Mitigating Ammonia, Methane, and Carbon Dioxide Emissions from Stored Pig Slurry Using Chemical and Biological Additives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Use of Additives
2.3. Dynamic Chamber
2.4. Sampling Frequency
2.5. Slurry Sample Analysis
2.6. Statistical Analysis
3. Results
3.1. Greenhouse Gas Emissions and Ammonia
3.2. Raw and Treated Pig Slurry Analysis
4. Discussion
4.1. Methane (CH4) Emissions
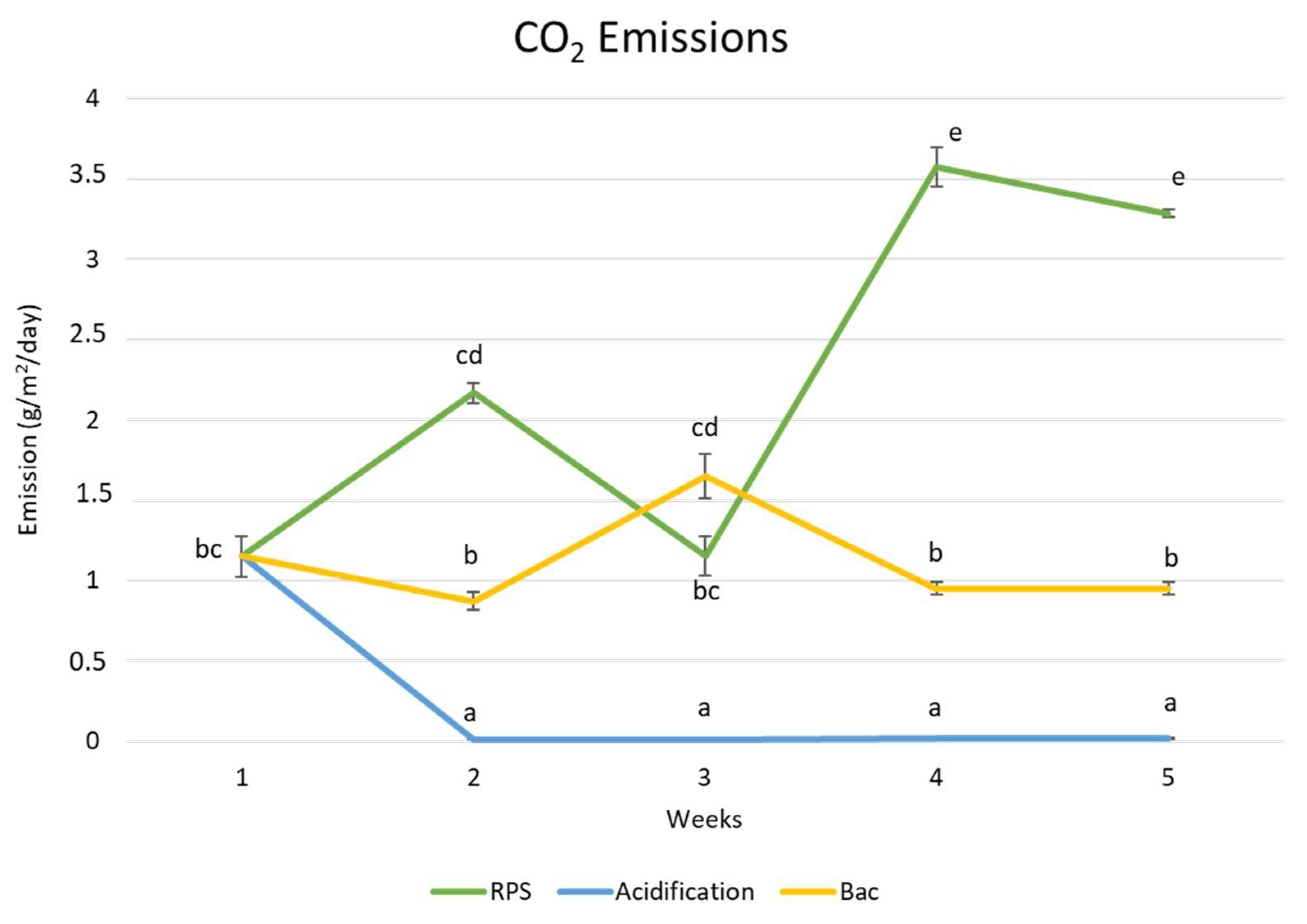
4.2. Carbon Dioxide (CO2) Emissions
4.3. Ammonia (NH3) Emissions
4.4. Compositions of the Treated and Control Raw Pig Slurry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whalen, J.K.; Thomas, B.W.; Sharifi, M. Novel Practices and Smart Technologies to Maximize the Nitrogen Fertilizer Value of Manure for Crop Production in Cold Humid Temperate Regions. Adv. Agron. 2019, 153, 1–85. [Google Scholar] [CrossRef]
- STATISTA. Number of Pigs Worldwide in 2023, by Leading Country (in Million Head); STATISTA: Hamburg, Germany, 2023. [Google Scholar]
- Zhang, L.; Sun, X. Addition of Seaweed and Bentonite Accelerates the Two-Stage Composting of Green Waste. Bioresour. Technol. 2017, 243, 154–162. [Google Scholar] [CrossRef] [PubMed]
- EPA United States Environmental Protection Agency. Available online: https://iaspub.epa.gov/tdb/pages/treatment/treatmentOverview.do?treatmentProcessId=1934681921 (accessed on 1 November 2023).
