Comparison of Solubilization Treatment Technologies for Phosphorus Release from Anaerobic Digestate of Livestock Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaerobic Digestate Source and Characterization
2.2. Experiment Setup and Conditions
2.2.1. pH Treatment
2.2.2. Sonication
2.2.3. Ozonation
2.2.4. Cold Plasma Treatment
2.2.5. Microwave Treatment
2.2.6. UV/H2O2 Treatment
2.2.7. Milling Treatment
2.3. Analytical Methods
2.4. Calculation Methods
2.5. Visual MINTEQ Software 3.1 Setup
3. Results and Discussion
3.1. Effects of Pretreatment on P Release
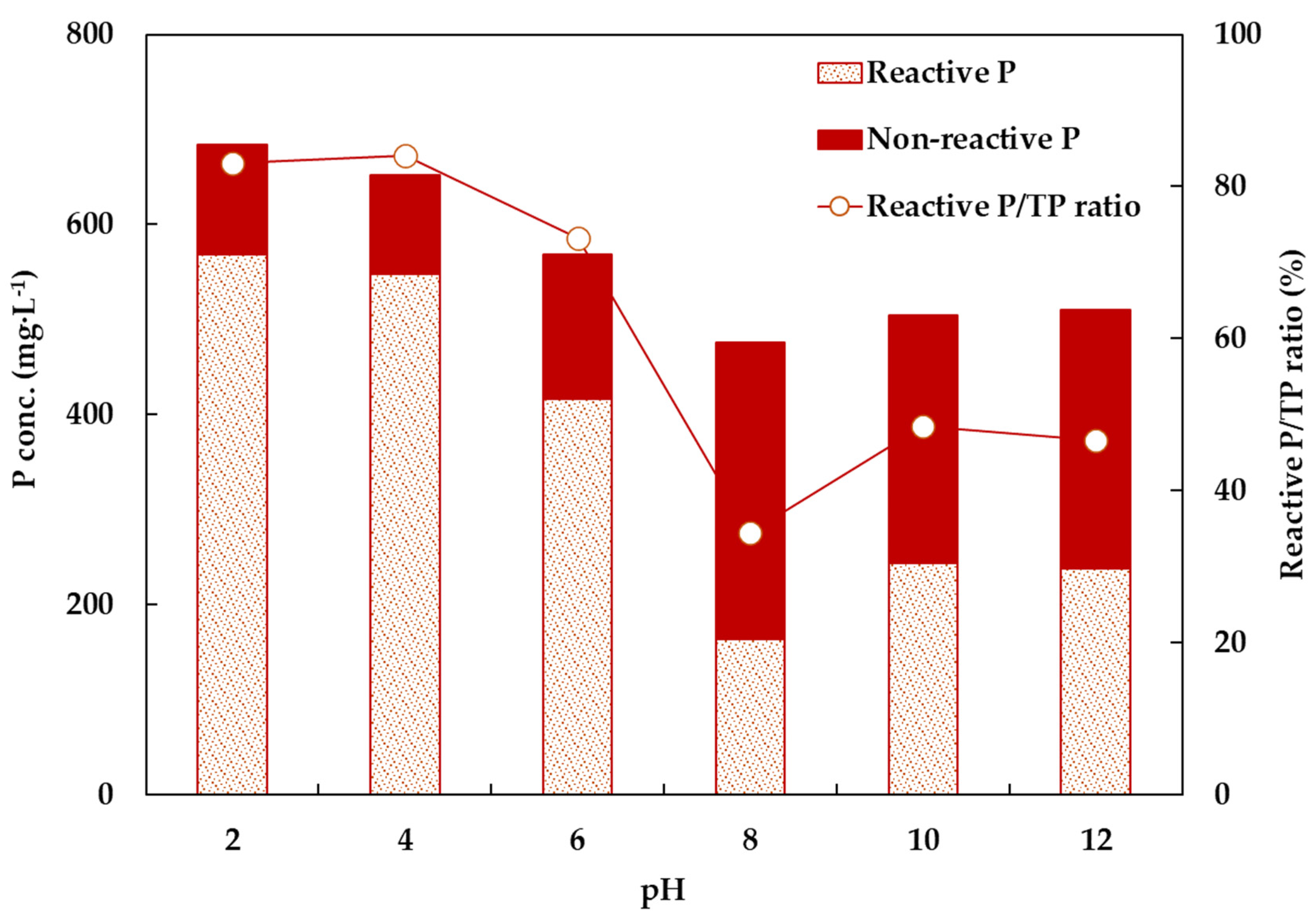
3.1.1. pH Pretreatment
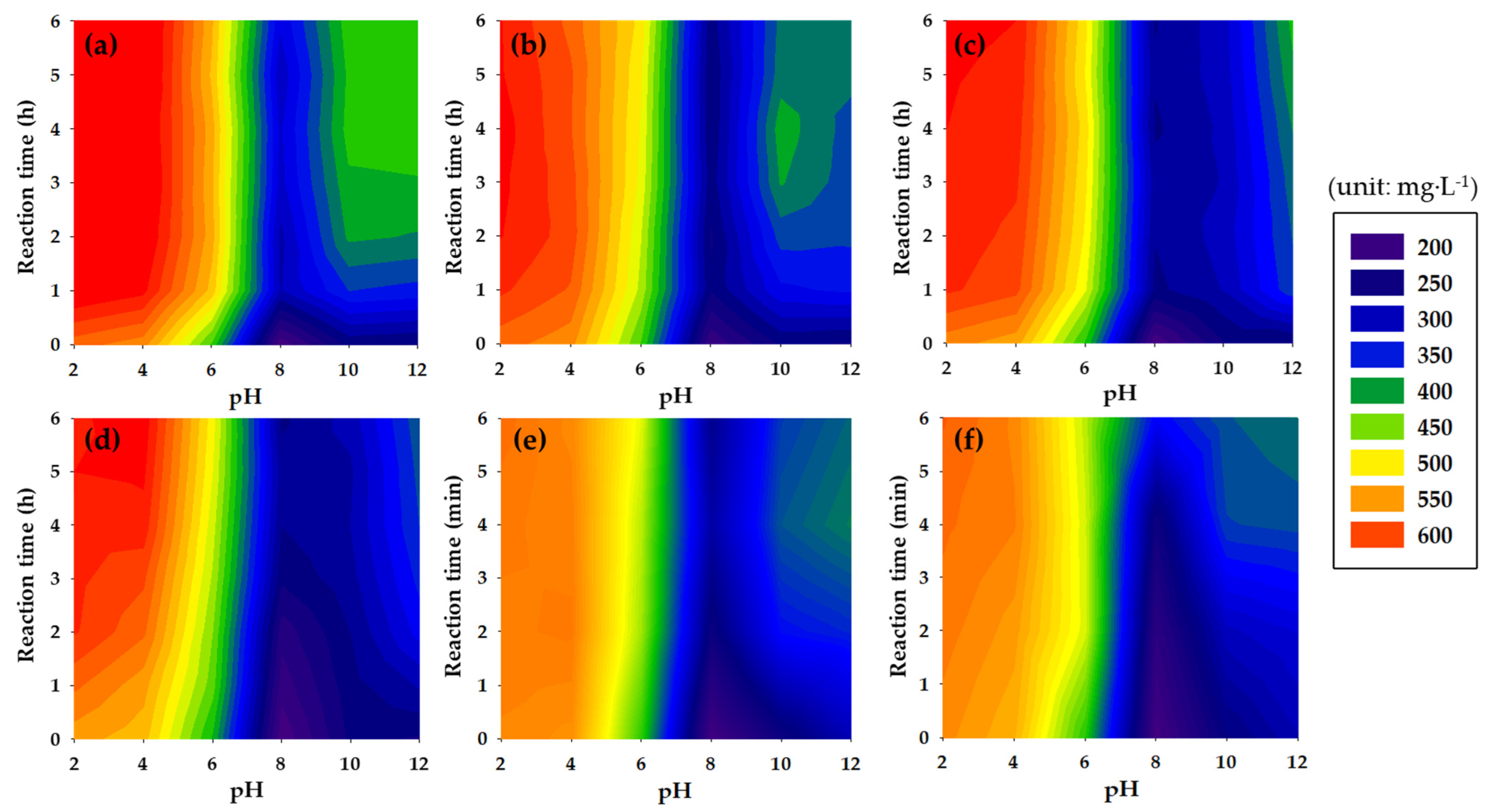
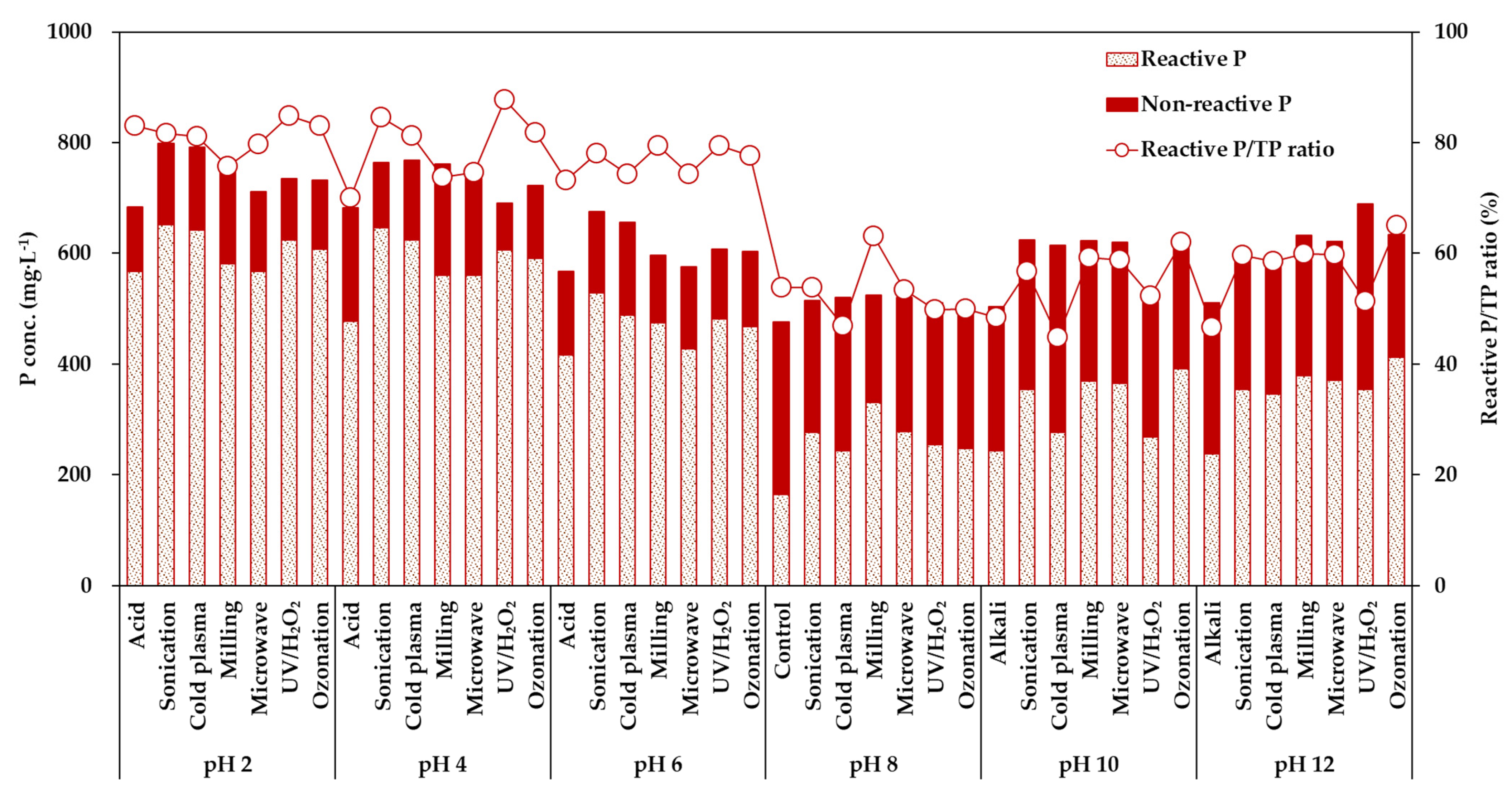
3.1.2. Pretreatment Processes and pH Combinations Process
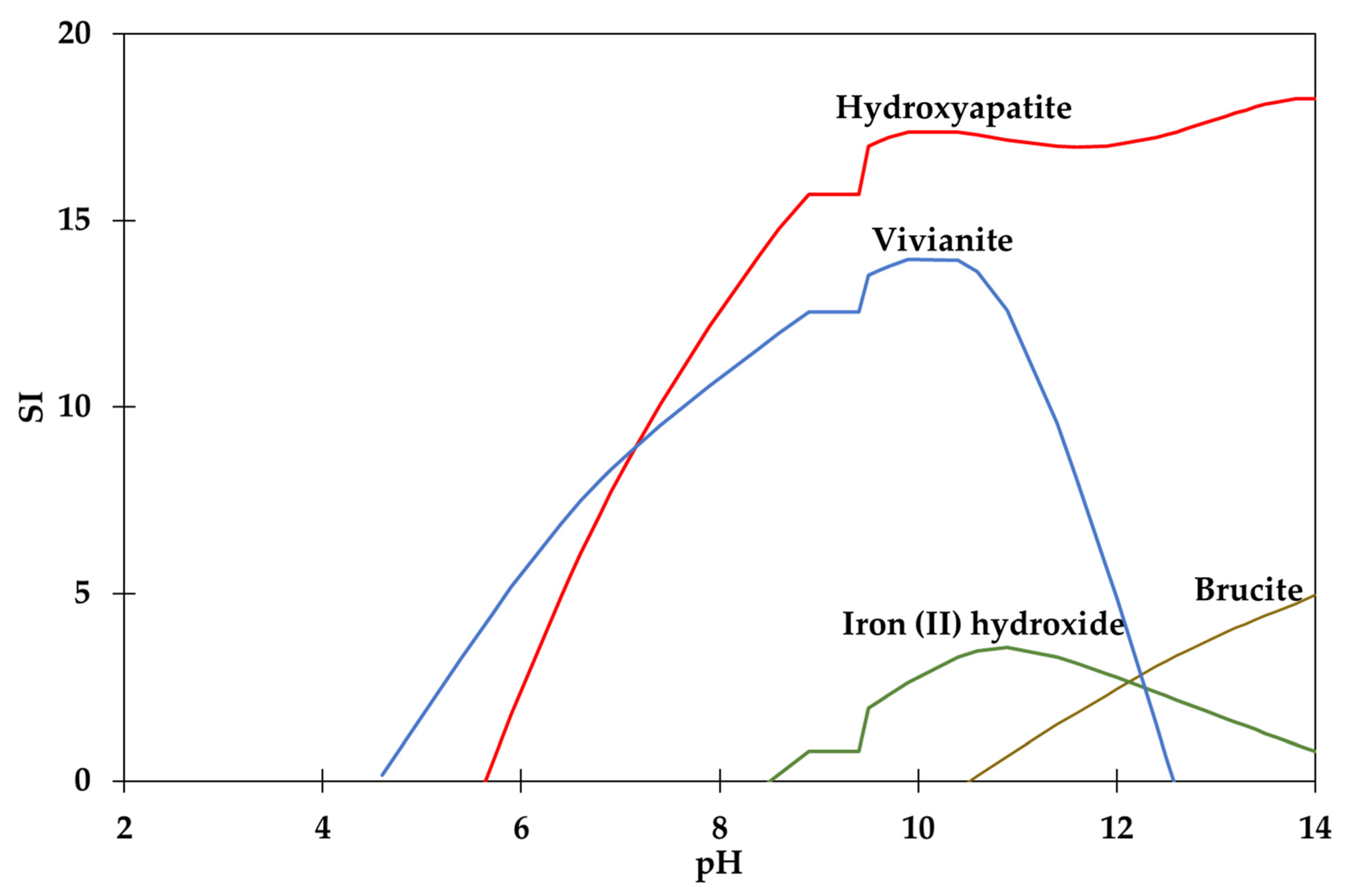
3.2. Visual MINTEQ Software Simulations
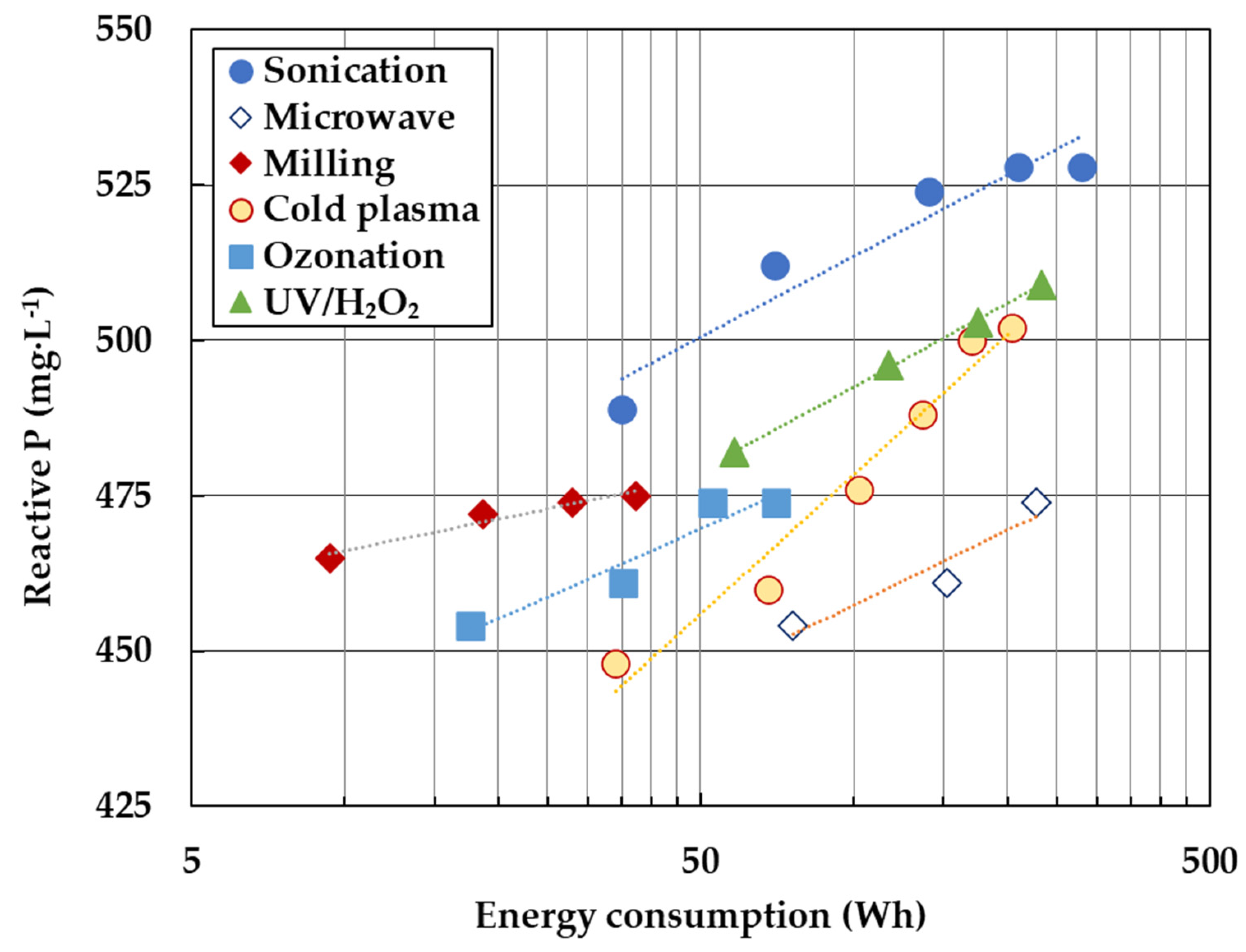
3.3. Electrical Energy Consumption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef]
- Hellal, F.; El-Sayed, S.; Zewainy, R.; Amer, A. Importance of phosphate pock application for sustaining agricultural production in Egypt. Bull. Natl. Res. Cent. 2019, 43, 11. [Google Scholar] [CrossRef]
- Fang, L.; Wang, Q.; Li, J.S.; Poon, C.S.; Cheeseman, C.R.; Donatello, S.; Tsang, D.C. Feasibility of wet-extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 939–971. [Google Scholar] [CrossRef]
- Cosgun, S.; Semerci, N. Combined and individual applications of ozonation and microwave treatment for waste activated sludge solubilization and nutrient release. J. Environ. Manag. 2019, 241, 76–83. [Google Scholar] [CrossRef]
