Facet-Dependent Adsorption of Phosphate on Hematite Nanoparticles: Role of Singly Coordinated Hydroxyl
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material Preparation
2.3. Characterization
2.4. Adsorption Experiments of Phosphate
2.5. Hematite NPs Surface Hydroxyl Density Titration
2.6. Estimation of the Densities of Singly Coordinated Hydroxyl Group
2.7. Data Analysis
3. Results and Discussion
3.1. Characteristics of Hematite NPs
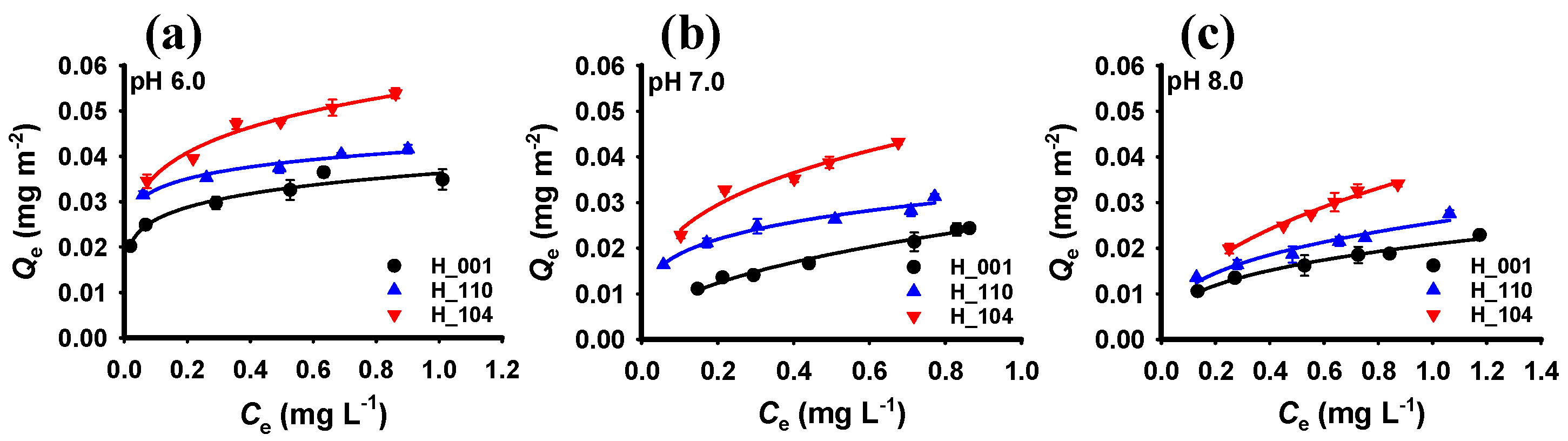
3.2. Adsorption of Phosphate by Hematite with Different Crystal Facets
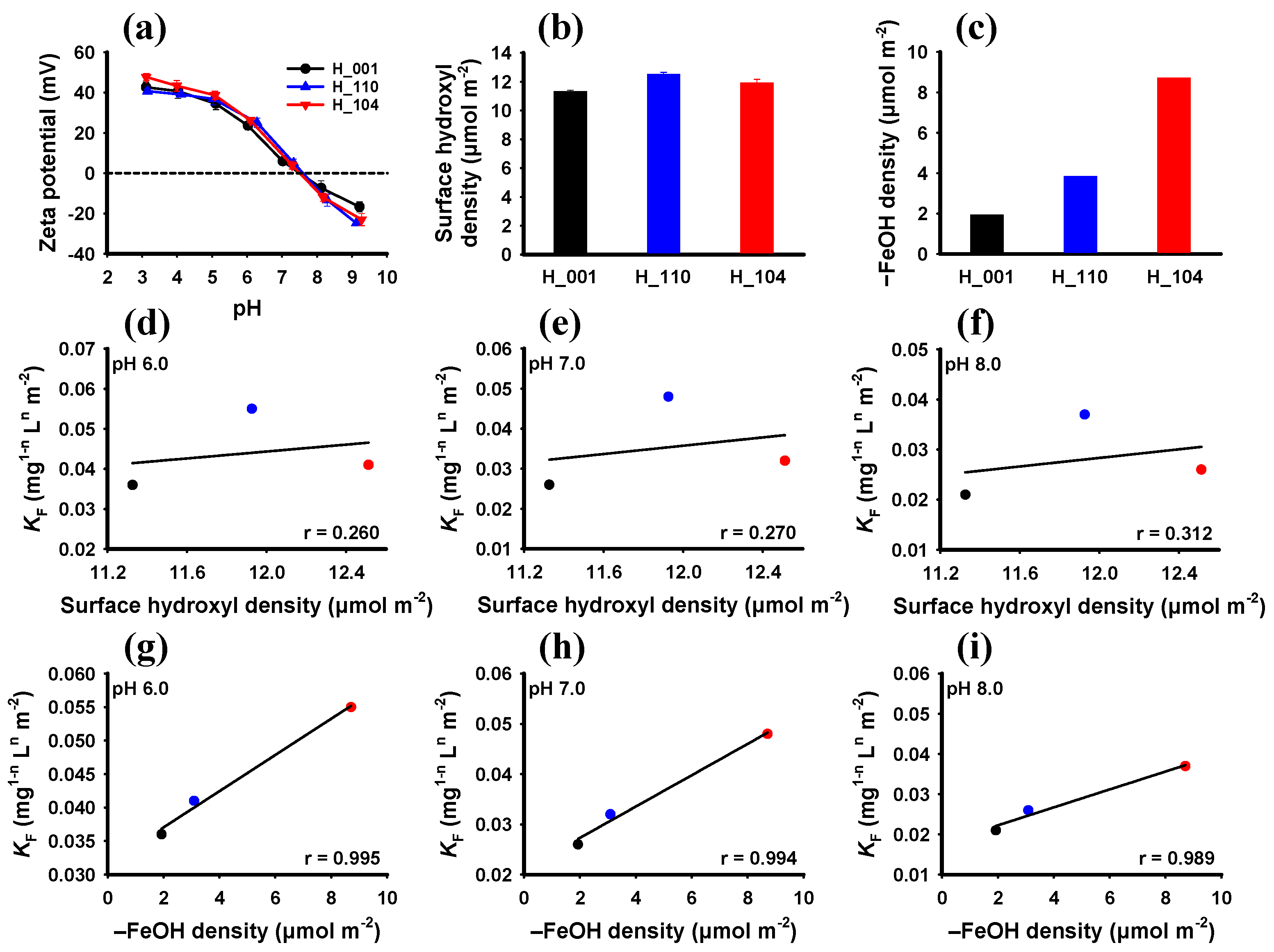
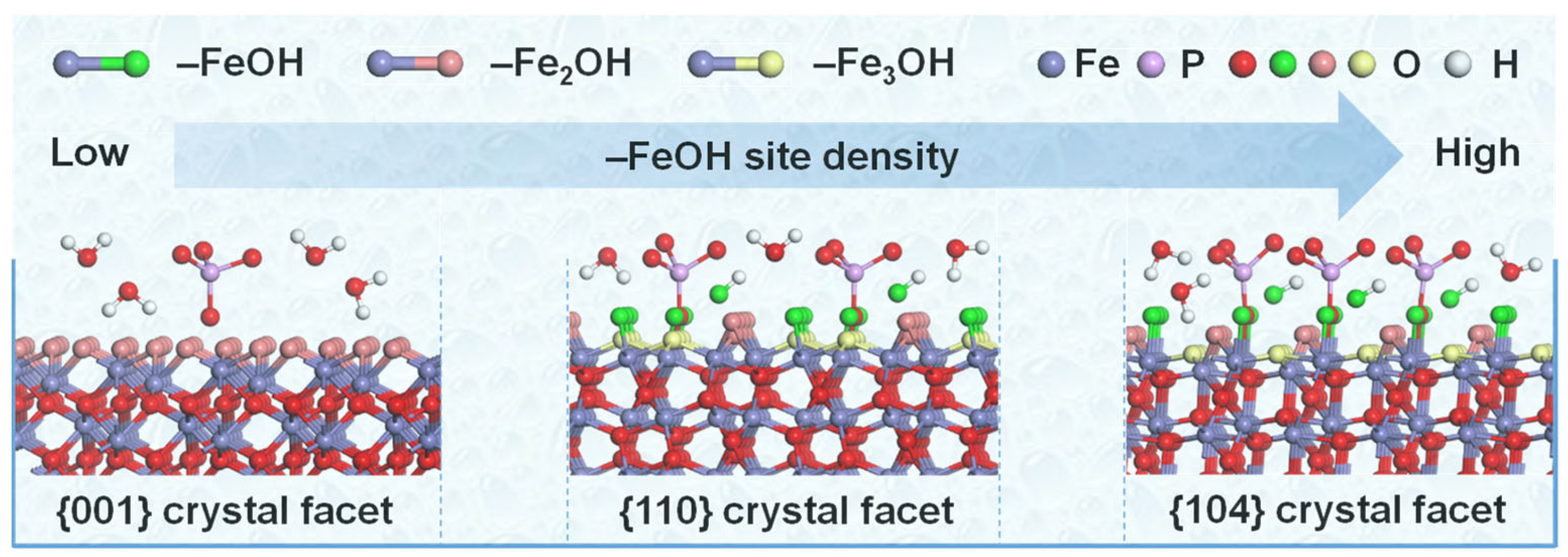
3.3. Facet-Dependent Adsorption Mechanism of Hematite to Phosphate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Choi, M.; Police, A.K.R.; Lee, J.; Bae, S. Removal of phosphorus by ferric ion-rich solutions prepared using various Fe(III)-containing minerals. Water 2022, 14, 3765. [Google Scholar] [CrossRef]
- Wilfert, P.; Kumar, P.S.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. The relevance of phosphorus and iron chemistry to the recovery of phosphorus from wastewater: A review. Environ. Sci. Technol. 2015, 49, 9400–9414. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, N.N.; Borham, A.; Zhang, S.W.; Xie, R.Q.; Zhao, C.; Hu, J.W.; Wang, J.J. The effect of iron-modified biochar on phosphorus adsorption and the prospect of synergistic adsorption between biochar and iron-oxidizing bacteria: A review. Water 2023, 15, 3315. [Google Scholar] [CrossRef]
- Wang, R.G.; Zhao, X.; Wang, T.C.; Guo, Z.Z.; Hu, Z.; Zhang, J.; Wu, S.B.; Wu, H.M. Can we use mine waste as substrate in constructed wetlands to intensify nutrient removal? A critical assessment of key removal mechanisms and long-term environmental risks. Water Res. 2022, 210, 118009. [Google Scholar] [CrossRef]