- European Union. Directive 91/676/EEC. Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources; European Union: Brussels, Belgium, 1991; Volume 375, pp. 1–8. [Google Scholar]
- European Union. Water Framework Directive (2000/60/EC); European Union: Brussels, Belgium, 2000. [Google Scholar]
- Varma, V.S.; Parajuli, R.; Scott, E.; Canter, T.; Lim, T.T.; Popp, J.; Thoma, G. Dairy and Swine Manure Management—Challenges and Perspectives for Sustainable Treatment Technology. Sci. Total Environ. 2021, 778, 146319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sheng, L.; Xu, J. Clogging Mechanisms of Constructed Wetlands: A Critical Review. J. Clean. Prod. 2021, 295, 126455. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, H.; Zhu, Z.; Gerber, P.J.; Xin, H.; Smith, P.; Opio, C.; Steinfeld, H.; Chadwick, D. Mitigating Greenhouse Gas and Ammonia Emissions from Swine Manure Management: A System Analysis. Environ. Sci. Technol. 2017, 51, 4503–4511. [Google Scholar] [CrossRef] [PubMed]
- Raj, T.; Verma, S.; Kumar, N.; Agrawal, R. Chapter 11—Role of Woody Biomass in Carbon Capture, Circular Bioeconomy, and Biomanufacturing. In Sustainable Biorefining of Woody Biomass to Biofuels and Biochemicals; Kumar, D., Kumar, S., Rajendran, K., Ray, R.C., Eds.; Applied Biotechnology Reviews; Woodhead Publishing: Cambridge, UK, 2023; pp. 291–318. ISBN 978-0-323-91187-0. [Google Scholar]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Solid—Liquid Separation of Animal Slurry in Theory and Practice. A Review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef]
- Loyon, L.; Guiziou, F.; Béline, F.; Peu, P. Gaseous Emissions (NH3, N2O, CH4, CO2) during Pig Slurry Biological Aerobic Treatment and Treatment by-Product Storages. Int. Congr. Ser. 2006, 1293, 299–302. [Google Scholar] [CrossRef]
- Lymperatou, A.; Rasmussen, N.B.; Gavala, H.N.; Skiadas, I.V. Improving the Anaerobic Digestion of Swine Manure through an Optimized Ammonia Treatment: Process Performance, Digestate and Techno-Economic Aspects. Energies 2021, 14, 787. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Sommer, S.G.; Christensen, M.L.; Schmidt, T.; Stoumann Jensen, L. Animal Manure Recycling; John Wiley & Sons Ltd.: London, UK, 2013. [Google Scholar]
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Alternatives to Sulfuric Acid for Slurry Acidification: Impact on Slurry Composition and Ammonia Emissions during Storage. J. Clean. Prod. 2016, 131, 296–307. [Google Scholar] [CrossRef]
- Fuchs, A.; Dalby, F.R.; Liu, D.; Kai, P.; Feilberg, A. Improved Effect of Manure Acidification Technology for Gas Emission Mitigation by Substituting Sulfuric Acid with Acetic Acid. Clean. Eng. Technol. 2021, 4, 100263. [Google Scholar] [CrossRef]
- Ottosen, L.; Poulsen, H.; Nielsen, D.; Finster, K.; Nielsen, L.P.; Revsbech, N. Observations on Microbial Activity in Acidified Pig Slurry. Biosyst. Eng. 2009, 102, 291–297. [Google Scholar] [CrossRef]
- McCrory, D.F.; Hobbs, P.J. Additives to Reduce Ammonia and Odor Emissions from Livestock Wastes: A Review. J. Environ. Qual. 2001, 30, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Provolo, G.; Finzi, A.; Perazzolo, F.; Mattachini, G.; Riva, E. Effect of a Biological Additive on Nitrogen Losses from Pig Slurry during Storage. J. Environ. Qual. 2016, 45, 1460–1465. [Google Scholar] [CrossRef]
- Wheeler, E.F.; Adviento-Borbe, M.A.A.; Brandt, R.C.; Topper, P.A.; Topper, D.A.; Elliott, H.A.; Graves, R.E.; Wheeler, E.F.; Arlene, M.; Adviento-Borbe, A.; et al. Manure Amendments for Mitigation of Dairy Ammonia and Greenhouse Gas Emissions: Preliminary Screening. Agric. Eng. Int. CIGR J. 2001, 13, 1–14. [Google Scholar]
- Van der Stelt, B.; Temminghoff, E.J.M.; Van Vliet, P.C.J.; Van Riemsdijk, W.H. Volatilization of Ammonia from Manure as Affected by Manure Additives, Temperature and Mixing. Bioresour Technol 2007, 98, 3449–3455. [Google Scholar] [CrossRef]
- International VERA Secretariat. Verification of Environmental Technologies for Agricultural Production Vera Test Protocol Covers and Other Mitigation Technologies for Reduction of Gaseous Emissions from Stored Manure; International VERA Secretariat: Delft, The Netherlands, 2018. [Google Scholar]
