Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea
Abstract
:1. Introduction
2. Materials and Methods
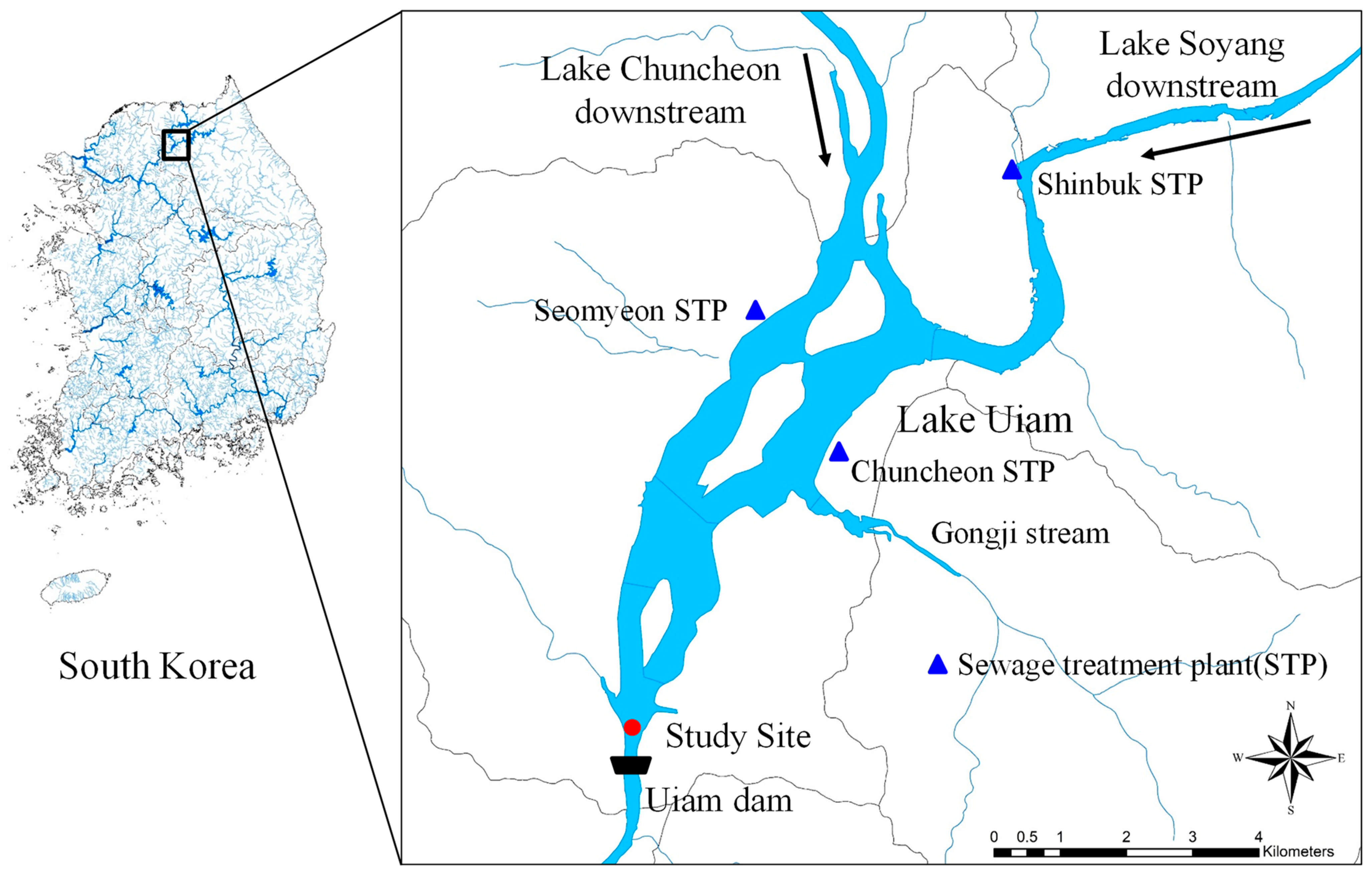
2.1. Study Site
2.2. Sample Collection and Water Quality Analysis
2.3. Phytoplankton Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Lake Uiam
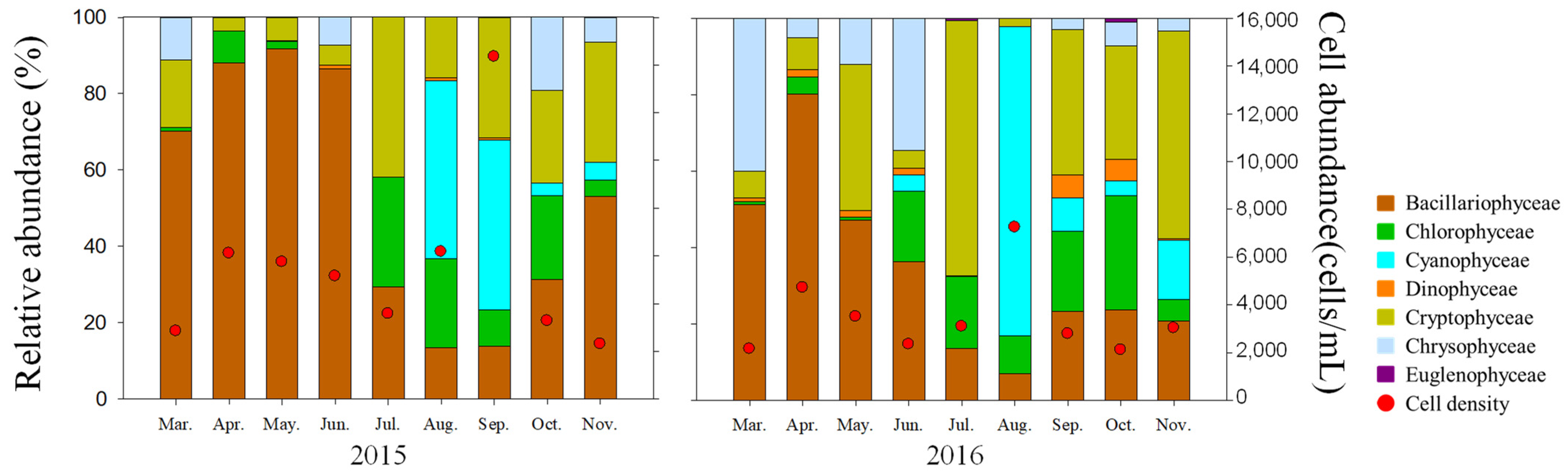
3.2. Phytoplankton Composition of Taxa
3.3. Seasonal Variation of Phytoplankton Abundance and Characteristics
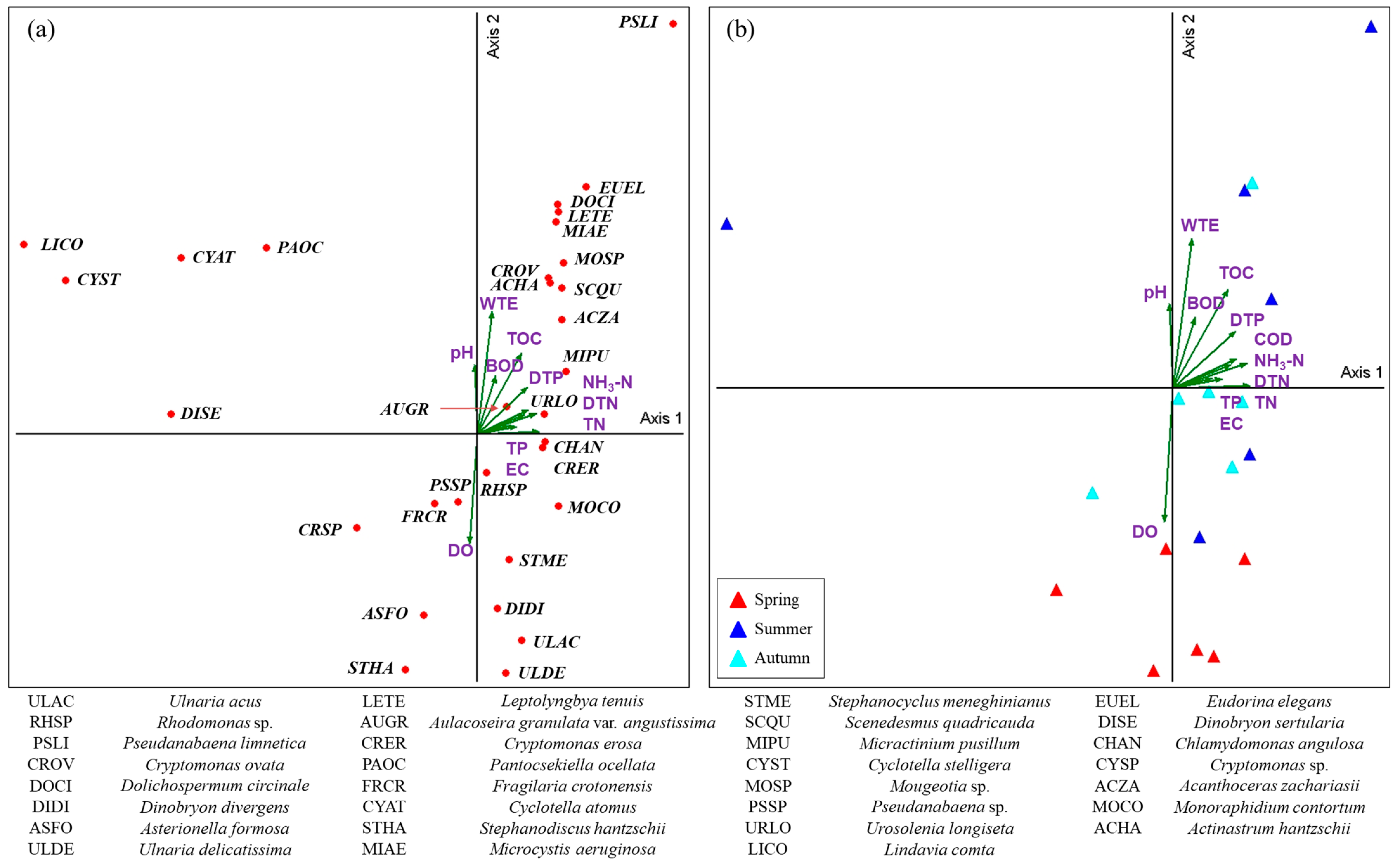
3.4. CCA of the Phytoplankton Community and Environmental Factors in Lake Uiam
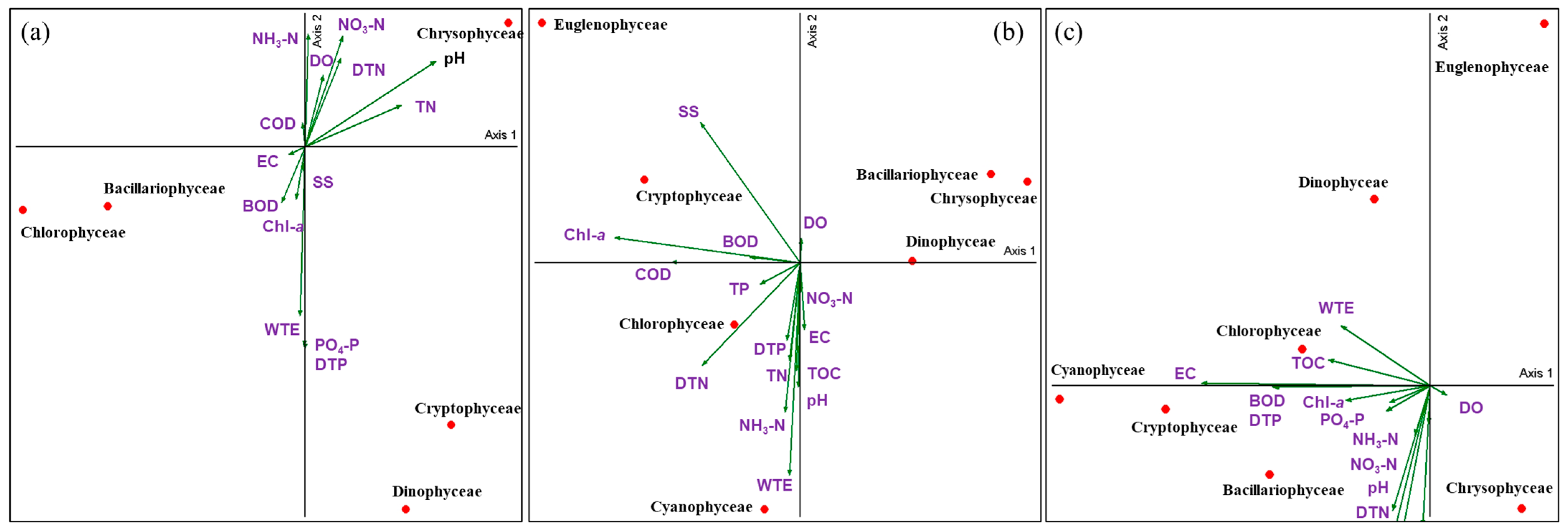
3.5. Seasonal Influence of Water Environmental Factors on Phytoplankton Communities
3.6. PCA of Seasonal Phytoplankton Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, B.C.; Park, J.H.; Hwang, G.S.; Choi, K.S. Eutrophication of Large Freshwater Ecosystems in Korea. Kor. J. Lim. 1998, 15, 25–30. [Google Scholar]
- Shin, J.-K.; Kang, C.-K.; Kim, H.-S.; Hwang, S.-J. Limnological characteristics of the river-type Paltang Reservoir, Korea: Hydrological and environmental factors. Korean J. Ecol. Environ. 2003, 36, 242–256. [Google Scholar]
- Li, J.; Liao, L.; Dai, X. Economic and Agricultural Impacts of Building a Dam—Evidence from Natural Experience of the Three-Gorges Dam. Agriculture 2022, 12, 1372. [Google Scholar] [CrossRef]
- An, K.-G.; Jones, J.R. Factors regulating bluegreen dominance in a reservoir directly influenced by the Asian monsoon. Hydrobiologia 2000, 432, 37–48. [Google Scholar] [CrossRef]
- Jung, S.; Shin, M.; Kim, J.; Eum, J.; Lee, Y.; Lee, J.; Choi, Y.; You, K.; Owen, J.; Kim, B. The effects of Asian summer monsoons on algal blooms in reservoirs. Inland Waters 2016, 6, 406–413. [Google Scholar] [CrossRef]