- Han, C.N.; Dai, Y.; Sun, N.N.; Wu, H.; Tang, Y.; Dai, T.H. Algae bloom and decomposition changes the phosphorus cycle pattern in taihu lake. Water 2022, 14, 3607. [Google Scholar] [CrossRef]
- Fahmi, A.; Hairani, A.; Alwi, M.; Nurzakiah, S. Fe-P pools as phosphorus source for rice in acid sulfate soils. Chilean J. Agric. Res. 2023, 83, 626–634. [Google Scholar] [CrossRef]
- Lin, J.W.; Li, Y.; Zhan, Y.H.; Wu, X.G. Combined amendment and capping of sediment with ferrihydrite and magnetite to control internal phosphorus release. Water Res. 2023, 235, 119899. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, 1414. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.X.; Zhou, Q.X.; Zheng, T.; Mo, F.; Ouyang, S.H. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Gonzalez-Carreño, T.; Veintemillas-Verdaguer, S.; Serna, C.J. The iron oxides strike back: From biomedical applications to energy storage devices and photoelectrochemical water splitting. Adv. Mater. 2011, 23, 5243–5249. [Google Scholar] [CrossRef]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-driven structural and thermodynamic complexity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Zhu, R.L.; Zhu, J.X.; Chen, Q.Z.; Liang, X.L.; He, H.P. Photoreductive dissolution of iron (hydr)oxides and its geochemical significance. ACS Earth Space Chem. 2022, 6, 811–829. [Google Scholar] [CrossRef]
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and Earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.F.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, X.J.; Zhang, X.; Rosso, K.M.; Zhang, L.Z. Facet-dependent contaminant removal properties of hematite nanocrystals and their environmental implications. Environ. Sci. Nano 2018, 5, 1790–1806. [Google Scholar] [CrossRef]
