Feasibility Study of Using Excess Sludge Fermentation Broth as a Co-Metabolic Carbon Source for 2,4,6-Trichlorophenol Degradation
Abstract
:1. Introduction
2. Materials and Methods
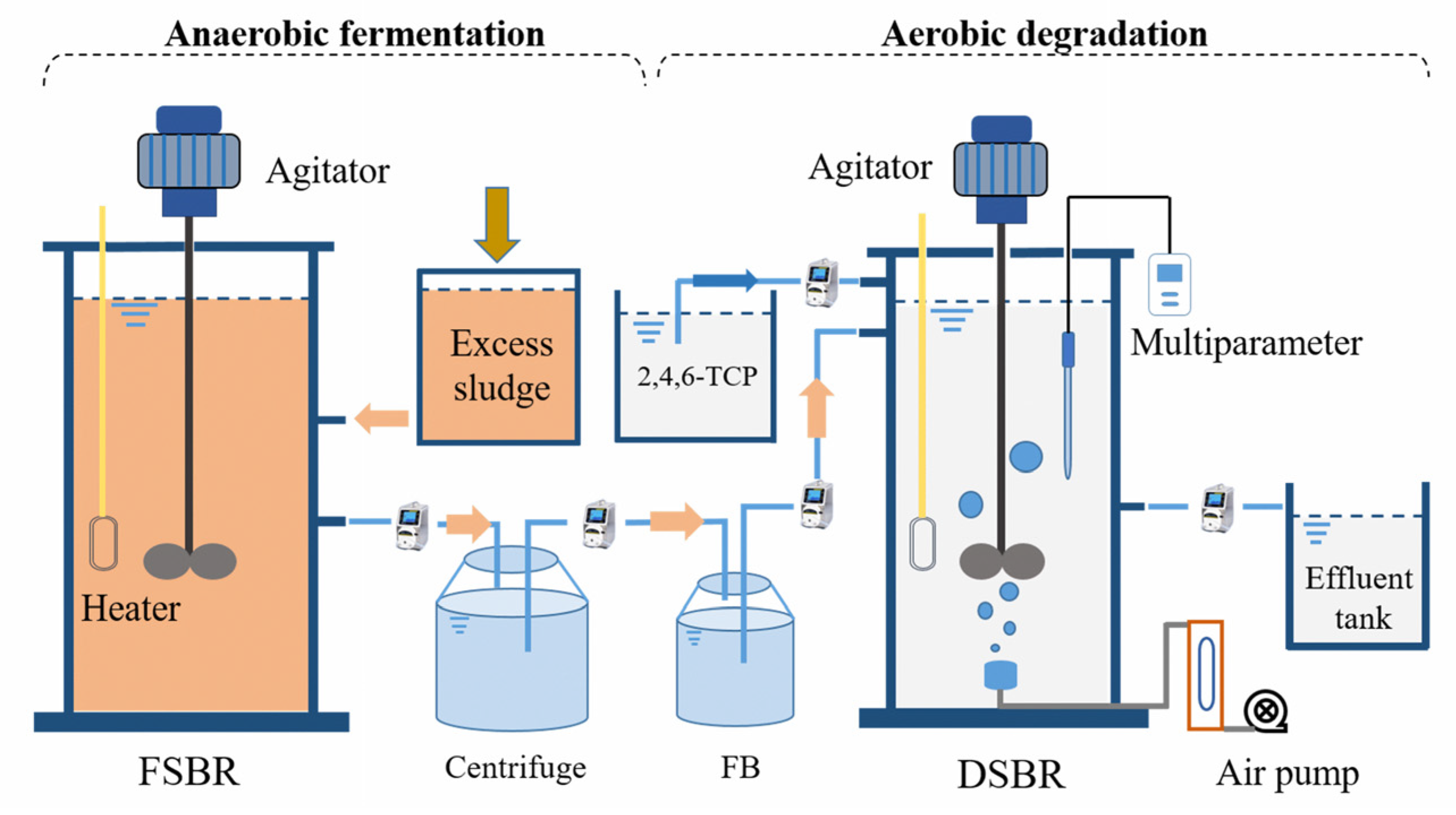
2.1. System Setup and the Reactor Operation
2.1.1. Long-Term Operation of FSBR
2.1.2. Long-Term Operation of DSBR
2.2. Seeding Sludge and Influent Wastewater Composition
2.3. The Batch Experiment
2.3.1. Mineralization and Chlorine Ion Removal Experiments of 2,4,6-TCP
2.3.2. Ammonia Nitrogen Concentration Influence Experiment
2.4. Analytical Methods
2.5. Calculation
3. Results and Discussion
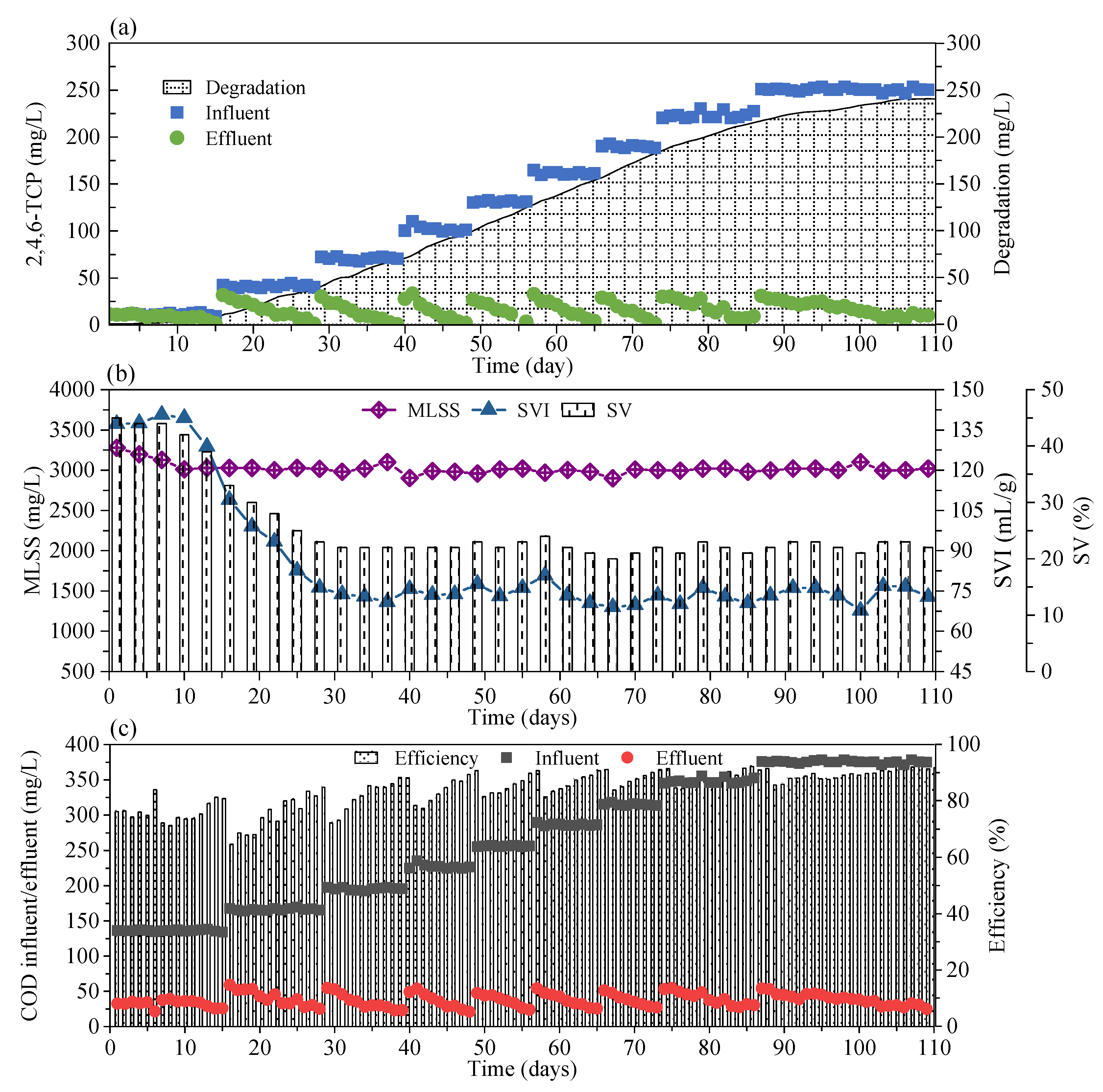
3.1. The Performance of FSBR in the Long-Term Operation
3.2. DSBR Performance in Long-Term Acclimation
3.2.1. 2,4,6-TCP Degradation Performance
3.2.2. COD Removal Performance
3.2.3. Sludge Characteristics
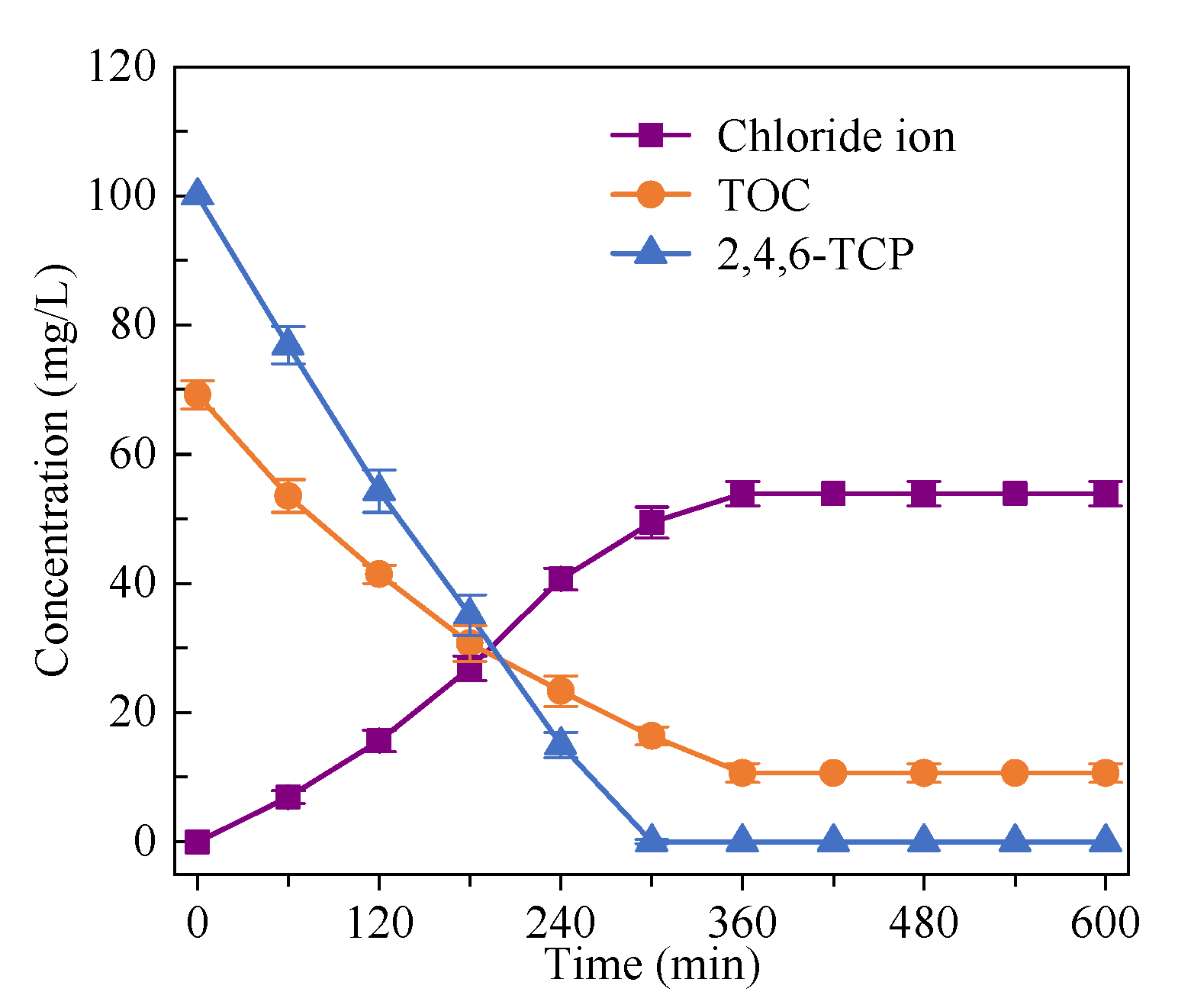
3.3. Mineralization
3.4. The Impact of Ammonia Nitrogen Influent Concentration on the Degradation of 2,4,6-TCPl
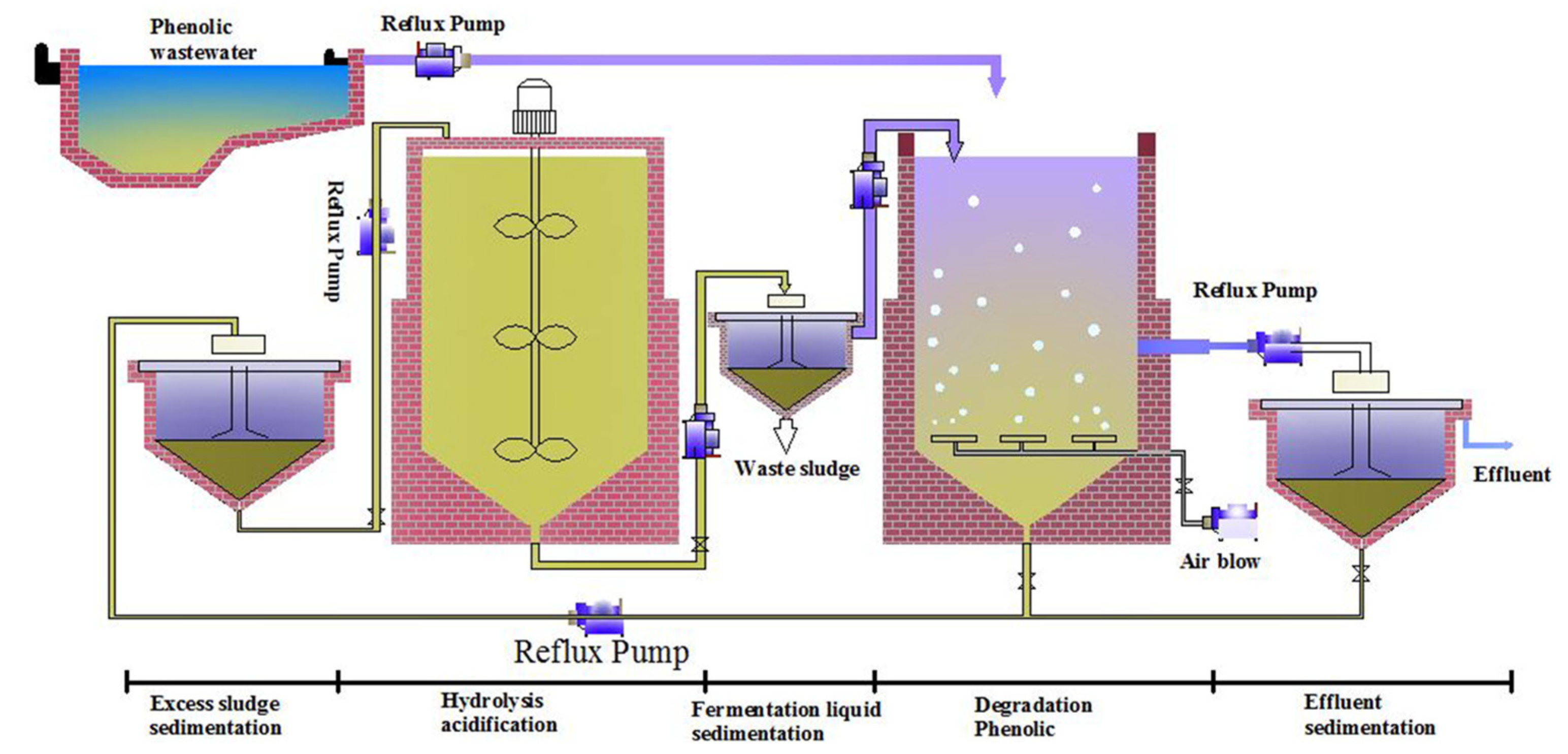
3.5. Novel Strategy for Degrading 2,4,6-TCP in a Pilot-Scale Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eker, S.; Kargi, F. COD, 2,4,6-trichlorophenol (TCP) and toxicity removal from synthetic wastewater in a rotating perforated-tubes biofilm reactor. J. Hazard. Mater. 2008, 159, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Gaya, U.I.; Abdullah, A.H.; Hussein, M.Z.; Zainal, Z. Photocatalytic removal of 2,4,6-trichlorophenol from water exploiting commercial ZnO powder. Desalination 2010, 263, 176–182. [Google Scholar] [CrossRef]