- Kim, D.; Kim, Y.; Kim, B. Simulation of eutrophication in a reservoir by CE-QUAL-W2 for the evaluation of the importance of point sources and summer monsoon. Lake Reserv. Manag. 2019, 35, 64–76. [Google Scholar] [CrossRef]
- Kocer, M.A.T.; ŞEN, B. Some factors affecting the abundance of phytoplankton in an unproductive alkaline lake (Lake Hazar, Turkey). Turk. J. Bot. 2014, 38, 790–799. [Google Scholar] [CrossRef]
- Bhat, N.A.; Wanganeo, A.; Raina, R. Seasonal dynamics of phytoplankton community in a tropical wetland. Environ. Monit. Assess. 2015, 187, 4136. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, S. Characteristics of phytoplankton distribution for assessment of water quality in the Zhalong Wetland, China. Int. J. Environ. Sci. Technol. 2015, 12, 3657–3664. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Chai, F.; Yu, H.; Sun, X.; Liu, D. Phytoplankton community structure dynamics in relation to water environmental factors in Zhalong Wetland. Int. J. Environ. Res. Public Health 2022, 19, 14996. [Google Scholar] [CrossRef]
- Mesquita, M.C.; Prestes, A.C.C.; Gomes, A.M.; Marinho, M.M. Direct effects of temperature on growth of different tropical phytoplankton species. Microb. Ecol. 2020, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marzetz, V.; Spijkerman, E.; Striebel, M.; Wacker, A. Phytoplankton community responses to interactions between light intensity, light variations, and phosphorus supply. Front. Environ. Sci. 2020, 8, 539733. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Jankowiak, J.; Hattenrath-Lehmann, T.; Kramer, B.J.; Ladds, M.; Gobler, C.J. Deciphering the effects of nitrogen, phosphorus, and temperature on cyanobacterial bloom intensification, diversity, and toxicity in western Lake Erie. Limnol. Oceanogr. 2019, 64, 1347–1370. [Google Scholar] [CrossRef]
