Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Experimental Design
2.2.1. The Change Pattern of the DO Concentration in an RAS
2.2.2. Construction of UROxygen and QOxygen Models for the U-Tube Oxygenator
2.2.3. The Analysis Method
3. Results and Discussion
3.1. The Change Pattern of the DO Concentration in an RAS
3.1.1. Effect of the Water-Treatment Units on the DO Concentration
3.1.2. The OCR of the APs
3.2. Models
3.2.1. Response-Surface Models Related Data Analysis
3.2.2. The UROxygen RSM
3.2.3. The QOxygen RSM
3.2.4. The QOxygen Feedback-Control Model
3.3. Analysis of the RAS Oxygenation Cost
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DO | dissolved oxygen |
| RAS | recirculating aquaculture system |
| UROxygen | oxygen-utilization rate |
| QOxygen | pure oxygen addition |
| QRAS | RAS flow |
| MFeeding | daily feeding rate |
| MDF | microscreen drum filter |
| TMDF | MDF backwashing period |
| RSM | response-surface model |
| AP | aquaculture ponds |
| FS | foam separator |
| MBBR | moving bed biofilm reactor |
| OCR | oxygen-consumption rate |
| CCD | central composite design |
| CV | coefficient of variation |
| AP | adequate precision |
| mshrimp | the average weight of shrimp |
References
- Suantika, G.; Situmorang, M.L.; Saputra, F.I.; Putri, S.L.E.; Putri, S.P.; Aditiawati, P.; Fukusaki, E. Metabolite profiling of whiteleg shrimp Litopenaeus vannamei from super-intensive culture in closed aquaculture systems: A recirculating aquaculture system and a hybrid zero water discharge–recirculating aquaculture system. Metabolomics 2020, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Kooloth Valappil, R.; Stentiford, G.D.; Bass, D. The rise of the syndrome–sub-optimal growth disorders in farmed shrimp. Rev. Aquac. 2021, 13, 1888–1906. [Google Scholar] [CrossRef]
- Albalat, A.; Zacarias, S.; Coates, C.J.; Neil, D.M.; Planellas, S.R. Welfare in farmed decapod crustaceans, with particular reference to Penaeus vannamei. Front. Mar. Sci. 2022, 677, 886024. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.; Zhou, L.; Chen, F.; Qiu, T.; Sun, J. Retrofitting sea cucumber nursery tanks to recirculating aquaculture systems for highly intensive Litopenaeus vannamei aquaculture. Appl. Sci. 2021, 11, 9478. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, H.; Chang, Z.; Song, X.; Zhao, F.; Li, J. Nitrogen budget in recirculating aquaculture and water exchange systems for culturing Litopenaeus Vannamei. J. Ocean Univ. China 2018, 17, 905–912. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Du, Y.; Sun, J. Nitrogen migration law and recycling strategy in an innovative recirculating aquaculture system: Enhancing performance through electrocoagulation. J. Water Process Eng. 2022, 50, 103275. [Google Scholar] [CrossRef]