- Chanikya, P.; Nidheesh, P.V.; Syam Babu, D.; Gopinath, A.; Suresh Kumar, M. Treatment of dyeing wastewater by combined sulfate radical based electrochemical advanced oxidation and electrocoagulation processes. Sep. Purif. Technol. 2021, 254, 117570. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, J. Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater. 2021, 404, 124191. [Google Scholar] [CrossRef] [PubMed]
- Ouali, S.; Loulergue, P.; Biard, P.; Nasrallah, N.; Szymczyk, A. Ozone compatibility with polymer nanofiltration membranes. J. Membr. Sci. 2021, 618, 118656. [Google Scholar] [CrossRef]
- Correa, J. Aerobic degradation of 2,4,6-TCP content in ECF bleached effluent. Environ. Int. 2003, 29, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Stirling, D.I.; Dalton, H. The fortuitous oxidation and cometabolism of various carbon compounds by whole-cell suspensions of Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 1979, 5, 315–318. [Google Scholar] [CrossRef]
- Alvarez-Cohen, L.; Speitel, G.E., Jr. Kinetics of aerobic cometabolism of chlorinated solvents. Biodegradation 2001, 12, 105–126. [Google Scholar] [CrossRef]
- Goltz, M.N.; Bouwer, E.J.; Huang, J. Transport issues and bioremediation modeling for the in situ aerobic co-metabolism of chlorinated solvents. Biodegradation 2001, 12, 127–140. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Z. Effects of different carbon sources on 2,4,6-trichlorophenol degradation in the activated sludge process. Bioprocess Biosyst. Eng. 2020, 43, 2143–2152. [Google Scholar] [CrossRef]