- Verhamme, E.M.; Redder, T.M.; Schlea, D.A.; Grush, J.; Bratton, J.F.; DePinto, J.V. Development of the Western Lake Erie Ecosystem Model (WLEEM): Application to connect phosphorus loads to cyanobacteria biomass. J. Great Lakes Res. 2016, 42, 1193–1205. [Google Scholar] [CrossRef]
- Youn, S.J.; Im, J.K.; Byeon, M.S.; Yu, S.J. Characteristics of cyanobacteria and odorous compounds production in lake uiam and lower gonji stream. J. Environ. Sci. Int. 2019, 35, 99–104. [Google Scholar]
- Byeon, J.H.; You, M.N.; Lee, E.J.; You, S.J.; Kim, B.H.; Byeon, M.S. Temporal and Spatial Distribution of Microbial Community and Odor Compounds in the Bukhan River System. Korean J. Ecol. Environ. 2018, 51, 299–310. [Google Scholar] [CrossRef]
- Byeon, J.H.; Hwang, S.-J.; Kim, B.H.; Park, J.R.; Lee, J.K.; Lim, B.J. Relationship between a Dense Population of Cyanobacteria and Odorous Compounds in the North Han River System in 2014 and 2015. Korean J. Ecol. Environ. 2015, 48, 263–271. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, Y.; Cho, H.; Hwang, S.-J. Molecular probes to evaluate the synthesis and production potential of an odorous compound (2-methylisoborneol) in cyanobacteria. Int. J. Environ. Res. Public Health 2020, 17, 1933. [Google Scholar] [CrossRef]
- Joh, G.J.; Choi, Y.S.; Shin, J.-K.; Lee, J. Problematic algae in the sedimentation and filtration process of water treatment plants. J. Water Supply Res. Technol. AQUA 2011, 60, 219–230. [Google Scholar] [CrossRef]
- Grover, J.P.; Chrzanowski, T.H. Seasonal dynamics of phytoplankton in two warm temperate reservoirs: Association of taxonomic composition with temperature. J. Plankton. Res. 2006, 28, 1–17. [Google Scholar] [CrossRef]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Baek, J.-S.; Youn, S.J.; Kim, H.N.; Sim, Y.; You, S.J.; Im, J.K. Effects of Environmental Factors on Phytoplankton Succession and Community Structure in Lake Chuncheon, South Korea. Korean J. Ecol. Environ. 2019, 52, 71–80. [Google Scholar] [CrossRef]
