Ecotoxicological Effects of Sodium Metasilicate on Two Hydra Species, Hydra viridissima Pallas, 1766 and Hydra oligactis Pallas, 1766
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Organisms
2.2. Toxicity Tests
2.3. Histomorphometric and Cytometric Analysis
2.4. Data Analysis
3. Results
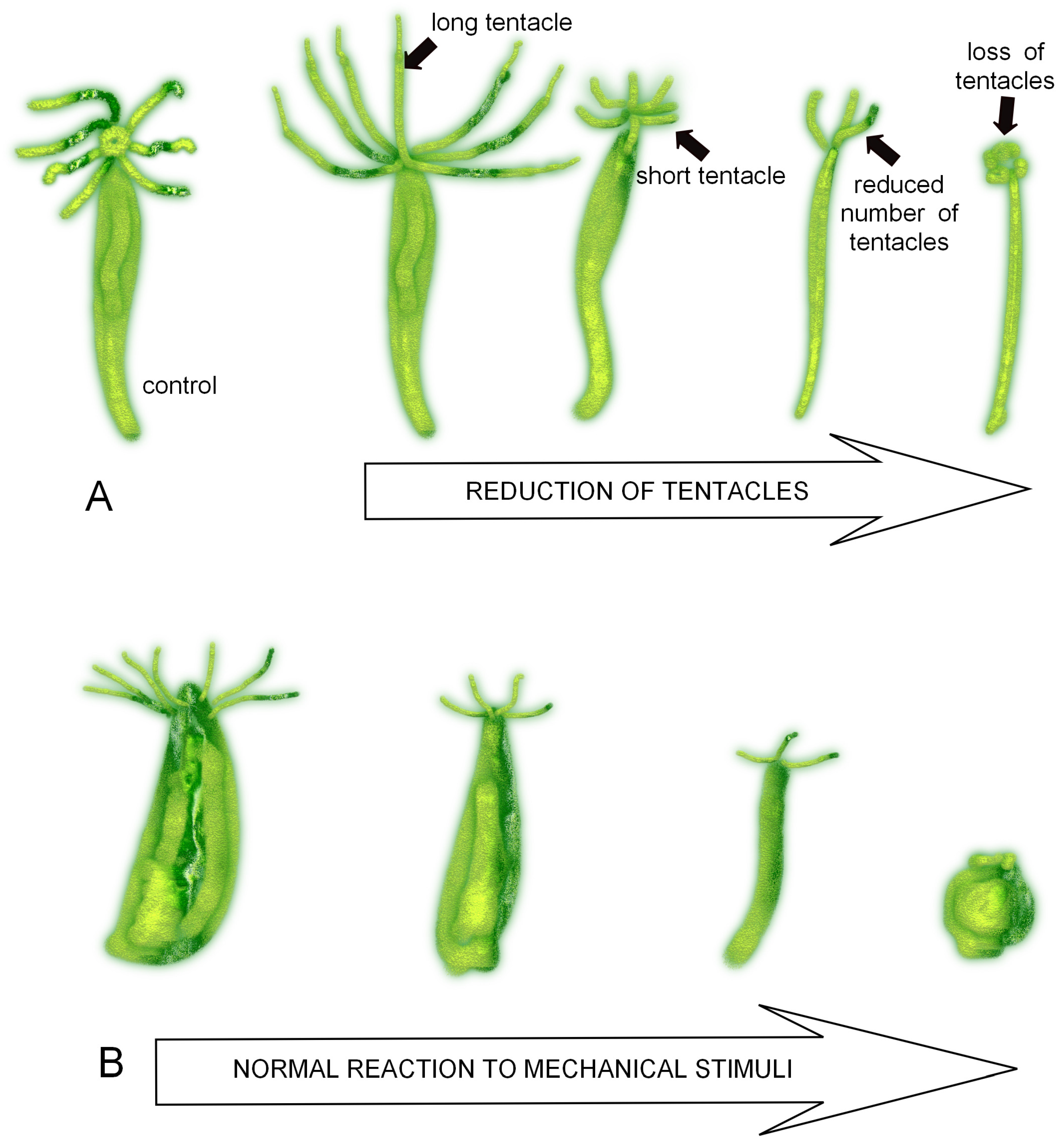
3.1. Morphological and Behavioural