- Majumder, P.S.; Gupta, S.K. Effect of carbon sources and shock loading on the removal of chlorophenols in sequential anaerobic-aerobic reactors. Bioresour. Technol. 2008, 99, 2930–2937. [Google Scholar] [CrossRef]
- Mun, C.H.; Ng, W.J.; He, J. Aclidogenic sequencing batch reactor start-up procedures for induction of 2,4,6-trichlorophenol dechlorination. Water Res. 2008, 42, 1675–1683. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.C.; Shi, H.C.; Liu, H.; Wang, J.L.; Qian, Y. Removal of 2,4-dichlorophenol in a conventional activated sludge system through bioaugmentation. Process Biochem. 2004, 39, 1701–1707. [Google Scholar] [CrossRef]
- Ziagova, M.; Kyriakou, G.; Liakopoulou-Kyriakides, M. Co-metabolism of 2,4-dichlorophenol and 4-Cl-m-cresol in the presence of glucose as an easily assimilated carbon source by Staphylococcus xylosus. J. Hazard. Mater. 2009, 163, 383–390. [Google Scholar] [CrossRef]
- Shin, J.; Rhee, C.; Shin, J.; Jang, H.M.; Shin, S.G.; Kim, Y.M. Determining the composition of bacterial community and relative abundance of specific antibiotics resistance genes via thermophilic anaerobic digestion of sewage sludge. Bioresour. Technol. 2020, 311, 123510. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y. Effects of Fe-Mn-modified biochar addition on anaerobic digestion of sewage sludge: Biomethane production, heavy metal speciation and performance stability. Bioresour. Technol. 2020, 313, 123695. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Wang, B.; Wang, S.; Li, B.; Peng, C.; Peng, Y. Integrating waste activated sludge (WAS) acidification with denitrification by adding nitrite (NO2−). Biomass Bioenergy 2014, 67, 460–465. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Shao, M.; Hu, F.; Wang, G.; Zhao, Y.; Gao, M.; Jin, C.; She, Z. Denitrification performance evaluation and kinetics analysis with mariculture solid wastes (MSW) derived carbon source in marine recirculating aquaculture systems (RAS). Bioresour. Technol. 2020, 313, 123649. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, A.S.; Henze, M. Biological hydrolysis and acidification of sludge under anaerobic conditions: The effect of sludge type and origin on the production and composition of volatile fatty acids. Water Res. 2008, 42, 3729–3738. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhou, Q. Waste activated sludge hydrolysis and short-chain fatty acids accumulation under mesophilic and thermophilic conditions: Effect of pH. Water Res. 2009, 43, 3735–3742. [Google Scholar] [CrossRef]
- Hu, C.; Guo, Y.; Guo, L.; Zhao, Y.; Jin, C.; She, Z.; Gao, M. Comparation of thermophilic bacteria (TB) pretreated primary and secondary waste sludge carbon sources on denitrification performance at different HRTs. Bioresour. Technol. 2020, 297, 122438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, K.; Su, Y.; Zheng, X.; Wang, Q. Continuous bioproduction of short-chain fatty acids from sludge enhanced by the combined use of surfactant and alkaline pH. Bioresour. Technol. 2013, 140, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z. Exploring the effects of carbon source level on the degradation of 2,4,6-trichlorophenol in the co-metabolism process. J. Hazard. Mater. 