- Bai, L.L.; Cao, C.C.; Wang, C.L.; Wang, C.H.; Zhang, H.; Jiang, H.L. Roles of phytoplankton- and macrophyte-derived dissolved organic matter in sulfamethazine adsorption on goethite. Environ. Pollut. 2017, 230, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, W.; Philips, B.L.; Grey, C.P. Phosphate adsorption on the iron oxyhydroxides goethite (α-FeOOH), akaganeite (β-FeOOH), and lepidocrocite (γ-FeOOH): A 31P NMR study. Energy Environ. Sci. 2011, 4, 4298–4305. [Google Scholar] [CrossRef]
- Siwek, H.; Bartkowiak, A.; Wlodarczyk, M. Adsorption of phosphates from aqueous solutions on alginate/goethite hydrogel composite. Water 2019, 11, 633. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, S.R.; Xu, Y.S.; Zheng, B.H.; Liu, J.Y. Molecular-scale study of aspartate adsorption on goethite and competition with phosphate. Environ. Sci. Technol. 2016, 50, 2938–2945. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, L.; Zhao, J.; Xing, B.S. Environmental processes and toxicity of metallic nanoparticles in aquatic systems as affected by natural organic matter. Environ. Sci. Nano 2016, 3, 240–255. [Google Scholar] [CrossRef]
- Parkinson, G.S. Iron oxide surfaces. Surf. Sci. Rep. 2016, 71, 272–365. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, X.J.; Jia, F.L.; Song, F.H.; Zhao, J.C.; Zhang, L.Z. Ascorbate-promoted surface iron cycle for efficient heterogeneous fenton alachlor degradation with hematite nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 8751–8758. [Google Scholar] [CrossRef] [PubMed]
- Vindedahl, A.M.; Strehlau, J.H.; Arnold, W.A.; Penn, R.L. Organic matter and iron oxide nanoparticles: Aggregation, interactions, and reactivity. Environ. Sci. Nano 2016, 3, 494–505. [Google Scholar] [CrossRef]
- Wang, M.L.; Kong, B.; Zhang, Y.Y.; Shan, C.; Pan, B.C. Facet-dependent phosphate adsorptive reactivity by lanthanum hydroxides of different crystal structure: Role of surface hydroxyl groups. Appl. Surf. Sci. 2021, 538, 147910. [Google Scholar] [CrossRef]
- Ge, X.F.; Wang, L.J.; Zhang, W.J. Molecular-scale determination of facet- and adsorbent-dependent phosphate adsorption by metal-based adsorbents. Environ. Sci. Nano 2022, 9, 3372–3384. [Google Scholar] [CrossRef]