- Peter, A.S. Effect of Acidification and Soil Injection of Animal Slurry on Ammonia and Odour Emission; Univerzita Veterinárskeho Lekárstva a Farmácie v Košiciach: Košice, Slovakia, 2013. [Google Scholar]
- Petersen, S.O.; Andersen, A.J.; Eriksen, J. Effects of Cattle Slurry Acidification on Ammonia and Methane Evolution during Storage. J. Environ. Qual. 2012, 41, 88–94. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of Animal Slurry—A Review. J. Envion. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Magrama Evaluación de Técnicas de Gestión de Deyecciones en Ganadería; Ministerio de Agricultura, Alimentación y Medio Am-biente, Centro de Publicaciones: Madrid, Spain, 2015; pp. 1–104.
- Wypych, G. Principles of Thermal Degradation. In PVC Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2015; pp. 79–165. [Google Scholar] [CrossRef]
- Kevin, K.P.R. Greenhouse Gas Fluxes—Recirculating Chamber Method; KBS LTER: East Lansing, MI, USA, 2019. [Google Scholar]
- Duchaufour, P. Precis de Pedologie; Masson: Paris, France, 1970. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Hjorth, M.; Cocolo, G.; Jonassen, K.; Abildgaard, L.; Sommer, S.G. Continuous In-House Acidification Affecting Animal Slurry Composition. Biosyst. Eng. 2015, 132, 56–60. [Google Scholar] [CrossRef]
- Shin, S.R.; Im, S.; Mostafa, A.; Lee, M.K.; Yun, Y.M.; Oh, S.E.; Kim, D.H. Effects of Pig Slurry Acidification on Methane Emissions during Storage and Subsequent Biogas Production. Water Res. 2019, 152, 234–240. [Google Scholar] [CrossRef]
- Overmeyer, V.; Trimborn, M.; Clemens, J.; Hölscher, R.; Büscher, W. Acidification of Slurry to Reduce Ammonia and Methane Emissions: Deployment of a Retrofittable System in Fattening Pig Barns. J. Environ. Manag. 2023, 331. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.-I.; Gelman, L.; Hukins, D.W.L. Two-Stage Thermoacoustic Electricity Generator for Waste Heat Recovery. In Proceedings of the International Association of Engineers World Congress on Engineering: WCE 2016, London, UK, 29 June–1 July 2016; Imperial College London: London, UK, 2016. ISBN 9789881404800. [Google Scholar]
- Berg, W.; Brunsch, R.; Pazsiczki, I. Greenhouse Gas Emissions from Covered Slurry Compared with Uncovered during Storage. Agric. Ecosyst. Environ. 2006, 112, 129–134. [Google Scholar] [CrossRef]
- Berg, W.; Pazsiczki, I. Mitigation of Methane Emissions during Manure Storage. Int. Congr. Ser. 2006, 1293, 213–216. [Google Scholar] [CrossRef]
- Petersen, S.O.; Højberg, O.; Poulsen, M.; Schwab, C.; Eriksen, J. Methanogenic Community Changes, and Emissions of Methane and Other Gases, during Storage of Acidified and Untreated Pig Slurry. J. Appl. Microbiol. 2014, 117, 160–172. [Google Scholar] [CrossRef]
- Chen, J.; Wade, M.J.; Dolfing, J.; Soyer, O.S. Increasing Sulfate Levels Show a Differential Impact on Synthetic Communities Comprising Different Methanogens and a Sulfate Reducer. J. R. Soc. Interface 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Wolter, M.; Prayitno, S.; Schuchardt, F. Greenhouse Gas Emission during Storage of Pig Manure on a Pilot Scale. Bioresour. Technol. 2004, 95, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Misselbrook, T.; Hunt, J.; Perazzolo, F.; Provolo, G. Greenhouse Gas and Ammonia Emissions from Slurry Storage: Impacts of Temperature and Potential Mitigation through Covering (Pig Slurry) or Acidification (Cattle Slurry). J. Environ. Qual. 2016, 45, 1520–1530. [Google Scholar] [CrossRef]
- Eriksen, J.; Andersen, A.J.; Poulsen, H.V.; Adamsen, A.P.S.; Petersen, S.O. Sulfur Turnover and Emissions during Storage of Cattle Slurry: Effects of Acidification and Sulfur Addition. J. Environ. Qual. 2012, 41, 1633–1641. [Google Scholar] [CrossRef]
- Joubin, M. Animal Slurry Acidification: Effects of Slurry Characteristics, Use of Different Acids, Slurry PH Buffering. Available online: https://www.semanticscholar.org/paper/Animal-slurry-acidification%3A-effects-of-slurry-use-Joubin/ecbe959322d9931936c9a9cba79a806e764fae4d (accessed on 26 November 2023).