2020, 392, 122293. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Chen, T.; Wang, L.; Wu, M.; Zhao, Q.; Wei, W. High-strength fermentable wastewater reclamation through a sequential process of anaerobic fermentation followed by microalgae cultivation. Bioresour. Technol. 2017, 227, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Mii, J.; Morono, Y.; Tanji, Y.; Unno, H. Kinetic analyses of trichloroethylene cometabolism by toluene-degrading bacteria harboring a tod homologous gene. Biochem. Eng. 2005, 26, 59–64. [Google Scholar] [CrossRef]
- Apha. Standard Methods for the Examination of Water and Wastewater, 12th ed.; United Book Press: Washington, DC, USA, 1998. [Google Scholar]
- Feng, L.; Yan, Y.; Chen, Y. Kinetic analysis of waste activated sludge hydrolysis and short-chain fatty acids production at pH 10. J. Environ. Sci. 2009, 21, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, Y.; Zhou, Q.; Gu, G. Biological short-chain fatty acids (SCFAs) production from waste-activated sludge affected by surfactant. Water Res. 2007, 41, 3112–3120. [Google Scholar] [CrossRef]
- Yan, Y.; Feng, L.; Zhang, C.; Wisniewski, C.; Zhou, Q. Ultrasonic enhancement of waste activated sludge hydrolysis and volatile fatty acids accumulation at pH 10.0. Water Res. 2010, 44, 3329–3336. [Google Scholar] [CrossRef]