- Stirner, T.; Scholz, D.; Sun, J.Z. Ab initio simulation of structure and surface energy of low-index surfaces of stoichiometric α-Fe2O3. Surf. Sci. 2018, 671, 11–16. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Li, T.; Zhong, W.; Jing, C.Y.; Li, X.G.; Zhang, T.; Jiang, C.J.; Chen, W. Enhanced hydrolysis of p-nitrophenyl phosphate by iron (hydr)oxide nanoparticles: Roles of exposed facets. Environ. Sci. Technol. 2020, 54, 8658–8667. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, D.D.; Liu, C.; Huang, S.H.; Zhou, Z.Y.; Wu, H.; Chen, X.R.; Huang, M.Y.; Zhou, S.D.; Gu, C. Facet effect of hematite on the hydrolysis of phthalate esters under ambient humidity conditions. Nat. Commun. 2022, 13, 6125. [Google Scholar] [CrossRef]
- Asif, A.H.; Rafique, N.; Hirani, R.A.K.; Wu, H.; Shi, L.; Zhang, S.; Wang, S.B.; Yin, Y.; Saunders, M.; Sun, H.Q. Morphology/facet-dependent photo-Fenton-like degradation of pharmaceuticals and personal care products over hematite nanocrystals. Chem. Eng. J. 2022, 432, 134429. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, X.J.; Zhao, J.C.; Zhang, L.Z. Hematite facet confined ferrous ions as high efficient Fenton catalysts to degrade organic contaminants by lowering H2O2 decomposition energetic span. Appl. Catal. B 2016, 181, 127–137. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, W.J.; Fan, Y.K.; Qu, C.C.; Ren, W.Y.; Huang, X.P.; Hong, M.; Liu, F.; Yin, H. Facet-dependent adsorption of aluminum(iii) on hematite nanocrystals and the influence on mineral transformation. Environ. Sci. Nano 2022, 9, 2073–2085. [Google Scholar] [CrossRef]
- Lin, M.; Tan, H.R.; Tan, J.P.Y.; Bai, S.Q. Understanding the growth mechanism of α-Fe2O3 nanoparticles through a controlled shape transformation. J. Phys. Chem. C 2013, 117, 11242–11250. [Google Scholar] [CrossRef]