Changes in Green and Brown Hydra Caused by SM
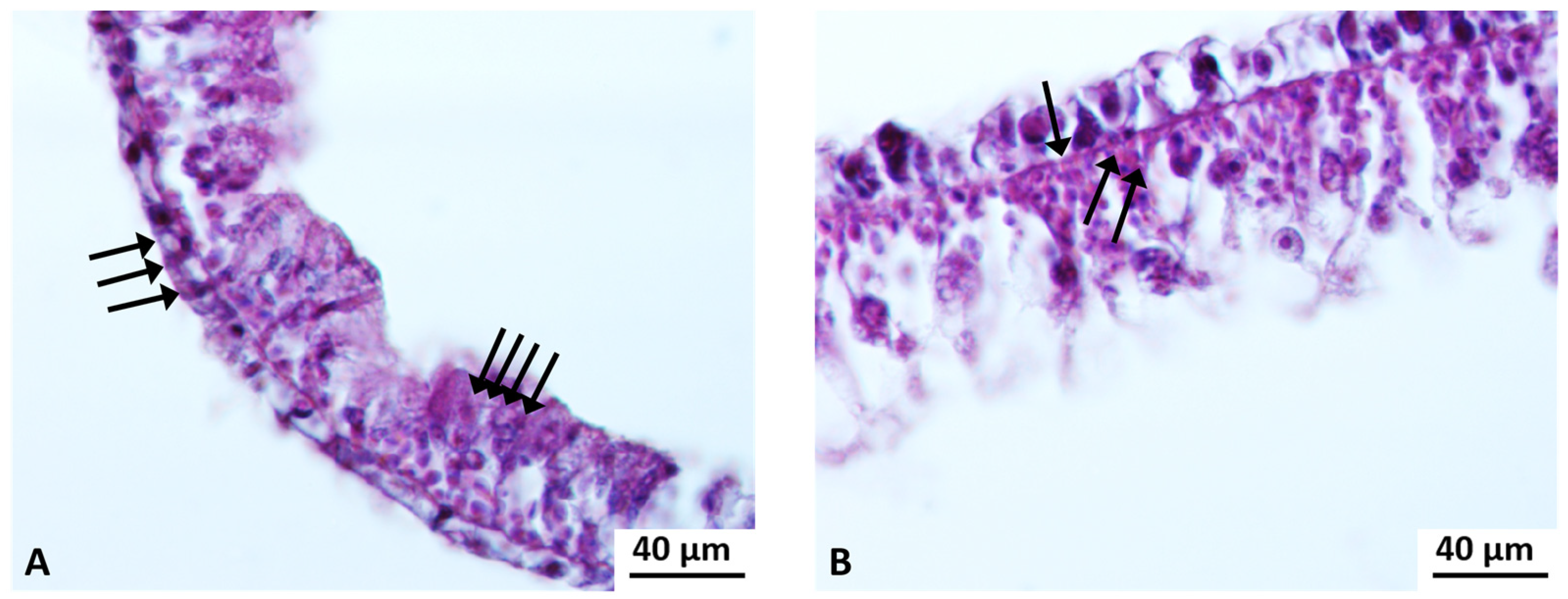
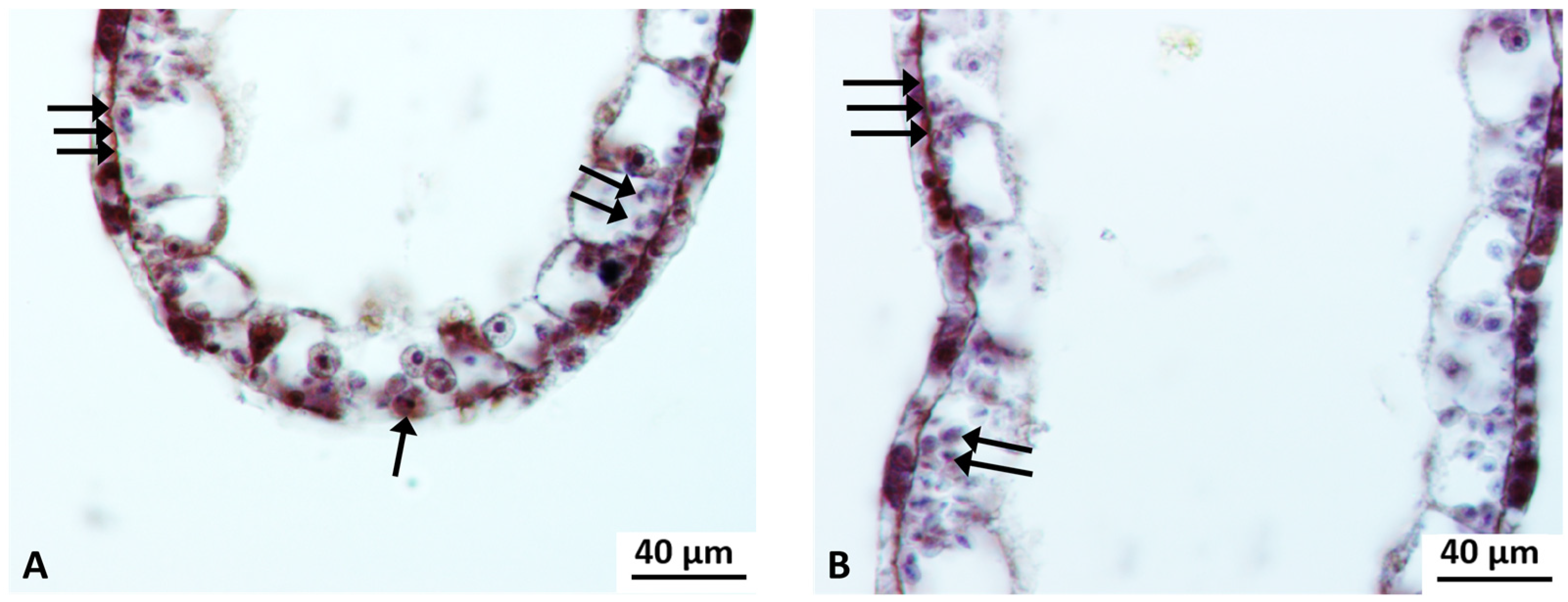