- Xiao, R.; Wei, Y.; An, D.; Li, D.; Ta, X.; Wu, Y.; Ren, Q. A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev. Aquac. 2019, 11, 863–895. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy use in recirculating aquaculture systems (RAS): A review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Kır, M.; Sunar, M.C.; Topuz, M.; Sarıipek, M. Thermal acclimation capacity and standard metabolism of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) at different temperature and salinity combinations. J. Therm. Biol. 2023, 112, 103429. [Google Scholar] [CrossRef]
- Colt, J.; Summerfelt, S.; Pfeiffer, T.; Fivelstad, S.; Rust, M. Energy and resource consumption of land-based Atlantic salmon smolt hatcheries in the Pacific Northwest (USA). Aquaculture 2008, 280, 94–108. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.P.; Aubin, J. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.P.; Belaud, A. Water quality and rainbow trout performance in a Danish Model Farm recirculating system: Comparison with a flow through system. Aquac. Eng. 2009, 40, 135–143. [Google Scholar] [CrossRef]
- Wei, Y.; Jiao, Y.; An, D.; Li, D.; Li, W.; Wei, Q. Review of dissolved oxygen detection technology: From laboratory analysis to online intelligent detection. Sensors 2019, 19, 3995. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Huang, L.; Li, D.; Duan, Q. Modelling and controlling dissolved oxygen in recirculating aquaculture systems based on mechanism analysis and an adaptive PID controller. Comput. Electron. Agric. 2022, 192, 106583. [Google Scholar] [CrossRef]
- Ta, X.; Wei, Y. Research on a dissolved oxygen prediction method for recirculating aquaculture systems based on a convolution neural network. Comput. Electron. Agric. 2018, 145, 302–310. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, X.; Li, W.; Wei, Y.; An, D. Research of dissolved oxygen prediction in recirculating aquaculture systems based on deep belief network. Aquac. Eng. 2020, 90, 102085. [Google Scholar] [CrossRef]