- Regueiro, I.; Coutinho, J.; Fangueiro, D. Comparison of Different Approaches for Ammonia Emissions Minimization by Acidification of Dairy and Pig Slurries. In Proceedings of the Ramiran 2013 Recycling of Agricultural and Industrial Residues in Agriculture, Versailles, France, 3–5 June 2013. [Google Scholar]
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing Ammonia Loss from Cattle Slurry by the Use of Acidifying Additives: The Role of the Buffer System. J. Sci. Food Agric. 1991, 57, 335–349. [Google Scholar] [CrossRef]
- Kumari, M.; Chandel, M.K. Anaerobic Co-Digestion of Sewage Sludge and Organic Fraction of Municipal Solid Waste: Focus on Mix Ratio Optimization and Synergistic Effects. J. Environ. Manag. 2023, 345. [Google Scholar] [CrossRef]
- Pongsopon, M.; Woraruthai, T.; Anuwan, P.; Amawatjana, T.; Tirapanampai, C.; Prombun, P.; Kusonmano, K.; Weeranoppanant, N.; Chaiyen, P.; Wongnate, T. Anaerobic Co-Digestion of Yard Waste, Food Waste, and Pig Slurry in a Batch Experiment: An Investigation on Methane Potential, Performance, and Microbial Community. Bioresour. Technol. Rep. 2023, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, N.; Liu, C.; Mi, T.; Li, J.; He, X.; Li, S.; Sun, Z.; Zhen, Y. Methanogenesis Pathways of Methanogens and Their Responses to Substrates and Temperature in Sediments from the South Yellow Sea. Sci. Total Environ. 2022, 815. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of Extreme PH Conditions on Methanogenesis: Methanogen Metabolism and Community Structure. Sci. Total Environ. 2023, 877. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wang, R.; Jiang, Q.; Zhang, Y.; Peng, X. Anaerobic Methane Oxidation Coupled to Denitrification Is an Important Potential Methane Sink in Deep-Sea Cold Seeps. Sci. Total Environ. 2020, 748, 142459. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Li, L.; Wang, W.; Xue, S.; Liu, J. Electro-Stimulated Anaerobic Oxidation of Methane with Synergistic Denitrification by Adding AQS: Electron Transfer Mode and Mechanism. Environ. Res. 2023, 229, 115997. [Google Scholar] [CrossRef] [PubMed]
- Bertora, C.; Alluvione, F.; Zavattaro, L.; van Groenigen, J.W.; Velthof, G.; Grignani, C. Pig Slurry Treatment Modifies Slurry Composition, N2O, and CO2 Emissions after Soil Incorporation. Soil Biol. Biochem. 2008, 40, 1999–2006. [Google Scholar] [CrossRef]
- Ambrose, H.W.; Dalby, F.R.; Feilberg, A.; Kofoed, M.V.W. Additives and Methods for the Mitigation of Methane Emission from Stored Liquid Manure. Biosyst. Eng. 2023, 229, 209–245. [Google Scholar] [CrossRef]
- Pedersen, J.; Feilberg, A.; Nyord, T. Effect of Storage and Field Acidification on Emissions of NH3, NMVOC, and Odour from Field Applied Slurry in Winter Conditions. J. Environ. Manag. 2022, 310, 114756. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Guàrdia, M.; Dai, X.R.; Nadimi, E.S. Emissions of NH3, CO2 and H2S during Swine Wastewater Management: Characterization of Transient Emissions after Air-Liquid Interface Disturbances. Atmos. Environ. 2012, 54, 408–418. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Sutton, A.L.; Kelly, D.T. Mechanisms of Gas Releases from Swine Wastes. Trans. ASABE 2009, 52, 2013–2025. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, D.; Yang, J.; Liu, X.; Zhang, K.; Fu, X.; Jiang, B.; Xue, R.; Fan, L.; Chen, W.; et al. Carbon Dioxide and Methane Emission of Denitrification Bioreactor Filling Waste Sawdust and Industrial Sludge for Treatment of Simulated Agricultural Surface Runoff. J. Environ. Manag. 2021, 289, 112503. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Wang, L.; Chen, Y.; Zheng, X.; Su, Y.; Tao, X. Insight into a Direct Carbon Dioxide Effect on Denitrification and Denitrifying Bacterial Communities in Estuarine Sediment. Sci. Total Environ. 2018, 643, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.R.; Blanes-Vidal, V. Emissions of Ammonia, Carbon Dioxide, and Hydrogen Sulfide from Swine Wastewater during and after Acidification Treatment: Effect of PH, Mixing and Aeration. J. Environ. Manag. 2013, 115, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kai, P.; Pedersen, P.; Jensen, J.E.; Hansen, M.N.; Sommer, S.G. A Whole-Farm Assessment of the Efficacy of Slurry Acidification in Reducing Ammonia Emissions. Eur. J. Agron. 2008, 28, 148–154. [Google Scholar] [CrossRef]
- Eihe, P.; Vebere, L.L.; Grinfelde, I.; Pilecka, J.; Sachpazidou, V.; Grinberga, L. The Effect of Acidification of Pig Slurry Digestate Applied on Winter Rapeseed on the Ammonia Emission Reduction. In IOP Conference Series: Earth and Environmental Science, Proceedings of the XVI-th International Youth Science and Environmental Baltic Region Countries Forum, Gdansk, Poland, 7–9 October 2019; Institute of Physics Publishing: Bristol, UK, 2019; Volume 390, p. 390. [Google Scholar]
- Kupper, T.; Häni, C.; Neftel, A.; Kincaid, C.; Bühler, M.; Amon, B.; VanderZaag, A. Ammonia and Greenhouse Gas Emissions from Slurry Storage—A Review. Agric. Ecosyst. Environ. 2020, 300, 106963. [Google Scholar] [CrossRef]
- Silva, A.A.; Fangueiro, D.; Carvalho, M. Slurry Acidification as a Solution to Minimize Ammonia Emissions from the Combined Application of Animal Manure and Synthetic Fertilizer in No-Tillage. Agronomy 2022, 12, 265. [Google Scholar] [CrossRef]
- Snoek, D.J.W.; Stigter, J.D.; Ogink, N.W.M.; Groot Koerkamp, P.W.G. Sensitivity Analysis of Mechanistic Models for Estimating Ammonia Emission from Dairy Cow Urine Puddles. Biosyst. Eng. 2014, 121, 12–24. [Google Scholar] [CrossRef]
- Bian, X.; Wu, Y.; Li, J.; Yin, M.; Li, D.; Pei, H.; Chang, S.; Guo, W. Effect of Dissolved Oxygen on High C/N Wastewater Treatment in Moving Bed Biofilm Reactors Based on Heterotrophic Nitrification and Aerobic Denitrification: Nitrogen Removal Performance and Potential Mechanisms. Bioresour. Technol. 2022, 365, 128147. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, J.; Wang, M.; Wu, Y.; Zhang, J.; Wang, S.; Pan, Y.; Cao, G. Nitrogen Removal from Wastewater for Heterotrophic Nitrification-Aerobic Denitrification Bacterium with the Combination of Bacteriophage DEY7 and Fe Nanoparticles. Biochem. Eng. J. 2023, 191. [Google Scholar] [CrossRef]
- Li, Y.; Jones, D.L.; Chen, Q.; Ge, T.; Chadwick, D.R. Acidification and Anaerobic Digestion Change the Phosphorus Forms and Distribution in Particle Fractions of Cattle Slurry and Phosphorus Dynamics in Soil after Application. Biosyst. Eng. 2020, 200, 101–111. [Google Scholar] [CrossRef]