- Prato, J.G.; Millán, F.C.; González, L.C.; Ríos, A.C.; López, E.; Ríos, I.; Navas, S.; Márquez, A.; Carrero, J.C.; Díaz, J.I. Adsorption of phosphate and nitrate ions on oxidic substrates prepared with a variable-charge lithological material. Water 2022, 14, 2454. [Google Scholar] [CrossRef]
- Tamura, H.; Tanaka, A.; Mita, K.Y.; Furuichi, R. Furuichi Surface hydroxyl site densities on metal oxides as a measure for the ion-exchange capacity. J. Colloid Interface Sci. 1999, 209, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Barrón, V.; Torrent, J. Surface hydroxyl configuration of various crystal faces of hematite and goethite. J. Colloid Interface Sci. 1996, 177, 407–410. [Google Scholar] [CrossRef]
- Lv, J.T.; Miao, Y.X.; Huang, Z.Q.; Han, R.X.; Zhang, S.Z. Facet-mediated adsorption and molecular fractionation of humic substances on hematite surfaces. Environ. Sci. Technol. 2018, 52, 11660–11669. [Google Scholar] [CrossRef]
- Li, X.G.; Li, T.; Zhang, T.; Gu, C.; Zheng, S.R.; Zhang, H.J.; Chen, W. Nano-TiO2-catalyzed dehydrochlorination of 1,1,2,2-tetrachloroethane: Roles of crystalline phase and exposed facets. Environ. Sci. Technol. 2018, 52, 4031–4039. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.T.; Ang, S.Y.; Ye, E.Y.; Sullivan, M.; Zhang, J.; Lin, M. Heterogeneous photo-Fenton reaction on hematite (α-Fe2O3) {104}, {113} and {001} surface facets. Phys. Chem. Chem. Phys. 2015, 17, 25333–25341. [Google Scholar] [CrossRef]
- Rashid, N.M.A.; Haw, C.; Chiu, W.; Khanis, N.H.; Rohaizad, A.; Khiew, P.; Rahman, S.A. Structural- and optical-properties analysis of single crystalline hematite (α-Fe2O3) nanocubes prepared by one-pot hydrothermal approach. CrystEngComm 2016, 18, 4720–4732. [Google Scholar] [CrossRef]
- Yu, H.G.; Liu, R.; Wang, X.F.; Wang, P.; Yu, J.G. Enhanced visible-light photocatalytic activity of Bi2WO6 nanoparticles by Ag2O cocatalyst. Appl. Catal. B 2012, 111, 326–333. [Google Scholar] [CrossRef]
