99 | 0.67 | 0.93 | 0.97 | 0.99 | 0.83 | 0.88 |
| Water Sample | HQ(NH4+) | HQ(NO3−) | HQMn | HI | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| TL1 | 0.013 | 0.015 | 0.035 | 0.041 | 0.016 | 0.018 | 0.064 | 0.075 |
| TL2 | 0.016 | 0.019 | 0.054 | 0.064 | 0.016 | 0.019 | 0.087 | 0.102 |
| GWPS | 0.007 | 0.008 | 0.079 | 0.093 | 0.016 | 0.018 | 0.101 | 0.120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Mihali, C.; Avram, A. Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania. Water 2023, 15, 3996. https://doi.org/10.3390/w15223996
Dippong T, Mihali C, Avram A. Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania. Water. 2023; 15(22):3996. https://doi.org/10.3390/w15223996
Chicago/Turabian StyleDippong, Thomas, Cristina Mihali, and Alexandra Avram. 2023. "Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania" Water 15, no. 22: 3996. https://doi.org/10.3390/w15223996
APA StyleDippong, T., Mihali, C., & Avram, A. (2023). Water Physico-Chemical Indicators and Metal Assessment of Teceu Lake and the Adjacent Groundwater Located in a Natura 2000 Protected Area, NW of Romania. Water, 15(22), 3996. https://doi.org/10.3390/w15223996