- Overmeyer, V.; Kube, A.; Clemens, J.; Büscher, W.; Trimborn, M. One-Time Acidification of Slurry: What Is the Most Effective Acid and Treatment Strategy? Agronomy 2021, 11, 1319. [Google Scholar] [CrossRef]
- Zhu, K.; El-Din, M.G.; Moawad, A.K.; Bromley, D. Physical and Chemical Processes for Removing Suspended Solids and Phosphorus from Liquid Swine Manure. Environ. Technol. 2004, 25, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Paz Pérez-Sangrador, M.; Cristina León-Cófreces, M.; Acítores-Benavente, M.; Cruz García-González, M. Solids and Nutrient Removal from Flushed Swine Manure Using Polyacrylamides. J. Environ. Manag. 2012, 93, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Umweltbundesamt GmbH. FINAL REPORT: Framework Service Contract ENV.D2/FRA/2012/0013 Support to the Implementation of the UWWTD: COD Substitution Scoping Study; Umweltbundesamt: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- El Bied, O.; García-Valero, A.; Fechtali, T.; Faz, Á.; Acosta, J.A. Purification Performance of Filtration Process for Pig Slurry Using Marine Sands, Silty Loam Soils, Fly Ash and Zeolite. Agronomy 2021, 11, 1608. [Google Scholar] [CrossRef]
- Gerardi, M.H.; Lytle, B. BOD and TSS. In The Biology and Troubleshooting of Facultative Lagoons; Wiley: Hoboken, NJ, USA, 2015; pp. 195–198. [Google Scholar]
- Alexander, R.T.; Cordat, E.; Chambrey, R.; Dimke, H.; Eladari, D. Acidosis and Urinary Calcium Excretion: Insights from Genetic Disorders. J. Am. Soc. Nephrol. 2016, 27, 3511–3520. [Google Scholar] [CrossRef]
- Wu, R.; Yao, F.; Li, X.; Shi, C.; Zang, X.; Shu, X.; Liu, H.; Zhang, W. Manganese Pollution and Its Remediation: A Review of Biological Removal and Promising Combination Strategies. Microorganisms 2022, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A. Complete Nitrification–Denitrification of Swine Manure in a Full-Scale, Non-Conventional Composting System. Waste Manag. 2015, 46, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Michaud, L.; Blancheton, J.P.; Bruni, V.; Piedrahita, R. Effect of Particulate Organic Carbon on Heterotrophic Bacterial Populations and Nitrification Efficiency in Biological Filters. Aquac. Eng. 2006, 34, 224–233. [Google Scholar] [CrossRef]
- Ouyang, C.F.; Chiou, R.J.; Lin, C.T. The Characteristics of Nitrogen Removal by the Biofilter System. Water Res. Technol. 2000, 42, 137–147. [Google Scholar] [CrossRef]
- Moyo, L.B.; Simate, G.S.; Mutsatsa, T. Biological Acidification of Pig Manure Using Banana Peel Waste to Improve the Dissolution of Particulate Phosphorus: A Critical Step for Maximum Phosphorus Recovery as Struvite. Heliyon 2022, 8. [Google Scholar] [CrossRef]
- Tiangco, K.; De Luna, M.D.; Vilando, A.; Lu, M.-C. Removal and Recovery of Calcium from Aqueous Solutions by Fluidized-Bed Homogeneous Crystallization. Process Saf. Environ. Prot. 2019, 128, 307–315. [Google Scholar] [CrossRef]