- Van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, F.; He, Z. Electrochemical phosphorus release and recovery from wastewater sludge: A review. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1359–1377. [Google Scholar]
- Jack, J.; Huggins, T.M.; Huang, Y.; Fang, Y.; Ren, Z.J. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Guo, H.G.; Chen, Q.L.; Hu, H.W.; He, J.Z. High-solid anaerobic co-digestion of pig manure with lignite promotes methane production. J. Clean. Prod. 2020, 258, 120695. [Google Scholar] [CrossRef]
- Ma, H.; Guo, Y.; Qin, Y.; Li, Y.Y. Nutrient recovery technologies integrated with energy recovery by waste biomass anaerobic digestion. Bioresour. Technol. 2018, 269, 520–531. [Google Scholar] [CrossRef]
- Kim, D.; Min, K.J.; Lee, K.; Yu, M.S.; Park, K.Y. Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater. Environ. Eng. Res. 2017, 22, 12–18. [Google Scholar] [CrossRef]
- Min, K.J.; Kim, D.; Lee, J.; Lee, K.; Park, K.Y. Characteristics of vegetable crop cultivation and nutrient releasing with struvite as a slow-release fertilizer. Environ. Sci. Pollut. Res. 2019, 26, 34332–34344. [Google Scholar] [CrossRef] [PubMed]
- Kamusoko, R.; Jingura, R.M.; Parawira, W.; Sanyika, W.T. Comparison of pretreatment methods that enhance biomethane production from crop residues—A systematic review. Biofuel Res. J. 2019, 6, 1080–1089. [Google Scholar] [CrossRef]
- Li, W.; Yu, N.; Liu, Q.; Li, Y.; Ren, N.; Xing, D. Enhancement of the sludge disintegration and nutrients release by a treatment with potassium ferrate combined with an ultrasonic process. Sci. Total Environ. 2018, 635, 699–704. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Chaudhari, P.K.; Ghosh, P. Effect of microwave treatment on maximizing biogas yield for anaerobic co-digestion of fruit and vegetable waste and anaerobic sludge. Biomass Convers. Biorefin. 2023, 1–13. [Google Scholar] [CrossRef]