- Liao, P.H.; Lo, K.V.; Chan, W.I.; Wong, W.T. Sludge reduction and volatile fatty acid recovery using microwave advanced oxidation process. J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2007, 42, 633–639. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, K.; Li, X.; Wang, D.; Zheng, W.; Zeng, G.; Liu, J. Enhanced efficiency of biological excess sludge hydrolysis under anaerobic digestion by additional enzymes. Bioresour. Technol. 2010, 101, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Uma Rani, R.; Kaliappan, S.; Adish Kumar, S.; Rajesh Banu, J. Combined treatment of alkaline and disperser for improving solubilization and anaerobic biodegradability of dairy waste activated sludge. Bioresour. Technol. 2012, 126, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, S.; Liu, Y.; Li, B.; Wang, B.; Peng, Y. Long-term effect of pH on short-chain fatty acids accumulation and microbial community in sludge fermentation systems. Bioresour. Technol. 2015, 197, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Z. Successful application of municipal domestic wastewater as a co-substrate in 2,4,6-trichlorophenol degradation. Chemosphere 2021, 280, 130707. [Google Scholar] [CrossRef]
- Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C. Identifying different types of bulking in an activated sludge system through quantitative image analysis. Chemosphere 2011, 85, 643–652. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Ma, B.; Liu, Y.; Wang, B.; Peng, Y. Improving municipal wastewater nitrogen and phosphorous removal by feeding sludge fermentation products to sequencing batch reactor (SBR). Bioresour. Technol. 2016, 222, 326–334. [Google Scholar] [CrossRef]