- Youn, S.J.; Kim, H.N.; Im, J.K.; Kim, Y.-J.; Baek, J.-S.; Lee, S.-W.; Lee, E.J.; Yu, S.J. Effect of environmental factors on phytoplankton communities and dominant species succession in Lake Cheongpyeong. J. Environ. Sci. Int. 2017, 26, 913–925. [Google Scholar] [CrossRef]
- Na, E.H.; Park, S.S. A hydrodynamic and water quality modeling study of spatial and temporal patterns of phytoplankton growth in a stratified lake with buoyant incoming flow. Ecol. Modell. 2006, 199, 298–314. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Lee, Y.; Kim, M.-S.; Kumar, K.S.; Shin, K.-H. Seasonal changes in mycosporine-like amino acid production rate with respect to natural phytoplankton species composition. Mar. Drugs 2015, 13, 6740–6758. [Google Scholar] [CrossRef]
- Sim, Y.; Byeon, M.S.; Kim, K.; Yu, S.J.; Im, J.K. Influence of Zooplankton and Environmental Factors on Clear-Water Phase in Lake Paldang, South Korea. Int. J. Environ. Res. Public Health 2021, 18, 7205. [Google Scholar] [CrossRef]
- MOE. Standard Methods for the Examination Water Quality; The Korean Ministry of Environment: Sejong, Republic of Korea, 2014. [Google Scholar]
- John, D.M.; Whitton, B.A.; Brook, A.J. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Hirose, H.; Yamagishi, T.; Akiyama, M. Illustrations of the Japanese Freshwater Algae; Uchidarokakuho Publ. Co., Ltd.: Tokyo, Japan, 1977; 927p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. Naviculaceae. Süßwasserflora von Mitteleuropa; Gustav Fischer Verlag: Stuttgart, Germany, 1986; Volume 1, p. 876. Available online: http://link.springer.com/book/9783827426154 (accessed on 4 September 2023).