- Dolan, E.; Murphy, N.; O’Hehir, M. Factors influencing optimal micro-screen drum filter selection for recirculating aquaculture systems. Aquac. Eng. 2013, 56, 42–50. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Sun, J.; Du, Y. Construction and application of an electrocoagulation and filtration linkage control system in a recirculating aquaculture system. J. Water Process Eng. 2021, 44, 102379. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Onukwuli, O.D.; Ighalo, J.O.; Menkiti, M.C. Bio-coagulation-flocculation (BCF) of municipal solid waste leachate using picralima nitida extract: RSM and ANN modelling. Curr. Res. Green Sustain. Chem. 2021, 4, 100078. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, T.; Chen, F.; Zhou, L.; Li, Y.; Sun, J.; Du, Y. Treating mariculture wastewater using electrocoagulation-microscreen drum filter technology: Electrode passivation and influencing factors. Environ. Eng. Sci. 2022, 39, 535–549. [Google Scholar] [CrossRef]
- Whangchai, N.; Klahan, R.; Balakrishnan, D.; Unpaprom, Y.; Ramaraj, R.; Pimpimol, T. Development of aeration devices and feeding frequencies for oxygen concentration improvement in 60-tones freshwater recirculating aquaculture and biofloc ponds of Asian seabass (Lates calcarifer) rearing. Chemosphere 2022, 307, 135761. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Olague, D.; Ponce-Palafox, J.T.; Castillo-Vargasmachuca, S.G.; Arámbul-Muñoz, E.; de los Santos, R.C.; Esparza-Leal, H.M. Effect of nursery system and stocking density to produce juveniles of whiteleg shrimp Litopenaeus Vannamei. Aquac. Rep. 2021, 20, 100709. [Google Scholar] [CrossRef]
- de Melo Filho ME, S.; Owatari, M.S.; Mouriño, J.L.P.; Carciofi BA, M.; Soares, H.M. Empirical modeling of feed conversion in Pacific white shrimp (Litopenaeus vannamei) growth. Ecol. Model. 2020, 437, 109291. [Google Scholar] [CrossRef]
- Weerasingha, R.; Park, G.; Yun, H.; Bai, S.C.; Lee, D.M.; Sung-Gun, K.; Jang, I.K. Effects of dietary administration of Macsumsuk® on growth and stress to low salinity and low dissolved oxygen in whiteleg shrimp, Litopenaeus vannamei (Boone, 1931) juveniles. Aquac. Res. 2020, 51, 1061–1068. [Google Scholar] [CrossRef]
- Walker, S.J.; Neill, W.H.; Lawrence, A.L.; Gatlin, D.M., III. Effect of salinity and body weight on ecophysiological performance of the Pacific white shrimp (Litopenaeus vannamei). J. Exp. Mar. Biol. Ecol. 2009, 380, 119–124. [Google Scholar] [CrossRef]