- Mawioo, P.M.; Rweyemamu, A.; Garcia, H.A.; Hooijmans, C.M.; Brdjanovic, D. Evaluation of a microwave based reactor for the treatment of blackwater sludge. Sci. Total Environ. 2016, 548, 72–81. [Google Scholar] [CrossRef]
- Dos Santos, L.C.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; de Aquino, S.F. Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresour. Technol. 2018, 263, 601–612. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Helong, Y.; Baojun, J.; Jinming, J. Comparison of sludge treatment by O3 and O3/H2O2. Water Sci. Technol. 2014, 70, 114. [Google Scholar] [CrossRef]
- Gahlot, P.; Balasundaram, G.; Tyagi, V.K.; Atabani, A.E.; Suthar, S.; Kazmi, A.A.; Štěpanec, L.; Juchelková, D.; Kumar, A. Principles and potential of thermal hydrolysis of sewage sludge to enhance anaerobic digestion. Environ. Res. 2022, 214, 113856. [Google Scholar] [CrossRef]
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, H.; Liu, J.; Luo, J.; Qian, G.; Wang, A. pH dependent phosphorus release from waste activated sludge: Contributions of phosphorus speciation. Chem. Eng. J. 2015, 267, 260–265. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Qi, X.; Chen, T.; Zhang, Y.; Gao, C.; Zhang, A. A new mechanical cutting pretreatment approach towards the improvement of primary sludge fermentation and anaerobic digestion. J. Environ. Chem. Eng. 2022, 10, 107163. [Google Scholar] [CrossRef]