- Huang, X.P.; Hou, X.J.; Song, F.H.; Zhao, J.C.; Zhang, L.Z. Facet-dependent Cr(VI) adsorption of hematite nanocrystals. Environ. Sci. Technol. 2016, 50, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Luengo, C.; Brigante, M.; Antelo, J.; Avena, M. Kinetics of phosphate adsorption on goethite: Comparing batch adsorption and ATR-IR measurements. J. Colloid Interface Sci. 2006, 300, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Beattie, J.K.; Coleman, L.M.; Jones, D.R. Phosphate ester hydrolysis facilitated by mineral phases. Environ. Sci. Technol. 1995, 29, 1706–1709. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Jing, C.Y. Molecular insights into glyphosate adsorption to goethite gained from ATR-FTIR, two-dimensional correlation spectroscopy, and DFT study. Environ. Sci. Technol. 2018, 52, 1946–1953. [Google Scholar] [CrossRef]
- Li, X.C.; Reich, T.; Kersten, M.; Jing, C.Y. Low-molecular-weight organic acid complexation affects antimony(III) adsorption by granular ferric hydroxide. Environ. Sci. Technol. 2019, 53, 5221–5229. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.L.; Hong, C.; Hu, H.Q.; Violante, A. Phosphate adsorption on uncoated and humic acid-coated iron oxides. J. Soils Sediments 2016, 16, 1911–1920. [Google Scholar] [CrossRef]
- Chen, M.N.; Xie, Z.M.; Yang, Y.; Gao, B.; Wang, J. Effects of calcium on arsenate adsorption and arsenate/iron bioreduction of ferrihydrite in stimulated groundwater. Int. J. Environ. Res. Public Health 2022, 19, 3465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mahmood, A.; Xie, X.F.; Wang, X.; Qiu, H.X.; Sun, J. Surface adsorption configurations of H3PO4 modified TiO2 and its influence on the photodegradation intermediates of gaseous o-xylene. Chem. Eng. J. 2020, 393, 124723. [Google Scholar] [CrossRef]