- Tahraoui, H.; Belhadj, A.E.; Triki, Z.; Boudellal, N.R.; Seder, S.; Amrane, A.; Jie, Z.; Nassim, M.; Amina, T.; Radhia, F.; et al. Mixed coagulant-flocculant optimization for pharmaceutical effluent pretreatment using response surface methodology and Gaussian process regression. Process Saf. Environ. Prot. 2023, 169, 909–927. [Google Scholar] [CrossRef]
- Sık, E.; Kobya, M.; Demirbas, E.; Gengec, E.; Oncel, M.S. Combined effects of co-existing anions on the removal of arsenic from groundwater by electrocoagulation process: Optimization through response surface methodology. J. Environ. Chem. Eng. 2017, 5, 3792–3802. [Google Scholar] [CrossRef]
- Dalla Santa, K.; Vinatea, L. Evaluation of respiration rates and mechanical aeration requirements in semi-intensive shrimp Litopenaeus vannamei culture ponds. Aquac. Eng. 2007, 36, 73–80. [Google Scholar] [CrossRef]
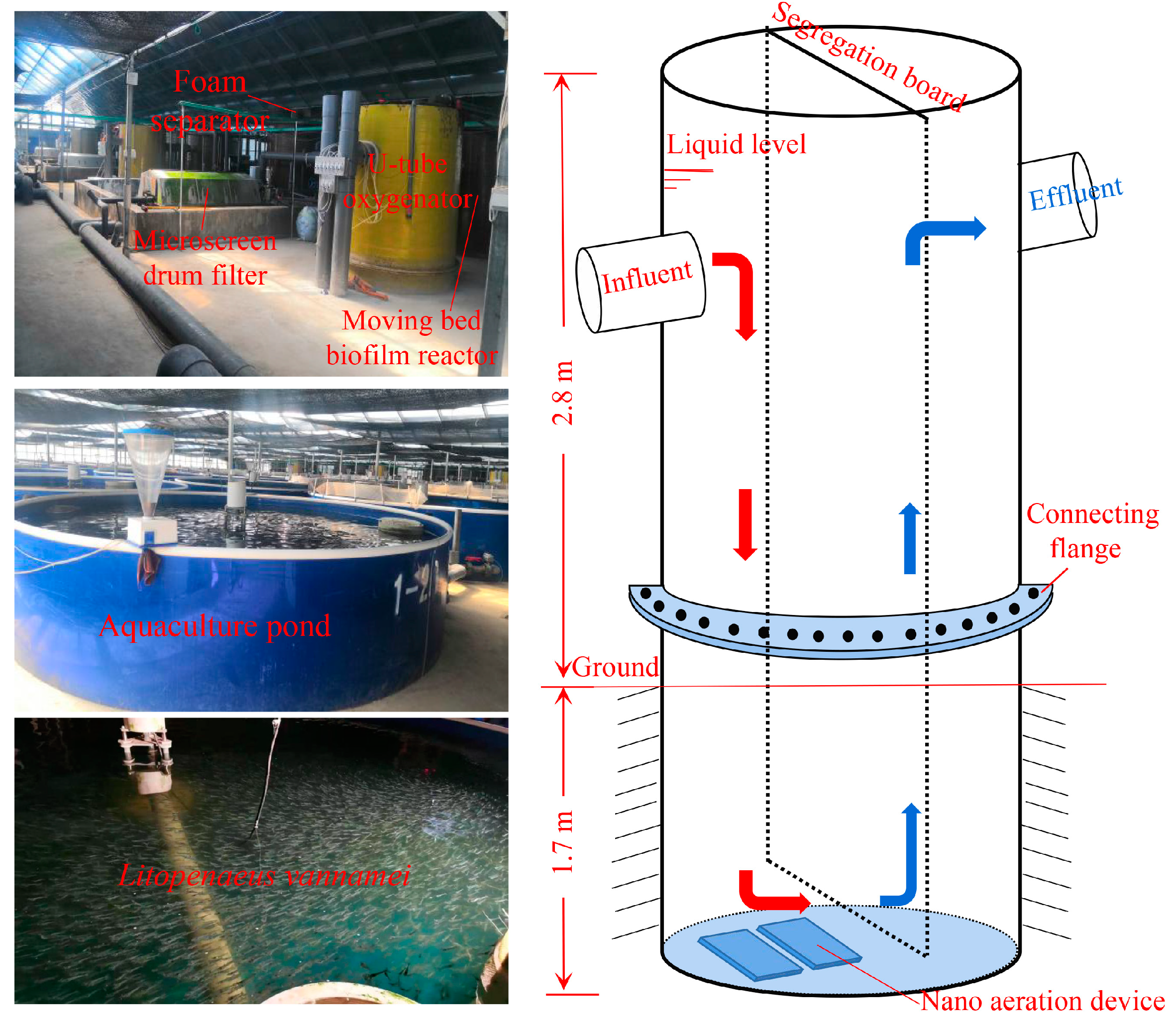

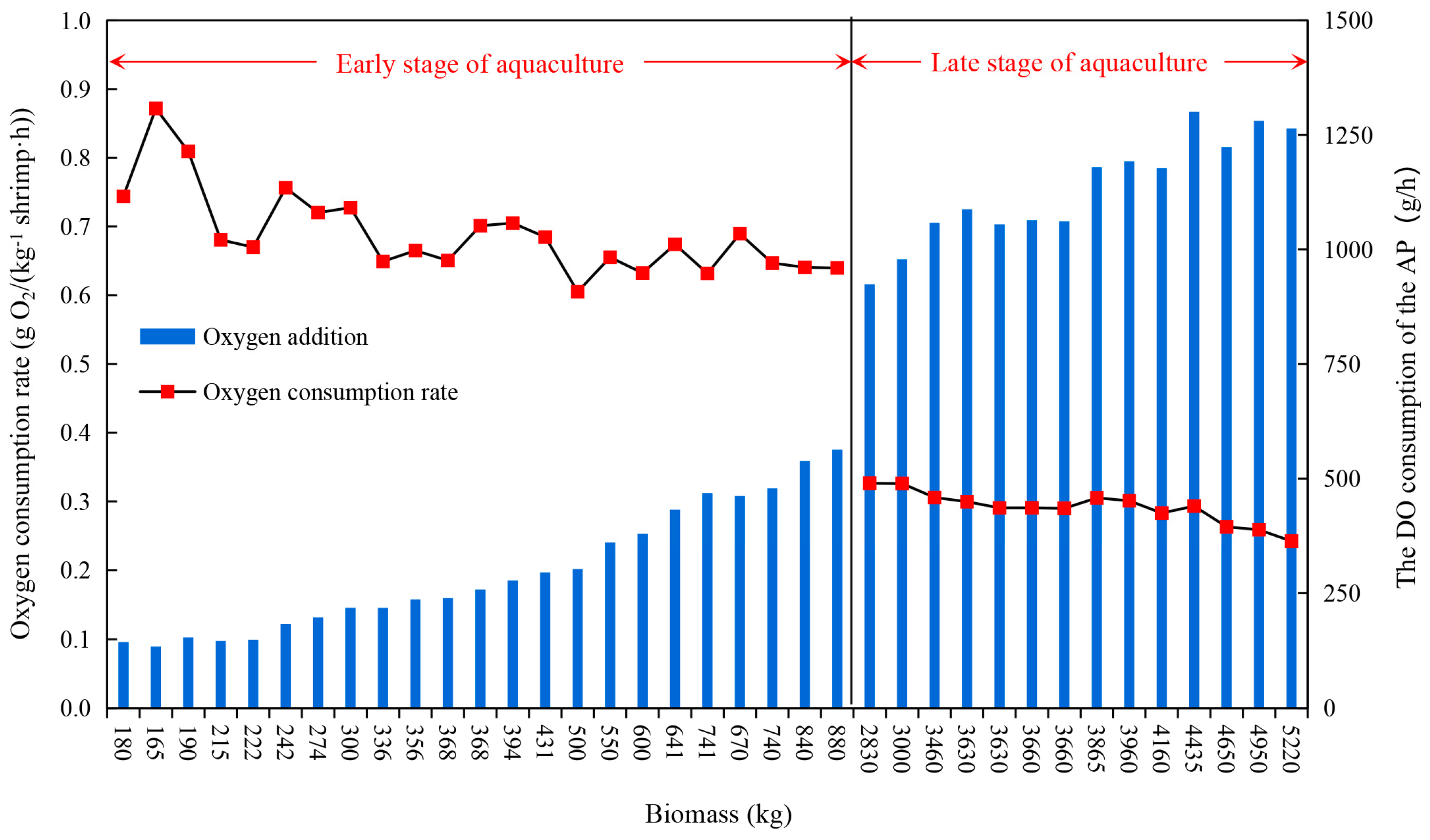

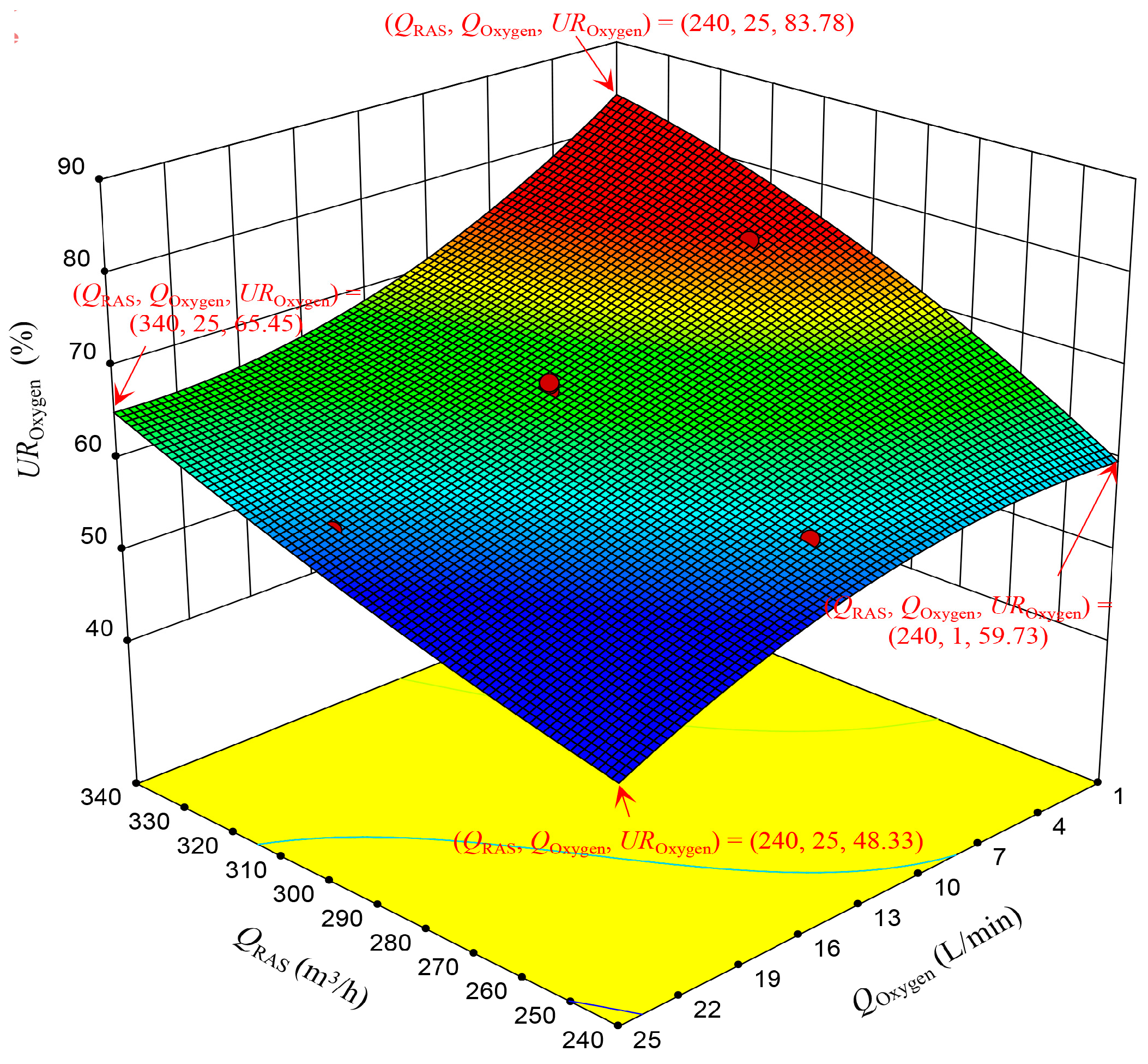


| Responses | Factors | Levels | |||
|---|---|---|---|---|---|
| Low | High | −alpha | +alpha | ||
| UROxygen (%) | QRAS (m3/h) | 266 | 337 | 251.3 | 351.7 |
| QOxygen (L/min) | 6.5 | 20.5 | 3.6 | 23.4 | |
| QOxygen (L/min) | QRAS (m3/h) | 250 | 320 | 235.5 | 334.5 |
| MFeeding (kg/d) | 40 | 80 | 31.7 | 88.3 | |
| Run | Factors | Response | Run | Factors | Response | ||
|---|---|---|---|---|---|---|---|
| QRAS (m3/h) | QOxygen (L/min) | UROxygen (%) | QRAS (m3/h) | MFeeding (kg/d) | QOxygen (L/min) | ||
| 1 | 301.5 | 23.4 | 58.16 | 1 | 334.5 | 60.0 | 14.5 |
| 2 | 301.5 | 13.5 | 66.57 | 2 | 250.0 | 40.0 | 7.5 |
| 3 | 301.5 | 13.5 | 65.12 | 3 | 235.5 | 60.0 | 19.0 |
| 4 | 337.0 | 6.5 | 75.65 | 4 | 250.0 | 80.0 | 26.5 |
| 5 | 337.0 | 20.5 | 65.17 | 5 | 285.0 | 31.7 | 4.5 |
| 6 | 301.5 | 13.5 | 66.78 | 6 | 285.0 | 60.0 | 16.0 |
| 7 | 266.0 | 20.5 | 55.12 | 7 | 285.0 | 60.0 | 16.5 |
| 8 | 266.0 | 6.5 | 65.78 | 8 | 285.0 | 88.3 | 25.0 |
| 9 | 351.7 | 13.5 | 70.35 | 9 | 320.0 | 40.0 | 5.0 |
| 10 | 301.5 | 13.5 | 65.85 | 10 | 285.0 | 60.0 | 16.5 |
| 11 | 301.5 | 3.6 | 78.12 | 11 | 285.0 | 60.0 | 16.5 |
| 12 | 301.5 | 13.5 | 64.78 | 12 | 285.0 | 60.0 | 16.0 |
| 13 | 251.3 | 13.5 | 60.12 | 13 | 320 | 80.0 | 21.5 |
| Response Value | R2 | Adj-R2 | Pred-R2 | CV (%) | Prob > F | AP |
|---|---|---|---|---|---|---|
| Model 1: UROxygen | 0.9906 | 0.9773 | 0.9001 | 1.28 | <0.0001 | 29.456 |
| Model 2: QOxygen | 0.9883 | 0.9799 | 0.9197 | 6.13 | <0.0001 | 34.757 |