- Zielewicz, E.; Tytła, M. Effects of ultrasonic disintegration of excess sludge obtained in disintegrators of different construction. Environ. Technol. 2016, 36, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.; Silva-Hernández, B.C.; Hooijmans, C.M.; Lopez-Vazquez, C.M.; Esparza-Soto, M.; Lucero-Chávez, M.; van Loosdrecht, M.C.M. Sludge reduction by ozone: Insights and modeling of the dose response effects. J. Environ. Manag. 2018, 206, 103–112. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.A.; Martín, M.A.; Chica, A.F.; Estévez-Pastor, F.S.; Toro-Baptista, E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renew. Energy 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Semblante, G.U.; Hai, F.I.; Dionysiou, D.D.; Fukushi, K.; Price, W.E.; Nghiem, L.D. Holistic sludge management through ozonation: A critical review. J. Environ. Manag. 2017, 185, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.J.; Van Loosdrecht, M.C. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef]
- Toor, U.A.; Kim, D.J. Effect of pH on phosphorus (P) dissolution and recovery from polyaluminum chlorides (PAC) sludge. J. Environ. Manag. 2019, 239, 142–149. [Google Scholar] [CrossRef]
- Chen, J.; Tang, S.; Yan, F.; Zhang, Z. Efficient recovery of phosphorus in sewage sludge through hydroxylapatite enhancement formation aided by calcium-based additives. Water Res. 2020, 171, 115450. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Liu, S.; Zhou, X.; Gao, L.; Le, Y.; Zhang, R. Phosphorus complexation of sewage sludge during thermal hydrolysis with different reaction temperature and reaction time by P K-edge XANES and (31)P NMR. Sci. Total Environ. 2019, 688, 1–9. [Google Scholar] [CrossRef]