- Saito, S.; Hamai, R.; Shiwaku, Y.; Hasegawa, T.; Sakai, S.; Tsuchiya, K.; Sai, Y.; Iwama, R.; Amizuka, N.; Takahashi, T.; et al. Involvement of distant octacalcium phosphate scaffolds in enhancing early differentiation of osteocytes during bone regeneration. Acta Biomater. 2021, 129, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Song, H.J.; Park, Y.J. Microwave treatment of calcium phosphate/titanium dioxide composite to improve protein adsorption. Materials 2022, 15, 4773. [Google Scholar] [CrossRef]
- Mäkie, P.; Persson, P.; Österlund, L. Adsorption of trimethyl phosphate and triethyl phosphate on dry and water pre-covered hematite, maghemite, and goethite nanoparticles. J. Colloid Interface Sci. 2013, 392, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.T.; Zhao, Z.J.; Dohnálek, Z.; Gong, J.L. Structural motifs of water on metal oxide surfaces. Chem. Soc. Rev. 2017, 46, 1785–1806. [Google Scholar] [CrossRef] [PubMed]
- Waiman, C.V.; Avena, M.J.; Regazzoni, A.E.; Zanini, G.P. A real time in situ ATR-FTIR spectroscopic study of glyphosate desorption from goethite as induced by phosphate adsorption: Effect of surface coverage. J. Colloid Interface Sci. 2013, 394, 485–489. [Google Scholar] [CrossRef]
- Ding, X.B.; Song, X.W.; Boily, J.F. Identification of fluoride and phosphate binding sites at FeOOH surfaces. J. Phys. Chem. C 2012, 116, 21939–21947. [Google Scholar] [CrossRef]
- Hiemstra, T.; Riemsdijk, W.H.V. A surface structural approach to ion adsorption: The charge distribution (CD) model. J. Colloid Interface Sci. 1996, 179, 488–508. [Google Scholar] [CrossRef]
- Boily, J.F.; Persson, P.; Sjöberg, S. Benzenecarboxylate surface complexation at the goethite (α-FeOOH)/water interface: III. The influence of particle surface area and the significance of modeling parameters. J. Colloid Interface Sci. 2000, 227, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Song, X.W.; Boily, J.F. Structural controls on OH site availability and reactivity at iron oxyhydroxide particle surfaces. Phys. Chem. Chem. Phys. 2012, 14, 2579–2586. [Google Scholar] [CrossRef] [PubMed]