| QRAS (m3/h) | MFeeding (kg/d) | MBiomass (kg) | QOxygen (L/min) | AP Effluent (mg/L) | MBBR Effluent (mg/L) | AP Influent (mg/L) | AP DO Loss (g/h) | O2 Addition (g/h) | RAS DO Increase (g/h) | UROxygen (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ai | Pi | ||||||||||
| 248 | 19.7 | 197 | 7.0 | 8.58 | 7.61 | 9.16 | 143.84 | 600.00 | 384.40 | 64.07 | 62.10 |
| 248 | 19.7 | 180 | 6.0 | 8.21 | 7.38 | 8.75 | 133.92 | 514.29 | 339.76 | 66.06 | 62.34 |
| 248 | 21.2 | 190 | 6.0 | 8.10 | 7.47 | 8.72 | 153.76 | 514.29 | 310.00 | 60.28 | 62.34 |
| 248 | 21.2 | 215 | 6.0 | 8.02 | 7.35 | 8.61 | 146.32 | 514.29 | 312.48 | 60.76 | 62.34 |
| 286 | 22.2 | 222 | 3.6 | 7.34 | 7.15 | 7.86 | 148.72 | 308.57 | 203.06 | 65.81 | 71.94 |
| 286 | 23.2 | 242 | 6.0 | 7.94 | 7.37 | 8.58 | 183.04 | 514.29 | 346.06 | 67.29 | 70.04 |
| 286 | 25.4 | 274 | 3.2 | 7.25 | 7.25 | 7.94 | 197.34 | 274.29 | 197.34 | 71.95 | 72.26 |
| 248 | 26.6 | 300 | 4.0 | 7.47 | 7.46 | 8.35 | 218.24 | 342.86 | 220.72 | 64.38 | 62.67 |
| 266 | 26.6 | 336 | 4.0 | 7.46 | 7.42 | 8.28 | 218.12 | 342.86 | 228.76 | 66.72 | 67.27 |
| 266 | 26.6 | 356 | 4.0 | 7.37 | 7.46 | 8.26 | 236.74 | 342.86 | 212.8 | 62.07 | 67.27 |
| 266 | 26.8 | 368 | 4.0 | 7.35 | 7.42 | 8.25 | 239.40 | 342.86 | 220.78 | 64.39 | 67.27 |
| 266 | 26.8 | 368 | 6.0 | 7.66 | 7.42 | 8.63 | 258.02 | 514.29 | 321.86 | 62.58 | 66.29 |
| 248 | 27.4 | 394 | 3.5 | 6.95 | 7.32 | 8.07 | 277.76 | 300.00 | 186.00 | 62.00 | 62.73 |
| 248 | 27.4 | 431 | 4.0 | 6.92 | 7.28 | 8.11 | 295.12 | 342.86 | 205.84 | 60.04 | 62.67 |
| 248 | 27.4 | 500 | 4.0 | 7.06 | 7.42 | 8.28 | 302.56 | 342.86 | 213.28 | 62.21 | 62.67 |
| 286 | 31.8 | 550 | 9.0 | 7.95 | 7.48 | 9.21 | 360.36 | 771.43 | 494.78 | 64.14 | 67.67 |
| 248 | 38.5 | 600 | 10.0 | 8.09 | 7.55 | 9.62 | 379.44 | 857.14 | 513.36 | 59.89 | 61.10 |
| 298 | 40.7 | 641 | 9.5 | 7.66 | 7.34 | 9.11 | 432.10 | 814.29 | 527.46 | 64.78 | 68.80 |
| 304 | 44.5 | 741 | 9.5 | 7.41 | 7.22 | 8.95 | 468.16 | 814.29 | 525.92 | 64.59 | 69.50 |
| 298 | 40.7 | 670 | 8.5 | 7.46 | 7.34 | 9.01 | 461.90 | 728.57 | 497.66 | 68.31 | 69.68 |
| 248 | 44.3 | 740 | 12.0 | 8.01 | 7.46 | 9.94 | 478.64 | 1028.57 | 615.04 | 59.80 | 60.21 |
| 248 | 50 | 840 | 16.0 | 8.28 | 7.31 | 10.45 | 538.16 | 1371.43 | 778.72 | 56.78 | 57.85 |
| 248 | 52.6 | 880 | 17.0 | 8.05 | 7.26 | 10.32 | 562.96 | 1457.14 | 758.88 | 52.08 | 57.14 |
| 286 | 68.6 | 2830 | 16.0 | 7.18 | 7.21 | 10.41 | 923.78 | 1371.43 | 915.20 | 66.73 | 62.21 |
| 286 | 68.6 | 3000 | 20.0 | 7.63 | 7.39 | 11.05 | 978.12 | 1714.29 | 1046.76 | 61.06 | 59.14 |