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd 2/2. Bacillariophyceae. 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae; Gustav Fischer: Jena, Germany, 1988. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 02/3: Bacillariophyceae: Teil 3: Centrales, Fragilariaceae, Eunotiaceae; Springer: Berlin/Heidelberg, Germany, 1991; Volume 2. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Süßwasserflora von Mitteleuropa, Bd. 02/4: Bacillariophyceae: Teil 4: Achnanthaceae, Kritische Ergänzungen zu Achnanthes sl, Navicula s. str., Gomphonema, Gesamtliteraturverzeichnis Teil 1-4, Ergänzter Nachdruck, 2004; Spektrum Akademischer Verlag: Heidelberg, Germany, 1999; Available online: http://link.springer.com/book/9783827408389 (accessed on 4 September 2023).
- Morabito, G.; Oggioni, A.; Caravati, E.; Panzani, P. Seasonal morphological plasticity of phytoplankton in Lago Maggiore (N. Italy). Hydrobiologia 2007, 578, 47–57. [Google Scholar] [CrossRef]
- Hu, R.; Han, B.; Naselli-Flores, L. Comparing biological classifications of freshwater phytoplankton: A case study from South China. Hydrobiologia 2013, 701, 219–233. [Google Scholar] [CrossRef]
- El-Zeiny, A.; Elagami, S.A.; Nour-Eldin, H.; El-Halawany, E.-S.F.; Bonanomi, G.; Abd-ElGawad, A.M.; Soufan, W.; El-Amier, Y.A. Wild plant habitat characterization in the last two decades in the Nile Delta coastal region of Egypt. Agriculture 2022, 12, 108. [Google Scholar] [CrossRef]
- Brown, J.D. Principal components analysis and exploratory factor analysis &ndash Definitions, differences, and choices. Statistics 2009, 13, 26–30. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Lokhande, S.; Tare, V. Spatio-temporal trends in the flow and water quality: Response of river Yamuna to urbanization. Environ. Monit. Assess. 2021, 193, 117. [Google Scholar] [CrossRef] [PubMed]
- Giao, N.T.; Nhien, H.T.H.; Anh, P.K.; Thuptimdang, P. Combination of water quality, pollution indices, and multivariate statistical techniques for evaluating the surface water quality variation in Can Tho City, Vietnam. Environ. Monit. Assess. 2022, 194, 844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.; Gao, D.; Yu, H. Source identification of surface water pollution using multivariate statistics combined with physicochemical and socioeconomic parameters. Sci. Total Environ. 2022, 806, 151274. [Google Scholar] [CrossRef] [PubMed]
- Jargal, N.; An, K.-G. Seasonal and interannual responses of blue-green algal taxa and chlorophyll to a monsoon climate, flow regimes, and N: P ratios in a temperate drinking-water reservoir. Sci. Total Environ. 2023, 896, 165306. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Eum, J.; Jung, S.; Choi, Y.; Owen, J.S.; Kim, B. Export of non-point source suspended sediment, nitrogen, and phosphorus from sloping highland agricultural fields in the East Asian monsoon region. Environ. Monit. Assess. 2016, 188, 692. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology; Kellogg Biological Station-Michigan State University: Hickory Corners, MI, USA, 1983. [Google Scholar]
- Reynolds, C.; Wiseman, S.; Godfrey, B.; Butterwick, C. Some effects of artificial mixing on the dynamics of phytoplankton populations in large limnetic enclosures. J. Plankton. Res. 1983, 5, 203–234. [Google Scholar] [CrossRef]
- Sze, P. A Biology of the Algae; WCB/McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- Lee, J.-H.; Park, J.-G.; Kim, E.-J. Trophic states and phytoplankton compositions of Dam Lakes in Korea. Algae 2002, 17, 275–281. [Google Scholar] [CrossRef]
- Wang, Z.; Li, R. Effects of light and temperature on the odor production of 2-methylisoborneol-producing Pseudanabaena sp. and geosmin-producing Anabaena ucrainica (cyanobacteria). Biochem. Syst. Ecol. 2015, 58, 219–226. [Google Scholar] [CrossRef]
