Enhanced Adsorption of Cu2+ from Aqueous Solution by Sludge Biochar Compounded with Attapulgite-Modified Fe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sludge Biochar Composites
2.2. Batch Sorption Experiments
2.3. Data Analysis
3. Results and Discussion
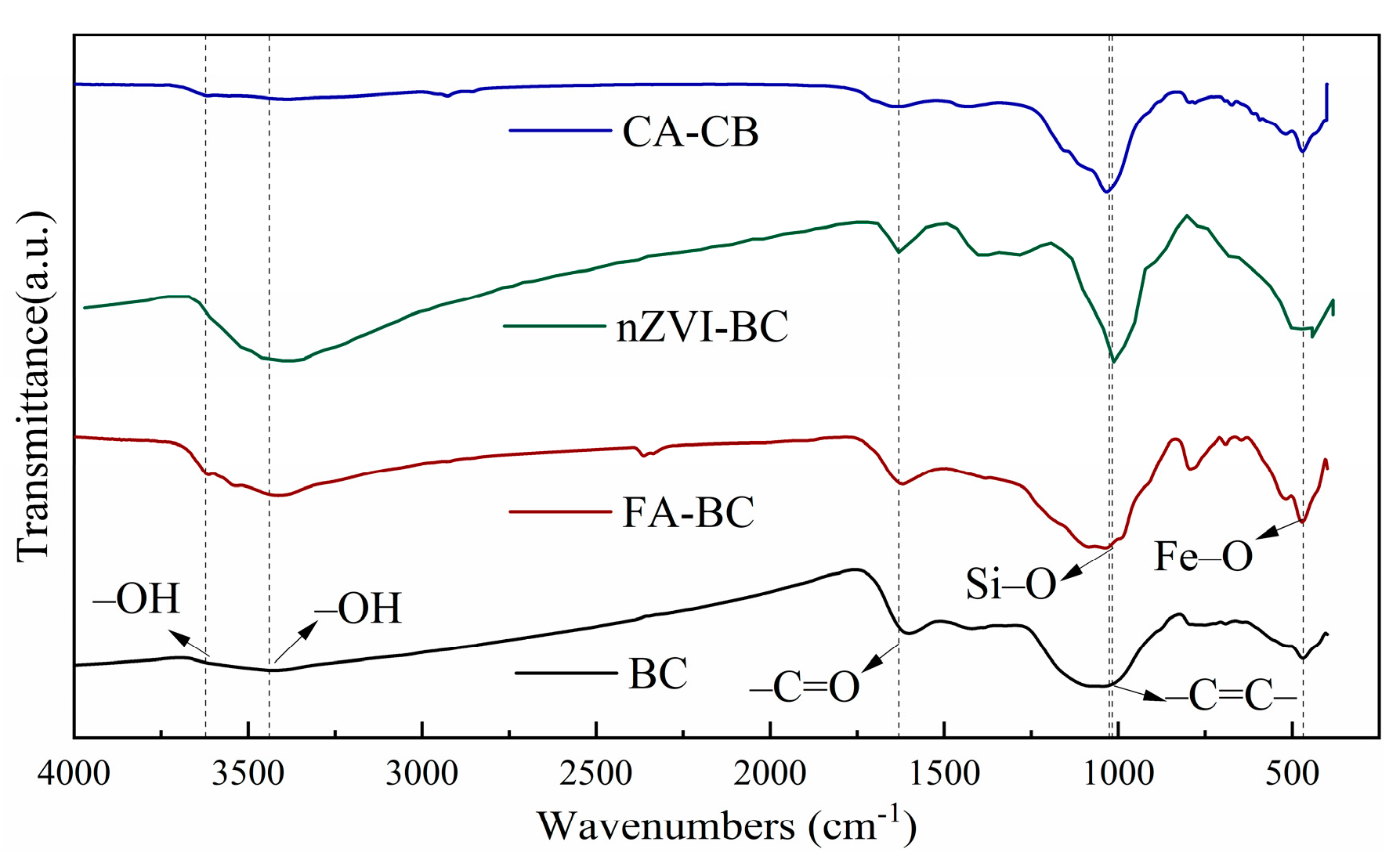
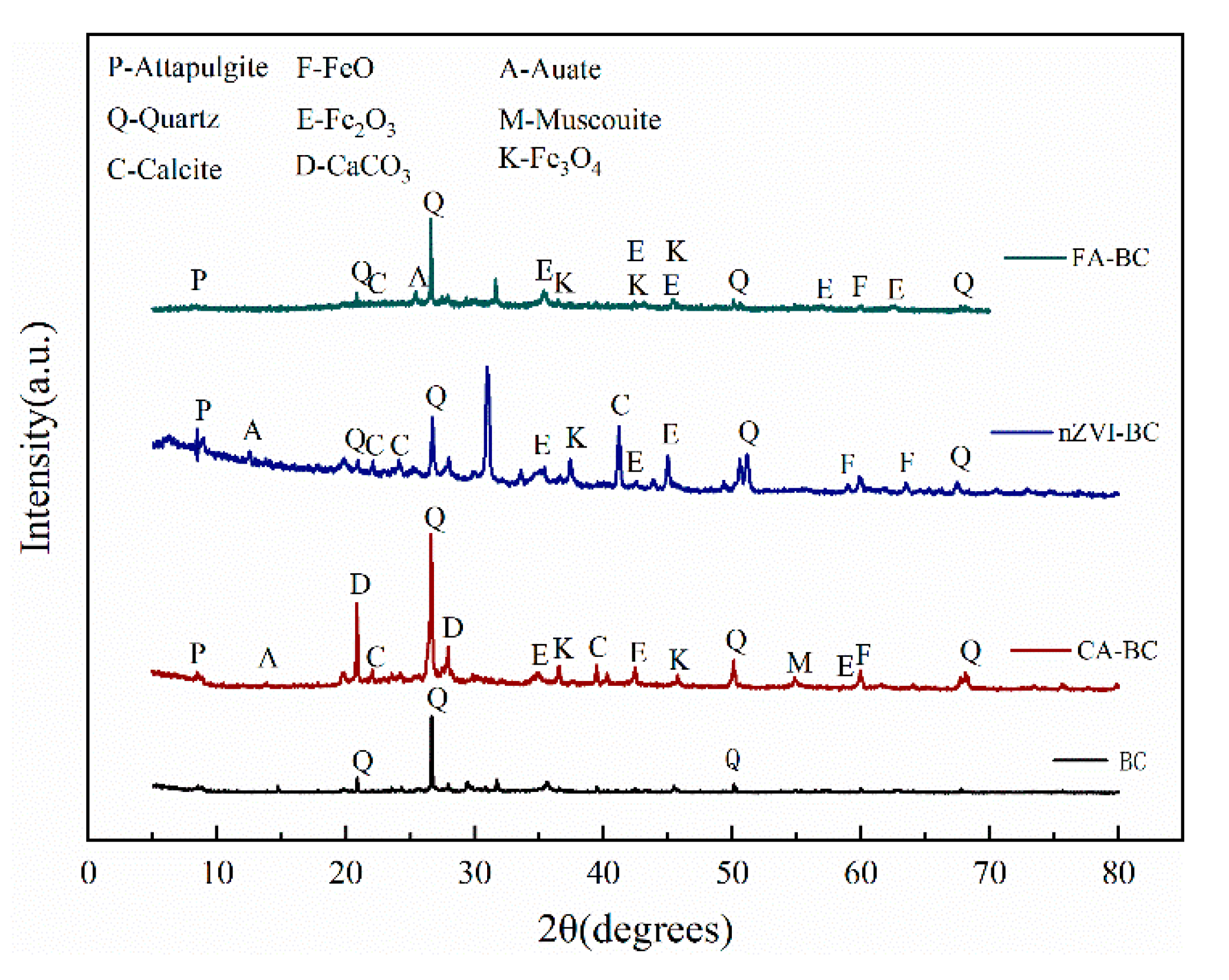
3.1. Characterization of Modified BC
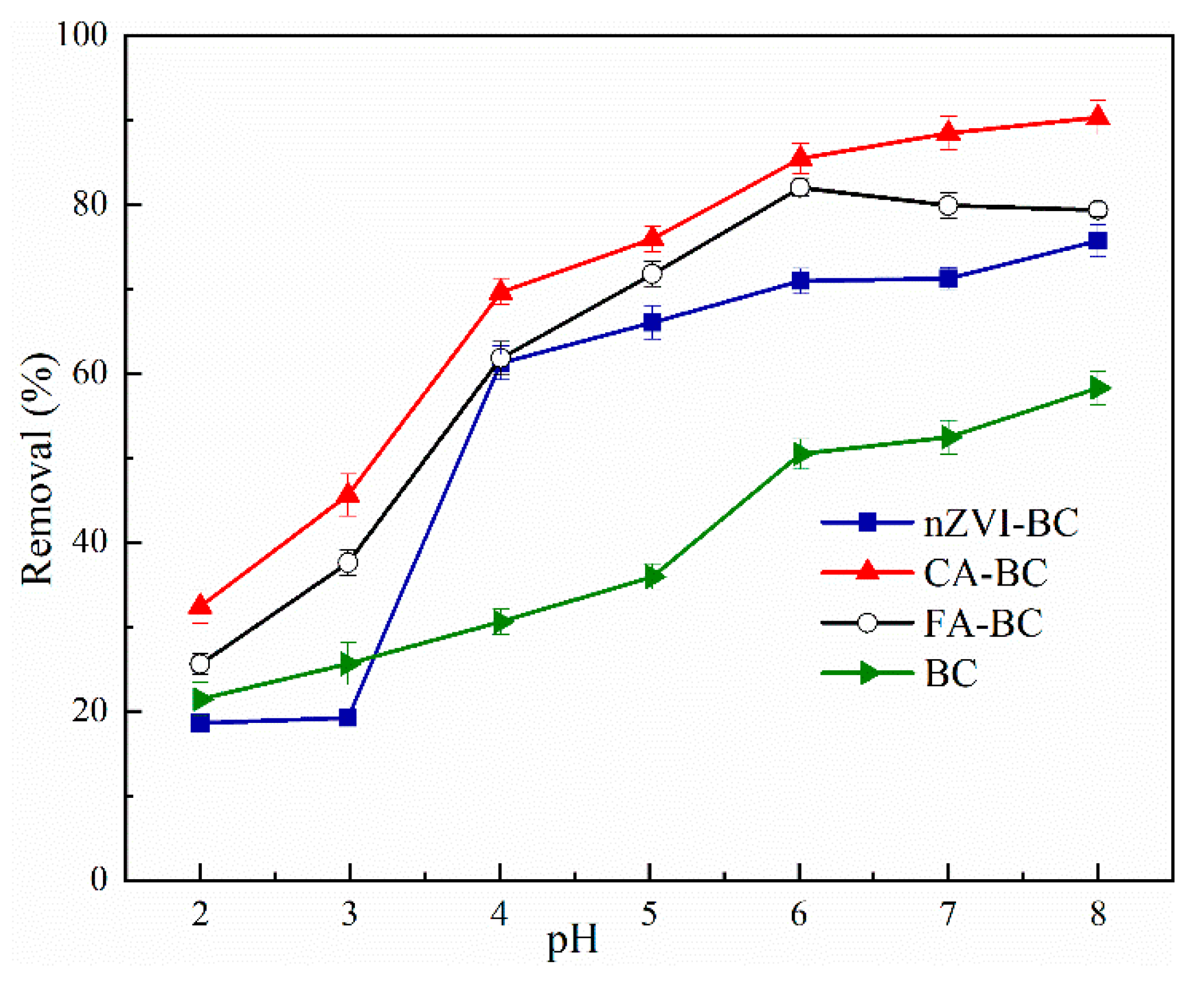
3.2. Cu2+ Removal
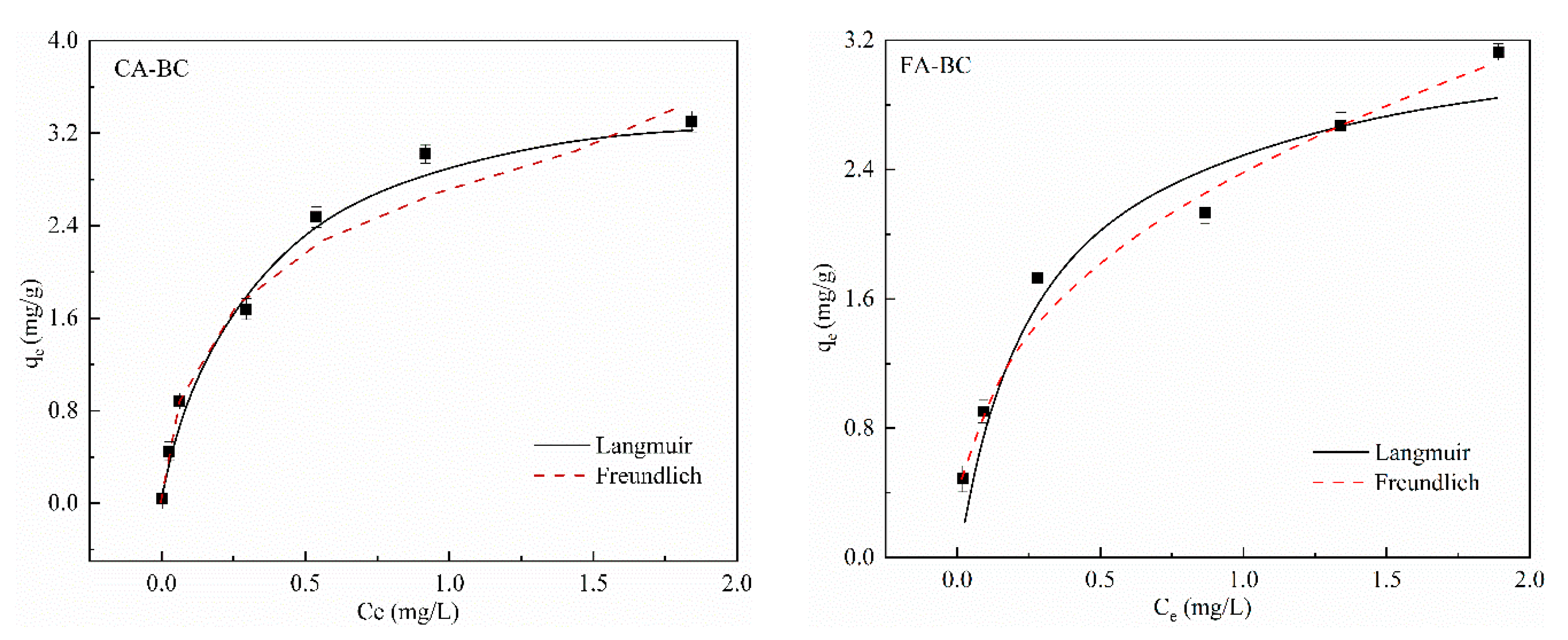
3.3. Sorption Models
3.4. Optimal Process Optimization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humelnicu, D.; Zinicovscaia, I.; Humelnicu, I.; Ignat, M.; Yushin, N.; Grozdov, D. Study on the SBA-15 silica and ETS-10 titanosilicate as efficient adsorbents for Cu(II) removal from aqueous solution. Water 2022, 14, 857. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.L.; Sun, Q.; Xia, L.Q.; Hui, Z.C. Adsorption properties of magnetic magnetite nanoparticle for coexistent Cr(VI) and Cu(II) in mixed solution. Water 2020, 12, 446. [Google Scholar] [CrossRef]
- Leiva, E.; Tapia, C.; Rodríguez, C. Highly efficient removal of Cu(II) ions from acidic aqueous solution using ZnO nanoparticles as nano-adsorbents. Water 2021, 13, 2960. [Google Scholar] [CrossRef]
- Taguba, M.A.M.; Ong, D.C.; Ensano, B.M.B.; Kan, C.-C.; Grisdanurak, N.; Yee, J.-J.; Luna, M.D.G. Nonlinear isotherm and kinetic modeling of Cu(II) and Pb(II) uptake from water by MnFe2O4/chitosan nanoadsorbents. Water 2021, 13, 1662. [Google Scholar] [CrossRef]