| 298 | 72.6 | 3460 | 23.0 | 8.01 | 7.48 | 11.56 | 1057.90 | 1971.43 | 1215.84 | 61.67 | 58.59 |
| 298 | 72.6 | 3630 | 24.5 | 8.21 | 7.67 | 11.86 | 1087.70 | 2100.00 | 1248.62 | 59.46 | 57.65 |
| 298 | 72.6 | 3630 | 25.0 | 8.25 | 7.68 | 11.79 | 1054.92 | 2142.86 | 1224.78 | 57.16 | 57.35 |
| 286 | 73.2 | 3660 | 18.0 | 6.73 | 7.18 | 10.45 | 1063.92 | 1542.86 | 935.22 | 60.62 | 60.67 |
| 286 | 73.2 | 3660 | 21.0 | 7.26 | 7.31 | 10.97 | 1061.06 | 1800.00 | 1046.76 | 58.15 | 58.37 |
| 337 | 77.3 | 3865 | 24.0 | 7.64 | 7.26 | 11.14 | 1179.50 | 2057.14 | 1307.56 | 63.56 | 64.83 |
| 290 | 79.2 | 3960 | 24.0 | 7.28 | 7.28 | 11.39 | 1191.90 | 2057.14 | 1191.9 | 57.94 | 56.70 |
| 290 | 79.2 | 4160 | 26.0 | 7.72 | 7.31 | 11.78 | 1177.40 | 2228.57 | 1296.3 | 58.17 | 55.28 |
| 301 | 84.7 | 4435 | 29.5 | 8.06 | 7.51 | 12.38 | 1300.32 | 2528.57 | 1465.87 | 57.97 | 55.54 |
| 337 | 85 | 4650 | 23.5 | 7.74 | 7.43 | 11.37 | 1223.31 | 2014.29 | 1327.78 | 65.92 | 64.86 |
| 337 | 85 | 4950 | 25.0 | 7.75 | 7.38 | 11.55 | 1280.60 | 2142.86 | 1405.29 | 65.58 | 64.82 |
| 301 | 86.4 | 5220 | 26.0 | 7.35 | 7.36 | 11.55 | 1264.20 | 2228.57 | 1261.19 | 56.59 | 57.32 |
| No. | MFeeding (kg) | QRAS (m3/h) | TMDF (s) | QOxygen (L/min) | No. | MFeeding (kg) | QRAS (m3/h) | TMDF (s) | QOxygen (L/min) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | c | a | b | c | ||||||||
| 1 | 18.5 | 241 | 1800 | 0.0 | - | - | 51 | 56.5 | 311 | 268 | 13.0 | 13.7 | 13.6 |
| 2 | 20.0 | 248 | 1674 | 0.0 | - | - | 52 | 58.5 | 311 | 257 | 14.0 | 14.5 | 14.1 |
| 3 | 19.7 | 248 | 1714 | 0.0 | - | - | 53 | 46.5 | 318 | 343 | 12.5 | 9.3 | 10.8 |
| 4 | 21.2 | 248 | 1565 | 0.0 | - | - | 54 | 46.5 | 318 | 339 | 12.5 | 9.3 | 10.9 |
| 5 | 27.9 | 248 | 1319 | 2.0 | - | - | 55 | 47.5 | 318 | 325 | 13.0 | 9.7 | 11.4 |
| 6 | 27.4 | 248 | 1241 | 2.0 | - | - | 56 | 49.0 | 318 | 300 | 13.0 | 10.4 | 12.3 |
| 7 | 28.2 | 248 | 1286 | 2.0 | - | - | 57 | 51.5 | 318 | 284 | 13.0 | 11.4 | 13.0 |
| 8 | 27.4 | 248 | 1000 | 2.0 | - | - | 58 | 54.0 | 318 | 260 | 13.5 | 12.4 | 14.0 |
| 9 | 27.4 | 248 | 1014 | 2.0 | - | - | 59 | 57.0 | 318 | 239 | 14.5 | 13.6 | 15.0 |
| 10 | 27.4 | 248 | 966 | 2.0 | - | - | 60 | 58.0 | 318 | 237 | 15.0 | 14.0 | 15.1 |
| 11 | 26.6 | 266 | 1047 | 1.5 | - | - | 61 | 60.0 | 318 | 225 | 16.5 | 14.8 | 15.7 |
| 12 | 27.0 | 266 | 1094 | 2.0 | - | - | 62 | 61.5 | 318 | 219 | 17.5 | 15.3 | 16.0 |