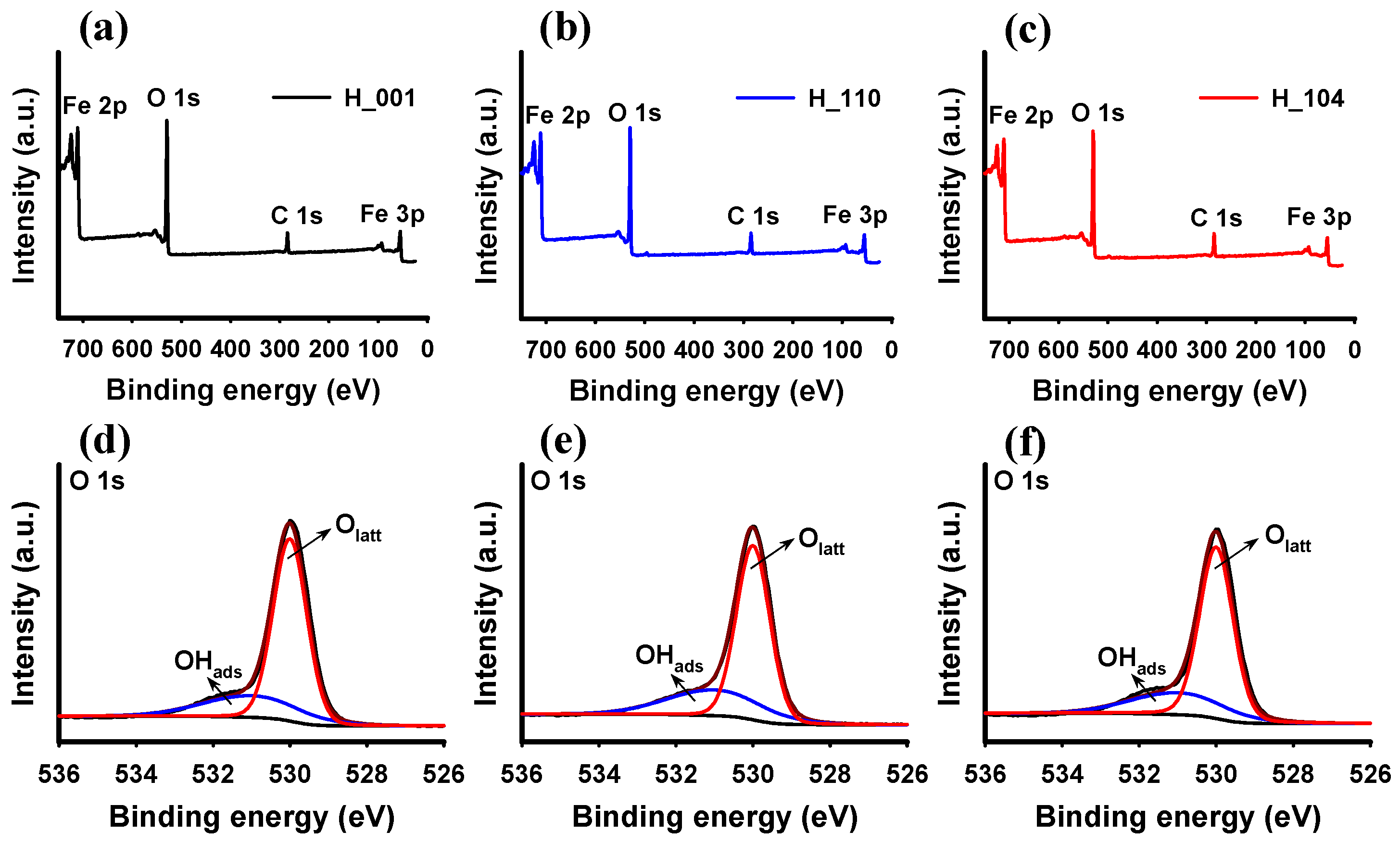

| Sample | SABET 1 (m2 g−1) | Proportion of Exposed Facets 2 | Surface Fe 3 (at %) | Surface O 3 (at %) | Fe/Olatt Ratio 3 | Isoelectric Point | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Olatt | OHads | |||||||||
| H_001 | 20.9 | 77.2% {001} | 22.8% {102} | - | - | 33.2 | 50.2 | 16.6 | 0.66 | 7.5 |
| H_110 | 24.2 | 37.2% {110} | 55.6% {001} | 3.6% {120} | 3.6% {210} | 32.4 | 48.1 | 19.5 | 0.67 | 7.6 |
| H_104 | 4.3 | 99.0% {104} | - | - | - | 31.2 | 51.4 | 17.4 | 0.61 | 7.5 |
| Sample | pH 6.0 | pH 7.0 | pH 8.0 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| KF (mg1−n Ln m−2) | n | R2 * | KF (mg1−n Ln m−2) | n | R2 * | KF (mg1−n Ln m−2) | n | R2 * | |
| H_001 | 0.036 | 0.144 | 0.952 | 0.026 | 0.454 | 0.982 | 0.021 | 0.350 | 0.981 |
| H_110 | 0.041 | 0.104 | 0.961 | 0.032 | 0.230 | 0.970 | 0.026 | 0.351 | 0.951 |
| H_104 | 0.055 | 0.185 | 0.971 | 0.048 | 0.308 | 0.959 | 0.037 | 0.456 | 0.987 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Shi, F.; Ju, Y.; Ding, Z. Facet-Dependent Adsorption of Phosphate on Hematite Nanoparticles: Role of Singly Coordinated Hydroxyl. Water 2023, 15, 4070. https://doi.org/10.3390/w15234070
Li T, Shi F, Ju Y, Ding Z. Facet-Dependent Adsorption of Phosphate on Hematite Nanoparticles: Role of Singly Coordinated Hydroxyl. Water. 2023; 15(23):4070. https://doi.org/10.3390/w15234070
Chicago/Turabian StyleLi, Tong, Fei Shi, Yiting Ju, and Zezhou Ding. 2023. "Facet-Dependent Adsorption of Phosphate on Hematite Nanoparticles: Role of Singly Coordinated Hydroxyl" Water 15, no. 23: 4070. https://doi.org/10.3390/w15234070
APA StyleLi, T., Shi, F., Ju, Y., & Ding, Z. (2023). Facet-Dependent Adsorption of Phosphate on Hematite Nanoparticles: Role of Singly Coordinated Hydroxyl. Water, 15(23), 4070. https://doi.org/10.3390/w15234070