- Therdkiattikul, N.; Ratpukdi, T.; Kidkhunthod, P.; Chanlek, N.; Siripattanakul-Ratpukdi, S. Manganese-Contaminated Groundwater Treatment by Novel Bacterial Isolates: Kinetic Study and Mechanism Analysis Using Synchrotron-Based Techniques. Sci. Rep. 2020, 10, 13391. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Zouboulis, A.I. Biological Treatment of Mn(II) and Fe(II) Containing Groundwater: Kinetic Considerations and Product Characterization. Water Res. 2004, 38, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, C.; Zhou, Z.; Cao, X.; Zhou, Y. Coupling between Nitrification and Denitrification as Well as Its Effect on Phosphorus Release in Sediments of Chinese Shallow Lakes. Water 2019, 11, 1809. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Chen, J.; Wu, Z.; Liu, Y.; Xie, S. Nitrifying Activity and Ammonia-Oxidizing Microorganisms in a Constructed Wetland Treating Polluted Surface Water. Sci. Total Environ. 2018, 628–629, 310–318. [Google Scholar] [CrossRef]



| 1st Week | 2nd Week | 3rd Week | 4th Week | 5th Week | ||
|---|---|---|---|---|---|---|
| Tª (°C) | RPS | 24.9 (0.07) d | 21.6 (0.05) c | 21.9 (0.11) c | 20.7 (0.05) b | 17.4 (0.15) a |
| ACID | 24.9 (0.07) d | 21.8 (0.18) c | 21.1 (0.11) c | 20.7 (0.16) b | 16.5 (0.17) a | |
| BAC | 24.9 (0.07) d | 21.3 (0.13) c | 21.7 (0.05) c | 21.6 (0.15) c | 17.7 (0.1) a | |
| pH | RPS | 7.41 (0.05) c | 7.67 (0.02) c | 7.79 (0.01) c | 8.13 (0.02) d | 8.19 (0.03) d |
| ACID | 7.41 (0.05) c | 6.2 (0.01) b | 5.56 (0.22) a | 5.18 (0.08) a | 5.12 (0.09) a | |
| BAC | 7.41 (0.05) c | 7.67 (0.1)c | 7.74 (0.01) c | 7.95 (0.01) bc | 8.2 (0.01) d | |
| EC (dSm−1) | RPS | 22.9 (0.36) b | 24.7 (0.13) c | 25.6 (0.64) c | 25.4 (0.04) c | 23.6 (0.53) b |
| ACID | 22.9 (0.36) b | 25.4 (0.44) c | 27.3 (0.16) d | 27.7 (0.16) d | 27.7 (0.16) d | |
| BAC | 22.9 (0.36) b | 22.7 (0.16) b | 21.6 (1.35) a | 23 (0.79) b | 22.1 (0.1) b | |
| TSSs (gL−1) | RPS | 77.7 (12.8) d | 39.2 (1.72) c | 43.2 (2.26) c | 22.3 (1.61) b | 22.2 (0.65) b |
| ACID | 77.7 (12.8) d | 43.1 (1.4) c | 22.4 (0.47) b | 24.1 (0.23) b | 24.1 (0.23) b | |
| BAC | 77.7 (12.8) d | 38.4 (3.28) c | 70.2 (2.42) d | 17 (0.75) a | 14.6 (0.15) a | |
| COD (gL−1) | RPS | 55 (6.56) d | 22 (0.64) c | 21.2 (0.2) b | 15.7 (0.31) a | 18.5 (0.52) a |
| ACID | 55 (6.56) d | 20.6 (0.8) b | 20.2 (1.17) b | 13.6 (0.15) a | 16.4 (0.8) a | |
| BAC | 55 (6.56) d | 23.2 (0.31) c | 21.3 (0.7) b | 15.8 (0.2) a | 12.2 (0.61) a | |
| BOD5 (gO2L−1) | RPS | 6.44 (0.16) b | 9.21 (0.11) c | 9.07 (0.1) c | 4.48 (0.06) a | 3.38 (0.02) a |
| ACID | 6.44 (0.16) b | 9.44 (0.08) c | 6.15 (0.02) b | 4.58 (0.03) a | 4.58 (0.03) a | |
| BAC | 6.44 (0.16) b | 10.4 (0.12) d | 8.49 (0.07) c | 4.66 (0.05) a | 3.44 (0.11) a |
| 1st Week | 2nd Week | 3rd Week | 4th Week | 5th Week | ||
|---|---|---|---|---|---|---|
| Total N (g L−1) | RPS | 3.52 (0.54) c | 2.94 (0.11) b | 2.52 (0.73) b | 2.94 (0.11) b | 2.46 (0.11) b |
| ACID | 3.24 (0.58) c | 3.02 (0.65) c | 2.45 (0.18) b | 2.69 (0.22) b | 2.55 (0.2) b | |
| BAC | 3.24 (0.58) c | 2.48 (0.1) b | 2.2 (0.31) b | 2.13 (0.37) b | 1.88 (0.26) a | |
| Na+ (mg L−1) | RPS | 1864 (141) a | 1988 (24.83) b | 2228 (55.63) c | 1988 (24.83) d | 2315 (86.14) cd |
| ACID | 1864 (141) a | 2038 (40.68) b | 2267 (14.35) c | 2207 (367.36) c | 1864 (141.8) a | |
| BAC | 1864 (141) a | 1966 (17.33) a | 2100 (67.37) b | 2068 (27.49) b | 2068 (27.49) b | |
| K+ (mg L−1) | RPS | 1966 (167) a | 2155 (39.34) ab | 2432 (68.44) b | 2155 (39.34) d | 2598 (95.75) c |
| ACID | 1966 (167) a | 2152 (26.84) ab | 2483 (24.97) c | 2432 (411.14) b | 1966 (167.69) a | |