- Yu, B.; Luo, J.; Xie, H.; Yang, H.; Chen, S.; Liu, J.; Zhang, R.; Li, Y.Y. Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.W.; Kim, J.J.; Kim, S.O. Analytical Methods of Hydroxyl Radical Produced by TiO2 Photo-catalytic Oxidation. J. Mineral. Soc. Korea 2015, 28, 245–253. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; McNamara, P.J.; Mayer, B.K. Meta-analysis of non-reactive phosphorus in water, wastewater, and sludge, and strategies to convert it for enhanced phosphorus removal and recovery. Sci. Total Environ. 2018, 644, 661–674. [Google Scholar] [CrossRef] [PubMed]
- He, Z.W.; Liu, W.Z.; Wang, L.; Tang, C.C.; Guo, Z.C.; Yang, C.X.; Wang, A.J. Clarification of phosphorus fractions and phosphorus release enhancement mechanism related to pH during waste activated sludge treatment. Bioresour. Technol. 2016, 222, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Y.; Wu, X.; Yuan, W.; Wang, H.; Deng, H.; Sun, L.; Li, R. Phosphorus Migration and Transformation in Activated Sludge by Ultrasonic Treatment. Sustainability 2022, 14, 11224. [Google Scholar] [CrossRef]
- Khanal, S.K.; Grewell, D.; Sung, S.; Van Leeuwen, J. Ultrasound applications in wastewater sludge pretreatment: A review. Crit. Rev. Environ. Sci. Technol. 2007, 37, 277–313. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Wolski, P.; Zawieja, I. Effect of Ultrasound Field on Dewatering of Sewage Sludge. Arch. Environ. Prot. 2012, 38, 25–31. [Google Scholar] [CrossRef]
- Tian, W.; Li, L.; Liu, F.; Zhang, Z.; Yu, G.; Shen, Q.; Shen, B. Assessment of the maturity and biological parameters of compost produced from dairy manure and rice chaff by excitation-emission matrix fluorescence spectroscopy. Bioresour. Technol. 2012, 110, 330–337. [Google Scholar] [CrossRef]