- Gao, L.-Y.; Deng, J.-H.; Huang, G.-F.; Li, K.; Cai, K.-Z.; Liu, Y.; Huang, F. Relative distribution of Cd2+ adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019, 272, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; O’Connor, D.; Wang, Y.A.; Jiang, L.; Xia, T.X.; Wang, L.W.; Tsang, D.; Ok, Y.S.; Hou, D.Y. A green biochar/iron oxide composite for methylene blue removal. J. Hazard. Mater. 2020, 384, 121286. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, V.; Balandinsky, D.; Romanov, G.; Rukin, G. Sorption treatment of water from chromium using biochar material. Arab. J. Basic Appl. Sci. 2023, 30, 299–306. [Google Scholar] [CrossRef]
- Wu, Q.; Dong, S.; Wang, L.; Li, X. Single and competitive adsorption behaviors of Cu2+, Pb2+ and Zn2+ on the biochar and magnetic biochar of pomelo peel in aqueous solution. Water 2021, 13, 868. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Bhatia, D.; Dhiman, J.; Samuel, J.; Prasad, R.; Singh, J. A sustainable paradigm of sewage sludge biochar: Valorization, opportunities, challenges and future prospects. J. Clean. Prod. 2020, 269, 122259. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, M.; Zhou, M.; Li, Y.C.; Wang, J.; Gao, B.; Sato, S.; Feng, K.; Yin, W.; Igalavithana, A.D.; et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J. Hazard. Mater. 2019, 373, 820–834. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Deng, J.; Xie, Y.; Zhang, C.; Jiang, Z.; Cheng, Y.; Hou, K.; Zeng, G. Stabilization of nanoscale zero-valent iron (nZVI) with modified biochar for Cr(VI) removal from aqueous solution. J. Hazard. Mater. 2017, 332, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Long, Y.; Li, H.; Wei, Z.; Liang, C.; Liu, R.; Chen, M. Unveiling the role of biochar in simultaneous removal of hexavalent chromium and trichloroethylene by biochar supported nanoscale zero-valent iron. Sci. Total Environ. 