| 13 | 26.8 | 266 | 1140 | 2.5 | - | - | 63 | 63.0 | 318 | 214 | 18.0 | 15.9 | 16.2 |
| 14 | 26.8 | 266 | 1125 | 2.5 | - | - | 64 | 50.4 | 325 | 237 | 13.5 | 10.7 | 15.1 |
| 15 | 20.7 | 286 | 947 | 0.0 | - | - | 65 | 52.3 | 325 | 235 | 14.0 | 11.5 | 15.2 |
| 16 | 21.2 | 286 | 930 | 0.0 | - | - | 66 | 54.5 | 325 | 243 | 15.5 | 12.3 | 14.8 |
| 17 | 21.2 | 286 | 905 | 0.0 | - | - | 67 | 56.5 | 325 | 224 | 16.5 | 13.1 | 15.7 |
| 18 | 22.2 | 286 | 889 | 0.5 | - | - | 68 | 59.0 | 325 | 214 | 17.0 | 14.1 | 16.2 |
| 19 | 23.2 | 286 | 882 | 1.0 | - | - | 69 | 60.5 | 325 | 210 | 17.5 | 14.6 | 16.5 |
| 20 | 25.4 | 286 | 857 | 1.5 | - | - | 70 | 62.0 | 325 | 205 | 17.5 | 15.2 | 16.7 |
| 21 | 27.0 | 286 | 828 | 2.0 | - | - | 71 | 63.4 | 325 | 201 | 18.0 | 15.7 | 17.0 |
| 22 | 29.4 | 297 | 688 | 2.0 | 1.6 | 2.7 | 72 | 64.8 | 325 | 199 | 18.0 | 16.1 | 17.1 |
| 23 | 30.5 | 297 | 667 | 2.5 | 2.1 | 3.1 | 73 | 65.4 | 325 | 192 | 17.5 | 16.4 | 17.5 |
| 24 | 31.4 | 297 | 649 | 3.5 | 2.6 | 3.4 | 74 | 64.6 | 337 | 190 | 17.0 | 15.5 | 17.6 |
| 25 | 33.0 | 297 | 617 | 4.0 | 3.5 | 4.0 | 75 | 61.0 | 337 | 208 | 16.0 | 14.3 | 16.6 |
| 26 | 34.2 | 297 | 591 | 4.5 | 4.1 | 4.5 | 76 | 62.0 | 337 | 202 | 16.5 | 14.6 | 16.9 |
| 27 | 35.5 | 297 | 576 | 5.0 | 4.7 | 4.8 | 77 | 62.5 | 337 | 202 | 16.5 | 14.8 | 16.9 |
| 28 | 37.4 | 297 | 541 | 5.5 | 5.7 | 5.5 | 78 | 62.0 | 337 | 205 | 16.5 | 14.6 | 16.7 |
| 29 | 39.5 | 297 | 522 | 5.5 | 6.7 | 5.9 | 79 | 63.0 | 337 | 201 | 17.0 | 15.0 | 17.0 |
| 30 | 40.7 | 297 | 497 | 5.5 | 7.3 | 6.5 | 80 | 64.5 | 337 | 198 | 17.0 | 15.5 | 17.1 |
| 31 | 37.9 | 297 | 539 | 5.5 | 5.9 | 5.5 | 81 | 65.5 | 337 | 190 | 17.0 | 15.8 | 17.6 |
| 32 | 42.5 | 297 | 466 | 6.5 | 8.2 | 7.2 | 82 | 67.5 | 337 | 186 | 17.5 | 16.5 | 17.9 |
| 33 | 44.8 | 297 | 407 | 9.5 | 9.2 | 8.8 | 83 | 67.5 | 337 | 183 | 17.5 | 16.5 | 18.0 |
| 34 | 44.7 | 297 | 424 | 7.0 | 9.2 | 8.3 | 84 | 64.0 | 337 | 195 | 17.0 | 15.3 | 17.3 |
| 35 | 44.5 | 304 | 402 | 9.5 | 8.9 | 8.9 | 85 | 64.0 | 337 | 200 | 17.0 | 15.3 | 17.0 |
| 36 | 46.5 | 304 | 391 | 9.5 | 9.8 | 9.3 | 86 | 65.0 | 337 | 190 | 17.5 | 15.6 | 17.6 |
| 37 | 46.5 | 304 | 382 | 10.0 | 9.8 | 9.5 | 87 | 66.0 | 337 | 190 | 18.0 | 16.0 | 17.6 |
| 38 | 47.5 | 304 | 365 | 10.0 | 10.2 | 10.1 | 88 | 69.0 | 337 | 185 | 18.0 | 16.9 | 17.9 |
| 39 | 48.5 | 304 | 350 | 10.5 | 10.7 | 10.5 | 89 | 72.5 | 337 | 175 | 18.0 | 18.0 | 18.6 |
| 40 | 49.5 | 304 | 342 | 10.5 | 11.1 | 10.8 | 90 | 74.0 | 337 | 168 | 18.5 | 18.4 | 19.0 |