- Zhang, H.; Victor, K.L.; Thompson, J.R.; Frederic, A.K.; Ping, H.L.; Sergey, L.; Donald, S.M.; James, A. Recovery of phosphorus from dairy manure: A pilot- scale study. Environ. Technol. 2014, 36, 1398–11404. [Google Scholar] [CrossRef]
- Fard, M.S.; Ehsani, A.; Soleimani, F. Optimization of Toluenediamine degradation in synthetic wastewater by a UV/H2O2 process using full factorial design. Water Resour. Ind. 2023, 30, 100218. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Abdul Aziz, A.R.; Wan Daud, W.M.A. Degradation performance and cost implication of UV-integrated advanced oxidation processes for wastewater treatments. Rev. Chem. Eng. 2015, 31, 263–302. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Palma, V. Enhanced removal of water pollutants by dielectric barrier discharge non-thermal plasma reactor. Sep. Purif. Technol. 2019, 215, 155–162. [Google Scholar] [CrossRef]
- Wang, J.; Sun, Y.; Feng, J.; Xin, L.; Ma, J. Degradation of triclocarban in water by dielectric barrier discharge plasma combined with TiO2/activated carbon fibers: Effect of operating parameters and byproducts identification. Chem. Eng. J. 2016, 300, 36–46. [Google Scholar] [CrossRef]
- Kostas, E.T.; Beneroso, D.; Robinson, J.P. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass. Renew. Sustain. Energy Rev. 2017, 77, 12–27. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.M.; Lim, J.S.; Park, T.J.; Byun, I.G. Enhancement of microwave effect with addition of chemical agents in solubilization of waste activated sludge. J. Ind. Eng. Chem. 2015, 24, 359–364. [Google Scholar] [CrossRef]