- Gilabert, J. Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon: The Mar Menor. J. Plankton. Res. 2001, 23, 207–218. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kim, S.-K. The use of winter water temperature and food composition by the copepod Cyclops vicinus (Uljanin, 1875) to provide a temporal refuge from fish predation. Biology 2021, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Lafarga-De la Cruz, F.; Valenzuela-Espinoza, E.; Millán-Núnez, R.; Trees, C.C.; Santamaría-del-Ángel, E.; Núnez-Cebrero, F. Nutrient uptake, chlorophyll a and carbon fixation by Rhodomonas sp.(Cryptophyceae) cultured at different irradiance and nutrient concentrations. Aquac. Eng. 2006, 35, 51–60. [Google Scholar] [CrossRef]
- Krivtsov, V.; Bellinger, E.; Sigee, D. Changes in the elemental composition of Asterionella formosa during the diatom spring bloom. J. Plankton. Res. 2000, 22, 169–184. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton. Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Gsell, A.S.; de Senerpont Domis, L.N.; Przytulska-Bartosiewicz, A.; Mooij, W.M.; van Donk, E.; Ibelings, B.W. Genotype-by-temperature interactions may help to maintain clonal diversity in Asterionella formosa (Bacillariophyceae). J. Phycol. 2012, 48, 1197–1208. [Google Scholar] [CrossRef]
- Vasconcelos, V.M. Species composition and dynamics of the phytoplankton in a recently-commissioned reservoir (Azibo-Portugal). Arch. Hydrobiol. 1991, 121, 67–78. [Google Scholar] [CrossRef]
- Bondarenko, N.; Guselnikova, N.Y. Studies on Synedra acus Kutz. var. radians (Kutz.) Hust.(Bacillariophyta) in culture. Int. J. Algae 2002, 4, 85–95. [Google Scholar]
- Michel, T.J.; Saros, J.E.; Interlandi, S.J.; Wolfe, A.P. Resource requirements of four freshwater diatom taxa determined by in situ growth bioassays using natural populations from alpine lakes. Hydrobiologia 2006, 568, 235–243. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Lai, Z.; Tan, X.; Pang, S.; Yang, W. Seasonal variations of Aulacoseira granulata population abundance in the Pearl River Estuary. Estuar. Coast. Shelf Sci. 2009, 85, 585–592. [Google Scholar] [CrossRef]
- Tsukada, H.; Tsujimura, S.; Nakahara, H. Seasonal succession of phytoplankton in Lake Yogo over 2 years: Effect of artificial manipulation. Limnology 2006, 7, 3–14. [Google Scholar] [CrossRef]
- Kamjunke, N.; Henrichs, T.; Gaedke, U. Phosphorus gain by bacterivory promotes the mixotrophic flagellate Dinobryon spp. during re-oligotrophication. J. Plankton. Res. 2007, 29, 39–46. [Google Scholar] [CrossRef]
- Vadrucci, M.; Barbone, E.; Ungaro, N.; Romano, A.; Bucci, R. Application of taxonomic and morpho-functional properties of phytoplankton communities to water quality assessment for artificial lakes in the Mediterranean Ecoregion. J. Plankton. Res. 2017, 39, 550–563. [Google Scholar] [CrossRef]
- Sabanci, F.Ç.; Koray, T. The dinoflagellat species distributed in izmir Bay (Aegean Sea) and the seasonal changes of species diversity. Rev. Hydrobiol. 2012, 5, 71–84. [Google Scholar]
- Gieskes, W.; Kraay, G. Dominance of Cryptophyceae during the phytoplankton spring bloom in the central North Sea detected by HPLC analysis of pigments. Mar. Biol. 1983, 75, 179–185. [Google Scholar] [CrossRef]
- Kasprzak, P.; Padisák, J.; Koschel, R.; Krienitz, L.; Gervais, F. Chlorophyll a concentration across a trophic gradient of lakes: An estimator of phytoplankton biomass? Limnologica 2008, 38, 327–338. [Google Scholar] [CrossRef]
- Walsby, A. Gas vesicles. Microbiol. Rev. 1994, 58, 94–144. [Google Scholar] [CrossRef]
- Paerl, H.; Fulton, R. Ecology of harmful cyanobacteria. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–109. [Google Scholar]
- Agostoni, M.; Waters, C.M.; Montgomery, B.L. Regulation of biofilm formation and cellular buoyancy through modulating intracellular cyclic di-GMP levels in engineered cyanobacteria. Biotechnol. Bioeng. 2016, 113, 311–319. [Google Scholar] [CrossRef]