| BAC | 1966 (167) a | 2011 (16.95) a | 2234 (66.26) b | 2206 (27.03) b | 2206 (27.03) b | |
| Ca+ (mg L−1) | RPS | 445 (33.5) b | 354 (28.7) a | 339 (7.74) a | 481 (26.33) b | 457 (11.16) b |
| ACID | 445 (33.5) b | 660 (23.98) c | 944 (24.01) e | 882 (89.81) d | 882 (89.81) d | |
| BAC | 445 (33.5) b | 323 (269.01) a | 370 (16.74) a | 533 (21.1) c | 397 (24.05) a | |
| Mg2+ (mg L−1) | RPS | 442 (18.15) a | 375 (5.53) a | 414 (9.03) a | 487 (118.51) a | 437 (10.45) a |
| ACID | 442 (18.15) a | 401 (19.3) a | 465 (8.11) a | 453 (70.77) a | 449 (10.15) a | |
| BAC | 442 (18.15) a | 376 (9.61) a | 470 (133.09) a | 420 (19.85) a | 342 (8.17) a | |
| P (mg L−1) | RPS | 136 (33.48) d | 46.1 (40.24) b | 91.7 (4.47) c | 75.3 (14.56) b | 153 (12.2) d |
| ACID | 136 (33.48) d | 95.2 (18.58) c | 64.8 (1.35) b | 96.9 (5.3) c | 96.9 (5.3) c | |
| BAC | 136 (33.48) d | 21.3 (37) a | 191 (173.48) e | 66.3 (0.47) b | 69.4 (1.59) b |
| 1st Week | 2nd Week | 3rd Week | 4th Week | 5th Week | ||
|---|---|---|---|---|---|---|
| Cu (μg L−1) | RPS | 0.7 (0.06) ab | 1.22 (1.06) b | 0.74 (0.12) ab | 1.19 (0.15) b | 1.24 (0.1) b |
| ACID | 0.7 (0.06) ab | 0.59 (0.07) ab | 0.53 (0.02) ab | 0.62 (0.18) ab | 0.62 (0.18) ab | |
| BAC | 0.7 (0.06) ab | 0.29 (0.05) a | 0.69 (0.05) ab | 0.88 (0.06) ab | 1.02 (0.12) ab | |
| Zn (μg L−1) | RPS | 1.57 (0.09) ab | 1.4 (0.18) ab | 2.03 (0.07) c | 2.23 (0.15) d | 2.04 (0.08) c |
| ACID | 1.57 (0.09) ab | 1.65 (0.38) b | 1.46 (0.23) ab | 1.29 (0.23) ab | 1.29 (0.23) ab | |
| BAC | 1.57 (0.09) ab | 1.05 (0.09) a | 1.55 (0.15) ab | 1.65 (0.04) b | 1.59 (0.24) ab | |
| Fe (μg L−1) | RPS | 6.72 (0.63) b | 4.9 (0.37) a | 7.88 (0.09) c | 11.89 (1.49) d | 9.68 (0.22) cd |
| ACID | 6.72 (0.63) b | 6.49 (0.96) b | 7.31 (0.36) c | 6.45 (1.04) b | 6.45 (1.04) b | |
| BAC | 6.72 (0.63) b | 4.5 (0.61) a | 7.47 (1.68) c | 8.85 (0.46) cd | 8.27 (0.68) c | |
| Mn (μg L−1) | RPS | 4.98 (0.25) c | 3.32 (0.41) b | 3.85 (0.11) b | 4.04 (1.34) b | 4.48 (0.17) c |
| ACID | 4.98 (0.25) c | 5.88 (0.42) cd | 6.18 (0.25) cd | 6.93 (1.02) d | 6.93 (1.02) d | |
| BAC | 4.98 (0.25) c | 3.66 (0.68) b | 4.24 (1.2) b | 3.7 (0.11) b | 2.48 (0.08) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El bied, O.; Turbí, M.A.T.; García-Valero, A.; Cano, Á.F.; Acosta, J.A. Mitigating Ammonia, Methane, and Carbon Dioxide Emissions from Stored Pig Slurry Using Chemical and Biological Additives. Water 2023, 15, 4185. https://doi.org/10.3390/w15234185
El bied O, Turbí MAT, García-Valero A, Cano ÁF, Acosta JA. Mitigating Ammonia, Methane, and Carbon Dioxide Emissions from Stored Pig Slurry Using Chemical and Biological Additives. Water. 2023; 15(23):4185. https://doi.org/10.3390/w15234185
Chicago/Turabian StyleEl bied, Oumaima, Martire Angélica Terrero Turbí, Amalia García-Valero, Ángel Faz Cano, and José A. Acosta. 2023. "Mitigating Ammonia, Methane, and Carbon Dioxide Emissions from Stored Pig Slurry Using Chemical and Biological Additives" Water 15, no. 23: 4185. https://doi.org/10.3390/w15234185
APA StyleEl bied, O., Turbí, M. A. T., García-Valero, A., Cano, Á. F., & Acosta, J. A. (2023). Mitigating Ammonia, Methane, and Carbon Dioxide Emissions from Stored Pig Slurry Using Chemical and Biological Additives. Water, 15(23), 4185. https://doi.org/10.3390/w15234185