- Min, K.J.; Lee, J.; Cha, H.Y.; Park, K.Y. Nutrient removal from secondary effluent using filamentous algae in raceway ponds. Membr. Water Treat. 2019, 10, 191–199. [Google Scholar] [CrossRef]
- Senn, A.C.; Kaegi, R.; Hug, S.J.; Hering, J.G.; Mangold, S.; Voegelin, A. Composition and structure of Fe (III)-precipitates formed by Fe (II) oxidation in water at near-neutral pH: Interdependent effects of phosphate, silicate and Ca. Geochim. Cosmochim. Acta 2015, 162, 220–246. [Google Scholar] [CrossRef]
- van Genuchten, C.M.; Pena, J.; Amrose, S.E.; Gadgil, A.J. Structure of Fe (III) precipitates generated by the electrolytic dissolution of Fe (0) in the presence of groundwater ions. Geochim. Cosmochim. Acta 2014, 127, 285–304. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, C.G.; Jeong, S.; Park, S.J. Synthesis of polysulfone beads impregnated with Ca-sepiolite for phosphate removal. Membr. Water Treat. 2020, 11, 69–77. [Google Scholar] [CrossRef]
- da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, S.; Jung, J.; Bae, S. Phosphorus removal by lime-natural mineral dissolved solutions. Membr. Water Treat. 2023, 14, 27. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Eiband, M.M.S.G.; de Melo, J.V.; Martínez-Huitle, C.A. Electrocoagulation and advanced electrocoagulation processes: A general review about the fundamentals, emerging applications and its association with other technologies. J. Electroanal. Chem. 2017, 801, 267–299. [Google Scholar] [CrossRef]
- Shaker, O.A.; Safwat, S.M.; Matta, M.E. Nickel removal from wastewater using electrocoagulation process with zinc electrodes under various operating conditions: Performance investigation, mechanism exploration, and cost analysis. Environ. Sci. Pollut. Res. 2023, 30, 26650–26662. [Google Scholar] [CrossRef] [PubMed]