- Wang, Z.; Akbar, S.; Sun, Y.; Gu, L.; Zhang, L.; Lyu, K.; Huang, Y.; Yang, Z. Cyanobacterial dominance and succession: Factors, mechanisms, predictions, and managements. J. Environ. Manag. 2021, 297, 113281. [Google Scholar] [CrossRef]
- Kromkamp, J.; Konopka, A.; Mur, L.R. Buoyancy Regulation in a Strain of Aphaniz. omenon flos-aquae (Cyanophyceae): The Importance of Carbohydrate Accumulation and Gas Vesicle Collapse. Microbiology 1986, 132, 2113–2121. [Google Scholar] [CrossRef]
- Tashiro, Y.; Monson, R.E.; Ramsay, J.P.; Salmond, G.P. Molecular genetic and physical analysis of gas vesicles in buoyant enterobacteria. Environ. Microbiol. 2016, 18, 1264–1276. [Google Scholar] [CrossRef]
- Wei, K.; Amano, Y.; Machida, M.; Asukabe, H.; Harada, K.-i. Effects of light and potassium ion on buoyancy regulation with gas vesicle in a Cyanobacterium Microcystis aeruginosa NIES-843. Water Air Soil Pollut. 2018, 229, 352. [Google Scholar] [CrossRef]
- Stewart, A.J.; Wetzel, R.G. Cryptophytes and other microflagellates as couplers in planktonic community dynamics. Arch. Hydrobiol. 1986, 106, 1–19. [Google Scholar] [CrossRef]
- Hammer, A.; Schumann, R.; Schubert, H. Light and temperature acclimation of Rhodomonas salina (Cryptophyceae): Photosynthetic performance. Aquat. Microb. Ecol. 2002, 29, 287–296. [Google Scholar] [CrossRef]


| Variables | Spring | Summer | Autumn |
|---|---|---|---|
| WTE (°C) | 12.3 ± 3.3 a | 24.6 ± 4.9 b | 17.5 ± 4.8 c |
| pH | 8.4 ± 0.5 a | 8.8 ± 0.6 b | 8.1 ± 0.4 a |
| DO (mg L−1) | 12.9 ± 1.6 a | 10.2 ± 1.2 b | 10.3 ± 1.7 b |
| EC (µS cm−1) | 117 ± 14 a | 110 ± 8 ab | 104 ± 26 a |
| BOD (mg L−1) | 1.7 ± 0.8 a | 2.0 ± 0.7 a | 1.8 ± 0.6 a |
| COD (mg L−1) | 3.3 ± 1.5 a | 3.9 ± 0.9 a | 3.5 ± 0.8 a |
| TSS (mg L−1) | 3.4 ± 17.7 a | 7.1 ± 1.1 a | 2.8 ± 1.4 a |
| TOC (mg L−1) | 2.1 ± 0.7 a | 2.6 ± 0.4 b | 2.3 ± 0.3 a |
| TN (mg L−1) | 2.257 ± 0.401 a | 2.103 ± 0.368 ab | 1.994 ± 0.379 b |
| DTN (mg L−1) | 2.080 ± 0.358 a | 1.921 ± 0.382 ab | 1.845 ± 0.409 b |
| NH3-N (mg L−1) | 0.147 ± 0.097 a | 0.107 ± 0.085 a | 0.094 ± 0.088 a |
| NO3-N (mg L−1) | 1.564 ± 0.198 a | 1.312 ± 0.274 b | 1.327 ± 0.361 b |
| TP (mg L−1) | 0.013 ± 0.021 a | 0.023 ± 0.011 b | 0.019 ± 0.004 ab |
| DTP (mg L−1) | 0.008 ± 0.009 a | 0.013 ± 0.004 b | 0.010 ± 0.004 ab |
| PO4-P (mg L−1) | 0.006 ± 0.005 a | 0.007 ± 0.002 a | 0.006 ± 0.003 a |
| Chl-a (mg m–3) | 9.6 ± 9.2 a | 11.6 ± 5.0 a | 10.5 ± 4.0 a |
| Date | Dominant Species | RA (%) | Subdominant Species | RA (%) |
|---|---|---|---|---|
| March 2015 | Stephanodiscus hantzschii | 43.2 | Rhodomonas sp. | 17.2 |
| April 2015 | Ulnaria acus | 72.2 | Monoraphidium contortum | 4.3 |
| May 2015 | Ulnaria acus | 55.7 | Asterionella formosa | 13.6 |
| June 2015 | Cyclotella atomus | 23.3 | Pantocsekiella ocellata | 21.4 |
| July 2015 | Rhodomonas sp. | 22.4 | Cryptomonas ovata | 19.6 |
| August 2015 | Leptolyngbya tenuis | 34.0 | Rhodomonas sp. | 11.3 |
| September 2015 | Dolichospermum circinale | 30.7 | Cryptomonas ovata | 23.2 |
| October 2015 | Rhodomonas sp. | 19.1 | Dinobryon divergens | 19.1 |
| November 2015 | Aulacoseira granulata var. angustissima | 23.8 | Rhodomonas sp. | 21.7 |
| March 2016 | Dinobryon divergens | 39.5 | Ulnaria acus | 34.4 |
| April 2016 | Ulnaria delicatissima | 37.7 | Ulnaria acus | 21.4 |
| May 2016 | Rhodomonas sp. | 34.9 | Fragilaria crotonensis | 17.7 |
| June 2016 | Dinobryon divergens | 34.6 | Ulnaria acus | 13.2 |
| July 2016 | Cryptomonas erosa | 36.9 | Rhodomonas sp. | 23.0 |
| August 2016 | Pseudanabaena limnetica | 79.3 | Ulnaria acus | 4.6 |
| September 2016 | Rhodomonas sp. | 21.2 | Cryptomonas erosa | 12.6 |
| October 2016 | Rhodomonas sp. | 17.5 | Aulacoseira granulata var. angustissima | 10.9 |
| November 2016 | Rhodomonas sp. | 33.7 | Cryptomonas sp. | 17.2 |
| Family | Species | Spring | Summer | Autumn |
|---|---|---|---|---|
| Bacillariophyceae | Ulnaria acus | * | * | * |
| Bacillariophyceae | Aulacoseira granulata var. angustissima | * | * | * |