2023, 889, 164243. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Meng, K.; Zhao, W.; Han, T.; Lei, Z.; Ma, G.; Wu, C.; Jia, H. Enhanced removal of Cd2+ by nano zero-valent iron modified attapulgite from aqueous solution: Optimal design, characterization and adsorption mechanism. J. Environ. Chem. Eng. 2022, 10, 107719. [Google Scholar] [CrossRef]
- Dai, C.; Yang, L.; Wang, J.; Li, D.; Zhang, Y.; Zhou, X. Enhancing anaerobic digestion of pharmaceutical industries wastewater with the composite addition of zero valent iron (ZVI) and granular activated carbon (GAC). Bioresour. Technol. 2022, 346, 126566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qian, L.; Ouyang, D.; Chen, Y.; Han, L.; Chen, M. Effective removal of Cr(VI) by attapulgite-supported nanoscale zero-valent iron from aqueous solution: Enhanced adsorption and crystallization. Chemosphere 2019, 221, 683–692. [Google Scholar] [CrossRef]
- Lv, N.; Li, X.; Qi, X.; Ren, Y. Calcium-modified granular attapulgite removed phosphorus from synthetic wastewater containing low-strength phosphorus. Chemosphere 2022, 296, 133898. [Google Scholar] [CrossRef]
- Sohrabi, N.; Mohammadi, R.; Ghassemzadeh, H.R.; Heris, S.S.S. Equilibrium, kinetic and thermodynamic study of diazinon adsorption from water by clay/GO/Fe3O4: Modeling and optimization based on response surface methodology and artificial neural network. J. Mol. Liq. 2021, 328, 115384. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; El-Monaem, E.M.A.; Mohy-Eldin, M.S.; Omer, A.M. Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci. Rep. 2021, 11, 16598. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]