- Cano, R.; Pérez-Elvira, S.I.; Fdz-Polanco, F. Energy feasibility study of sludge pretreatments: A review. Appl. Energy 2015, 149, 176–185. [Google Scholar] [CrossRef]
- Ariunbaatar, J.; Panico, A.; Esposito, G.; Pirozzi, F.; Lens, P.N. Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl. Energy 2014, 123, 143–156. [Google Scholar] [CrossRef]


| Unit | Concentration | Unit | Concentration | ||
|---|---|---|---|---|---|
| pH | - | 8.22 | Ca2+ | mg·L−1 | 60.3 |
| Suspended Solids (SS) | mg·L−1 | 34,360 | Mg2+ | mg·L−1 | 14.8 |
| Chemical Oxygen Demand (COD) | mg·L−1 | 34,650 | Fe2+ | mg·L−1 | 34.9 |
| Total Nitrogen (TN) | mg·L−1 | 4200 | K+ | mg·L−1 | 1971.6 |
| Ammoniacal Nitrogen (NH3-N) | mg·L−1 | 3820 | Ni2+ | mg·L−1 | 0.6 |
| Total Phosphorus (TP) | mg·L−1 | 476 | Cu2+ | mg·L−1 | 0.7 |
| Phosphate phosphorus (PO43−-P) | mg·L−1 | 164 | Cl- | mg·L−1 | 4191.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.H.; Min, K.J.; An, H.J.; Park, K.Y. Comparison of Solubilization Treatment Technologies for Phosphorus Release from Anaerobic Digestate of Livestock Manure. Water 2023, 15, 4033. https://doi.org/10.3390/w15234033
Lee JH, Min KJ, An HJ, Park KY. Comparison of Solubilization Treatment Technologies for Phosphorus Release from Anaerobic Digestate of Livestock Manure. Water. 2023; 15(23):4033. https://doi.org/10.3390/w15234033
Chicago/Turabian StyleLee, Jae Hwa, Kyung Jin Min, Hyo Jin An, and Ki Young Park. 2023. "Comparison of Solubilization Treatment Technologies for Phosphorus Release from Anaerobic Digestate of Livestock Manure" Water 15, no. 23: 4033. https://doi.org/10.3390/w15234033