| Bacillariophyceae | Asterionella formosa | * | * | * |
| Bacillariophyceae | Ulnaria delicatissima | * | * | * |
| Bacillariophyceae | Fragilaria crotonensis | * | * | * |
| Bacillariophyceae | Pantocsekiella ocellata | * | * | |
| Bacillariophyceae | Cyclotella atomus | * | * | |
| Bacillariophyceae | Stephanodiscus hantzschii | * | * | |
| Bacillariophyceae | Stephanocyclus meneghinianus | * | * | * |
| Bacillariophyceae | Discostella pseudostelligera | * | * | |
| Bacillariophyceae | Urosolenia longiseta | * | * | * |
| Bacillariophyceae | Lindavia comta | * | ||
| Bacillariophyceae | Acanthoceras zachariasii | * | * | |
| Chlorophyceae | Scenedesmus quadricauda | * | * | * |
| Chlorophyceae | Micractinium pusillum | * | * | * |
| Chlorophyceae | Mougeotia sp. | * | * | * |
| Chlorophyceae | Eudorina elegans | * | * | |
| Chlorophyceae | Chlamydomonas angulosa | * | * | |
| Chlorophyceae | Monoraphidium contortum | * | * | * |
| Chlorophyceae | Actinastrum hantzschii | * | * | * |
| Chlorophyceae | Monactinus simplex | * | ||
| Chlorophyceae | Coelastrum microporum | * | ||
| Cyanophyceae | Pseudanabaena limnetica | * | ||
| Cyanophyceae | Dolichospermum circinale | * | * | |
| Cyanophyceae | Leptolyngbya tenuis | * | * | |
| Cyanophyceae | Microcystis aeruginosa | * | * | |
| Cyanophyceae | Pseudanabaena sp. | * | * | * |
| Cyanophyceae | Dolichospermum planctonicum | * | * | |
| Cryptophyceae | Rhodomonas sp. | * | * | * |
| Cryptophyceae | Cryptomonas ovata | * | * | * |
| Cryptophyceae | Cryptomonas erosa | * | * | * |
| Cryptophyceae | Cryptomonas sp. | * | * | |
| Chrysophyceae | Dinobryon divergens | * | * | * |
| Chrysophyceae | Dinobryon sertularia | * | * | |
| Dinophyceae | Gymnodinium sp. | * | * | * |
| Variables | WTE | DO | BOD | COD | Chl-a | TN | DTN | NO3-N | NH3-N | TP | DTP | PO4-P | TOC | PH | EC | TSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillariophyceae | −0.338 | 0.299 | 0.081 | −0.104 | −0.137 | −0.225 | −0.186 | −0.164 | −0.152 | −0.295 | −0.195 | 0.130 | −0.408 | −0.169 | 0.247 | −0.013 |
| Chlorophyceae | 0.687 ** | −0.619 ** | 0.358 | 0.335 | 0.326 | −0.025 | 0.051 | −0.272 | 0.098 | 0.111 | 0.216 | −0.088 | 0.610 ** | 0.239 | 0.173 | 0.063 |
| Cyanophyceae | 0.536 * | −0.279 | 0.331 | 0.029 | 0.210 | 0.296 | 0.302 | 0.131 | 0.419 | 0.157 | 0.678 ** | 0.195 | 0.549 * | 0.414 | 0.145 | −0.166 |
| Dinophyceae | 0.269 | −0.265 | 0.151 | −0.419 | 0.099 | −0.370 | −0.378 | −0.144 | −0.349 | 0.494 * | 0.067 | 0.177 | 0.555 * | −0.414 | 0.059 | −0.232 |
| Cryptophyceae | 0.255 | −0.371 | 0.607 ** | 0.345 | 0.708 ** | −0.154 | −0.063 | −0.150 | −0.140 | 0.068 | 0.117 | 0.124 | 0.494 * | 0.201 | −0.095 | 0.202 |
| Chrysophyceae | −0.337 | 0.379 | −0.520 * | −0.343 | −0.390 | 0.392 | 0.179 | 0.324 | −0.022 | −0.160 | −0.293 | −0.137 | −0.238 | −0.144 | 0.047 | −0.101 |
| Euglenophyceae | 0.111 | −0.087 | −0.172 | −0.170 | 0.005 | −0.365 | −0.319 | −0.121 | −0.303 | 0.004 | 0.096 | 0.149 | −0.059 | −0.293 | −0.449 | 0.237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, J.-K.; Sim, Y.-B.; Hwang, S.-J.; Byeon, M.-S.; Kang, T.-G. Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea. Water 2023, 15, 4118. https://doi.org/10.3390/w15234118
Im J-K, Sim Y-B, Hwang S-J, Byeon M-S, Kang T-G. Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea. Water. 2023; 15(23):4118. https://doi.org/10.3390/w15234118
Chicago/Turabian StyleIm, Jong-Kwon, Youn-Bo Sim, Soon-Jin Hwang, Myeong-Seop Byeon, and Tae-Gu Kang. 2023. "Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea" Water 15, no. 23: 4118. https://doi.org/10.3390/w15234118
APA StyleIm, J.-K., Sim, Y.-B., Hwang, S.-J., Byeon, M.-S., & Kang, T.-G. (2023). Temporal and Seasonal Variations in a Phytoplankton Community Structure in Artificial Lake Uiam, South Korea. Water, 15(23), 4118. https://doi.org/10.3390/w15234118