- Dong, L.; Huang, Y.J.; Chen, H.; Liu, L.Q.; Liu, C.Q.; Xu, L.G.; Zha, J.R.; Wang, Y.X.; Liu, H. Magnetic γ-Fe2O3-loaded attapulgite sorbent for Hg0 removal in coal-fired flue gas. Energy Fuels 2019, 33, 7522–7533. [Google Scholar] [CrossRef]
- Xu, C.; Qi, J.; Yang, W.; Chen, Y.; Yang, C.; He, Y.; Wang, J.; Lin, A. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay. Sci. Total Environ. 2019, 686, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, P.; Wang, Y.; Wen, K.; Su, X.; Zhu, R.; He, H.; Xi, Y. Effect of acid activation of palygorskite on their toluene adsorption behaviors. Appl. Clay Sci. 2018, 159, 60–67. [Google Scholar] [CrossRef]
- Katiyar, R.; Patel, A.K.; Nguyen, T.-B.; Singhania, R.R.; Chen, C.-W.; Dong, C.-D. Adsorption of copper (II) in aqueous solution using biochars derived from Ascophyllum nodosum seaweed. Bioresour. Technol. 2021, 328, 124829. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, Z.; Zhang, J.; Jiao, C.; Ding, L.; Yang, S. Synthesis and adsorption properties of novel bacterial cellulose/graphene oxide/attapulgite materials for Cu and Pb Ions in aqueous solutions. Materials 2020, 13, 3703. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice straw biochar and magnetic rice straw biochar for safranin O adsorption from aqueous solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Tehreem, S.; Yousra, M.; Alamer, K.H.; Alsudays, I.M.; Sarwar, S.; Kamal, A.; Naeem, S. Analysis of the role of various biochar in the remediation of heavy metals in contaminated water and its kinetics study. J. Saudi Chem. Soc. 2022, 26, 101518. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.-C.; Li, Z.; Chen, Z.; Wang, Y. Co-pyrolysis of sewage sludge and organic fractions of municipal solid waste: Synergistic effects on biochar properties and the environmental risk of heavy metals. J. Hazard. Mater. 2021, 412, 125200. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Dai, Y. Strong adsorption of metolachlor by biochar prepared from walnut shells in water. Environ. Sci. Pollut. Res. 2021, 28, 48379–48391. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, L.; Huang, R.; Lin, H.; Wang, D. Immobilization of heavy metals in biochar derived from co-pyrolysis of sewage sludge and calcium sulfate. J. Hazard. Mater. 2021, 403, 123648. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Y.; Zhang, Y.; Lin, T.; Zeng, G.; Zhang, S.; Wang, Y.; He, W.; Zhang, M.; Long, H. An efficient adsorbent: Simultaneous activated and magnetic ZnO doped biochar derived from camphor leaves for ciprofloxacin adsorption. Bioresour. Technol. 2019, 288, 121511. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, J.; Zhang, D.; Wang, F.; Li, Y.; Yi, W. Effect of pyrolysis temperature on characteristics, chemical speciation and environmental risk of Cr, Mn, Cu, and Zn in biochars derived from pig manure. Sci. Total Environ. 2020, 704, 135283. [Google Scholar] [CrossRef]
- Wu, J.; Huang, R.; Zhou, Q.; Lu, H.; Li, F.; Wu, K.; Li, Z. Magnetic biochar reduces phosphorus uptake by Phragmites australis during heavy metal remediation. Sci. Total Environ. 2021, 758, 143643. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, R.; Pang, X.; Yu, C.; Jian, X. Adsorption of phosphorus in water by metal-modified large-size biochar: Realizing the recovery and recycling of phosphorus. Sustain. Chem. Pharm. 2023, 36, 101279. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, I.K.; Tsang, D.C.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.; Alessi, D.S.; Komárek, M.; Ok, Y.S.; et al. Multifunctional iron-biochar composites for the removal of potentially toxic elements, inherent cations, and hetero-chloride from hydraulic fracturing wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef]
- Xu, C.; Feng, Y.; Li, H.; Yang, Y.; Wu, R. Adsorption and immobilization of phosphorus from eutrophic seawater and sediment using attapulgite—Behavior and mechanism. Chemosphere 2023, 313, 137390. [Google Scholar] [CrossRef]
- Wang, H.; Lou, X.; Hu, Q.; Sun, T. Adsorption of antibiotics from water by using Chinese herbal medicine residues derived biochar: Preparation and properties studies. J. Mol. Liq. 2021, 325, 114967. [Google Scholar] [CrossRef]
- Qu, J.; Akindolie, M.S.; Feng, Y.; Jiang, Z.; Zhang, G.S.; Jiang, Q.; Deng, F.X.; Cao, B.; Zhang, Y. One-pot hydrothermal synthesis of NaLa(CO3)2 decorated magnetic biochar for efficient phosphate removal from water: Kinetics, isotherms, thermodynamics, mechanisms and reusability exploration. Chem. Eng. J. 2020, 394, 124915. [Google Scholar] [CrossRef]
- Anfar, Z.; Ahsaine, H.A.; Zbair, M.; Amedlous, A.; El Fakir, A.A.; Jada, A.; El Alem, N. Recent trends on numerical investigations of response surface methodology for pollutants adsorption onto activated carbon materials: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1043–1084. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, M.; Shao, S. Optimization of target biochar for the adsorption of target heavy metal ion. Sci. Rep. 2022, 12, 13662. [Google Scholar] [CrossRef] [PubMed]





| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | |||||
|---|---|---|---|---|---|---|
| k1 | qm1 | R2 | k2 | qm2 | R2 | |
| CA–BC | 0.0254 | 3.5174 | 0.9760 | 0.0082 | 3.9666 | 0.9940 |
| nZVI–BC | 0.0197 | 3.8460 | 0.9260 | 0.0050 | 4.5490 | 0.9790 |
| FA–BC | 0.0246 | 4.7535 | 0.8975 | 0.0061 | 4.7348 | 0.9875 |
| BC | 0.0936 | 2.4242 | 0.9523 | 0.0075 | 2.4456 | 0.9625 |
| T/K (298) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax | KL | R2 | KF | nf | R2 | |
| CA–BC | 3.988 | 0.0355 | 0.9922 | 3.907 | 3.2323 | 0.9839 |
| nZVI–BC | 3.226 | 0.0660 | 0.9360 | 2.408 | 3.7900 | 0.9780 |
| FA–BC | 3.070 | 0.0056 | 0.9892 | 3.037 | 3.2300 | 0.9830 |
| BC | 2.823 | 0.0203 | 0.9949 | 0.198 | 2.890 | 0.9726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ren, J.; Ren, H.; Tao, L.; Wu, C.; Sun, X.; Lv, M. Enhanced Adsorption of Cu2+ from Aqueous Solution by Sludge Biochar Compounded with Attapulgite-Modified Fe. Water 2023, 15, 4169. https://doi.org/10.3390/w15234169
Wang R, Ren J, Ren H, Tao L, Wu C, Sun X, Lv M. Enhanced Adsorption of Cu2+ from Aqueous Solution by Sludge Biochar Compounded with Attapulgite-Modified Fe. Water. 2023; 15(23):4169. https://doi.org/10.3390/w15234169
Chicago/Turabian StyleWang, Ruoan, Jun Ren, Hanru Ren, Ling Tao, Chaohui Wu, Xinni Sun, and Mairong Lv. 2023. "Enhanced Adsorption of Cu2+ from Aqueous Solution by Sludge Biochar Compounded with Attapulgite-Modified Fe" Water 15, no. 23: 4169. https://doi.org/10.3390/w15234169