| 41 | 50.5 | 304 | 336 | 10.5 | 11.5 | 11.0 | 91 | 75.0 | 337 | 162 | 19.5 | 18.7 | 19.5 |
| 42 | 52.0 | 304 | 313 | 11.0 | 12.2 | 11.8 | 92 | 75.5 | 337 | 157 | 19.5 | 18.9 | 19.8 |
| 43 | 52.5 | 304 | 310 | 11.5 | 12.4 | 11.9 | 93 | 76.5 | 337 | 158 | 19.5 | 19.2 | 19.7 |
| 44 | 53.5 | 304 | 300 | 11.5 | 12.8 | 12.3 | 94 | 75.5 | 337 | 154 | 19.0 | 18.9 | 20.0 |
| 45 | 53.5 | 311 | 287 | 11.5 | 12.5 | 12.8 | 95 | 76.0 | 337 | 155 | 19.5 | 19.0 | 20.0 |
| 46 | 53.5 | 311 | 290 | 12.0 | 12.5 | 12.7 | 96 | 78.5 | 337 | 150 | 20.5 | 19.7 | 20.3 |
| 47 | 53.5 | 311 | 291 | 12.5 | 12.5 | 12.7 | 97 | 78.0 | 337 | 152 | 20.5 | 19.6 | 20.2 |
| 48 | 53.0 | 311 | 292 | 12.5 | 12.3 | 12.6 | 98 | 75.0 | 337 | 155 | 20.0 | 18.7 | 20.0 |
| 49 | 54.0 | 311 | 279 | 12.5 | 12.7 | 13.2 | 99 | 76.0 | 337 | 154 | 21.0 | 19.0 | 20.0 |
| 50 | 56.0 | 311 | 269 | 13.0 | 13.5 | 13.6 | 100 | 76.0 | 337 | 150 | 21.0 | 19.0 | 20.4 |
| Aquaculture Mode | Oxygenation Method | Oxygen Addition/Energy Consumption | Total Feeding Amount (kg) | Shrimp Yield (kg) | Oxygenation Cost (CNY/kg Shrimp) | Reference |
|---|---|---|---|---|---|---|
| RAS (384 m3) | Pure oxygen | 2296 kg O2 | 4987 | 4488 | 0.42 | This study |
| RAS (338 m3) | Pure oxygen + Roots blower | 452 kg O2 + 4579 kWh | 4368 | 3956 | 1.25 | [4] |
| Factory water exchange farming (272 m3) | Roots blower | 3540 kWh | 2372 | 1590.8 | 2.33 | [5] |
| Semi-intensive pond farming (1 ha) | Paddle-wheel aerators | 2681 kWh | - | 2520 | 1.06 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Du, Y.; Su, G.; Wang, H.; Zhang, J.; Tian, H.; Zhou, L.; Qiu, T.; Sun, J. Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models. Water 2023, 15, 4019. https://doi.org/10.3390/w15224019
Xu J, Du Y, Su G, Wang H, Zhang J, Tian H, Zhou L, Qiu T, Sun J. Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models. Water. 2023; 15(22):4019. https://doi.org/10.3390/w15224019
Chicago/Turabian StyleXu, Jianping, Yishuai Du, Guogen Su, Hexiang Wang, Jiawei Zhang, Huiqin Tian, Li Zhou, Tianlong Qiu, and Jianming Sun. 2023. "Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models" Water 15, no. 22: 4019. https://doi.org/10.3390/w15224019
APA StyleXu, J., Du, Y., Su, G., Wang, H., Zhang, J., Tian, H., Zhou, L., Qiu, T., & Sun, J. (2023). Application of a U-Tube Oxygenator in a Litopenaeus vannamei Recirculating Aquaculture System: Efficiency and Management Models. Water, 15(22), 4019. https://doi.org/10.3390/w15224019