- Karakashev, D.; Schmidt, J.E.; Angelidaki, I. Innovative process scheme for removal of organic matter, phosphorus and nitrogen from pig manure. Water Res. 2008, 42, 4083–4090. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, Y.; Liu, Y.; Jin, B.; Wang, B.; Wang, S. Change of pH during excess sludge fermentation under alkaline, acidic and neutral conditions. Bioresour. Technol. 2014, 174, 1–5. [Google Scholar] [CrossRef]



| Components | TCOD | SCOD | SS | Proteins | Polysaccharide | VFAs (mg COD/L) | |||
|---|---|---|---|---|---|---|---|---|---|
| Influent | 11,770 | 4984 | 10,830 | 1130 | 155 | - | 75.7 | 23.6 | - |
| Effluent | 1223.5 | 1120.2 | - | - | - | 974.5 | 634.4 | 193.4 | 5.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Sun, Z.; Li, J. Feasibility Study of Using Excess Sludge Fermentation Broth as a Co-Metabolic Carbon Source for 2,4,6-Trichlorophenol Degradation. Water 2023, 15, 4008. https://doi.org/10.3390/w15224008
Wang J, Sun Z, Li J. Feasibility Study of Using Excess Sludge Fermentation Broth as a Co-Metabolic Carbon Source for 2,4,6-Trichlorophenol Degradation. Water. 2023; 15(22):4008. https://doi.org/10.3390/w15224008
Chicago/Turabian StyleWang, Jianguang, Zhirong Sun, and Jun Li. 2023. "Feasibility Study of Using Excess Sludge Fermentation Broth as a Co-Metabolic Carbon Source for 2,4,6-Trichlorophenol Degradation" Water 15, no. 22: 4008. https://doi.org/10.3390/w15224008
APA StyleWang, J., Sun, Z., & Li, J. (2023). Feasibility Study of Using Excess Sludge Fermentation Broth as a Co-Metabolic Carbon Source for 2,4,6-Trichlorophenol Degradation. Water, 15(22), 4008. https://doi.org/10.3390/w15224008