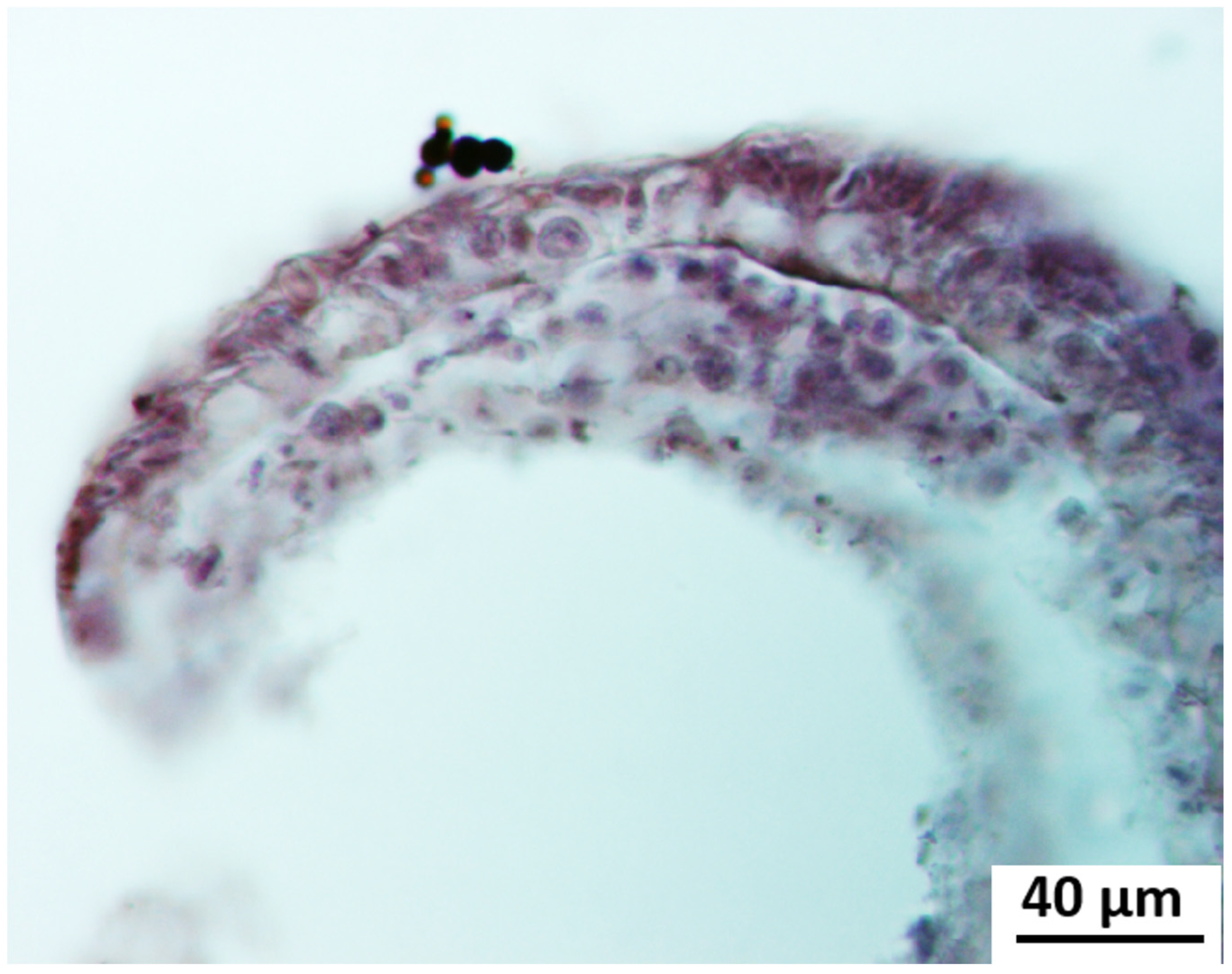
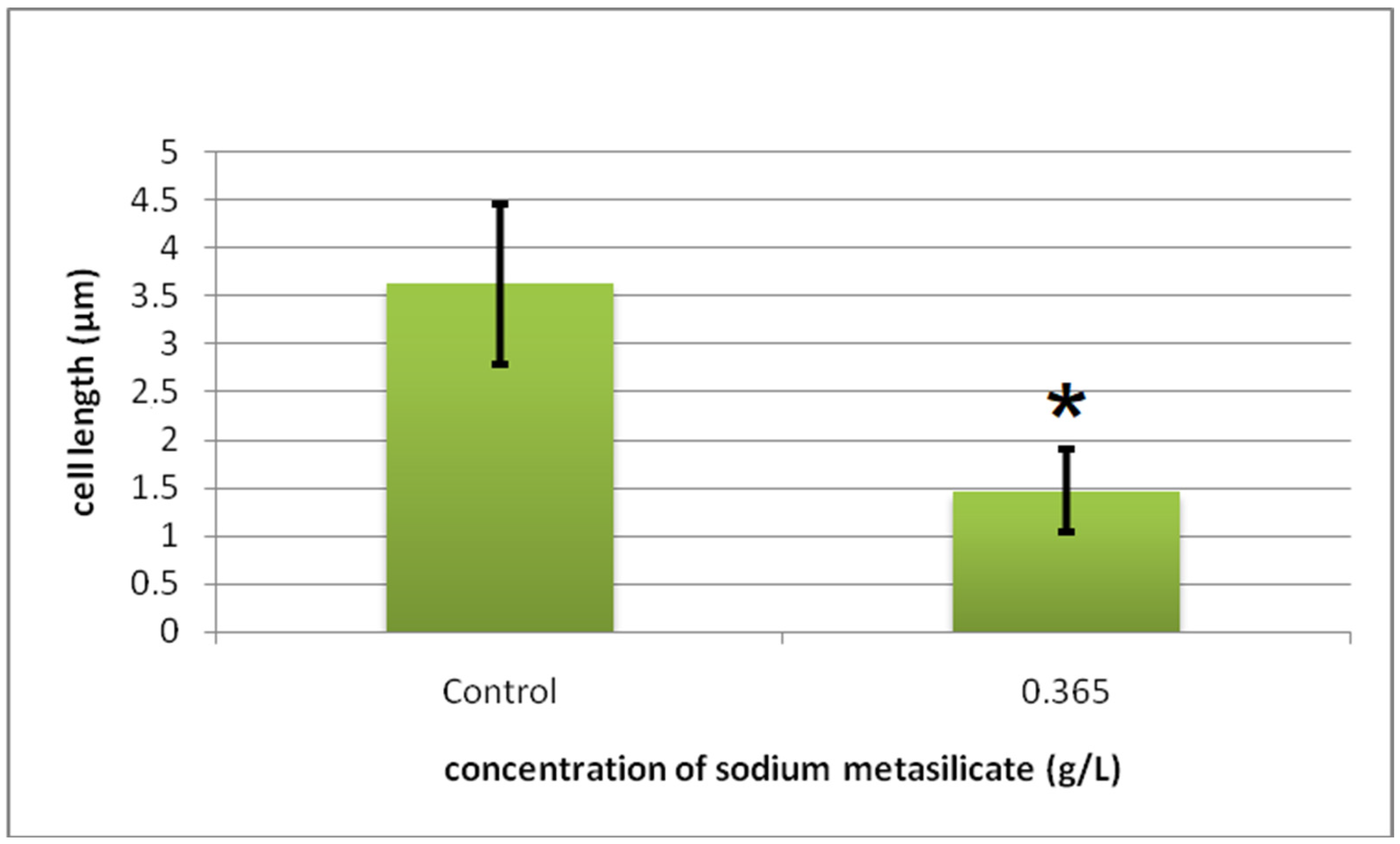
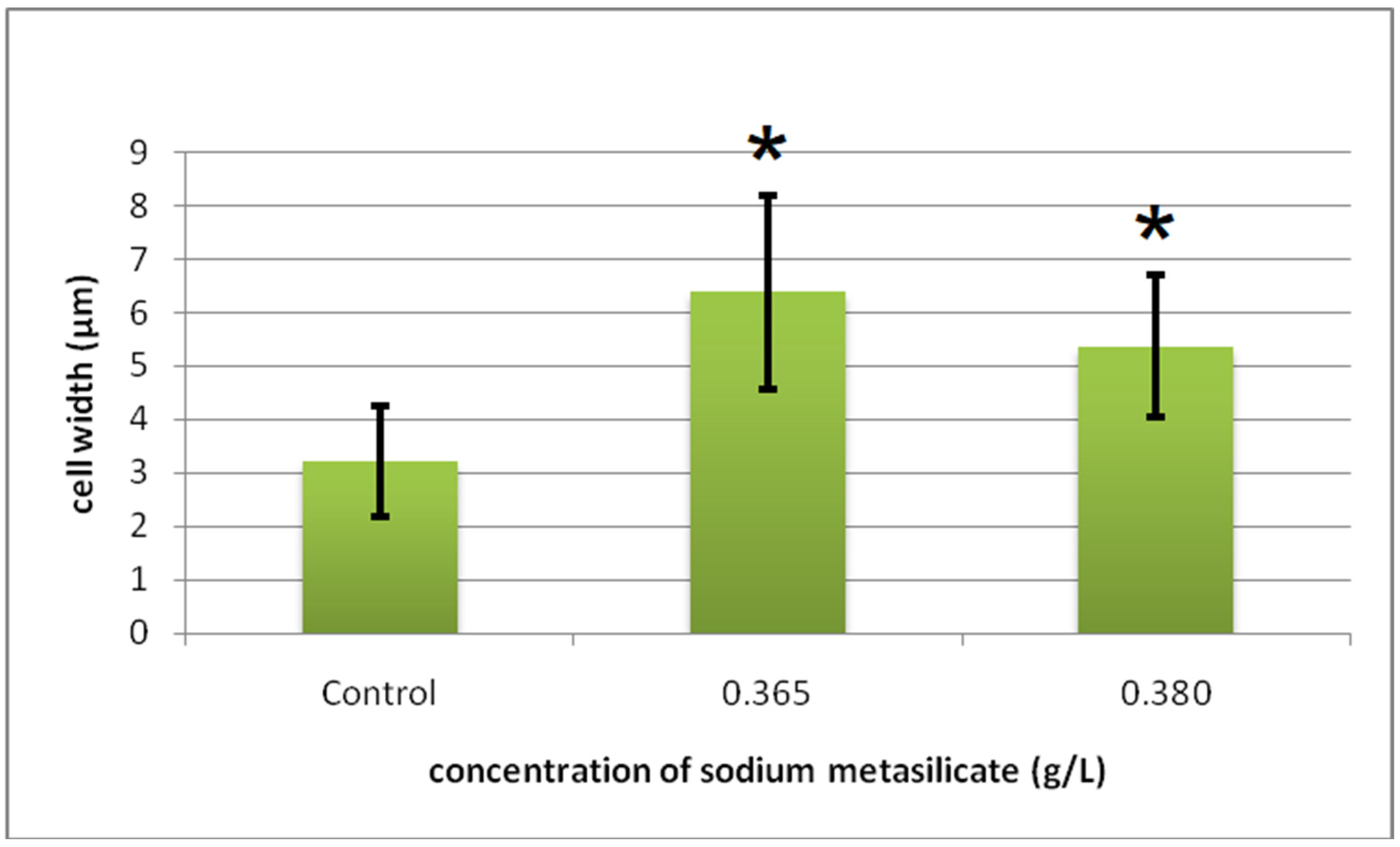
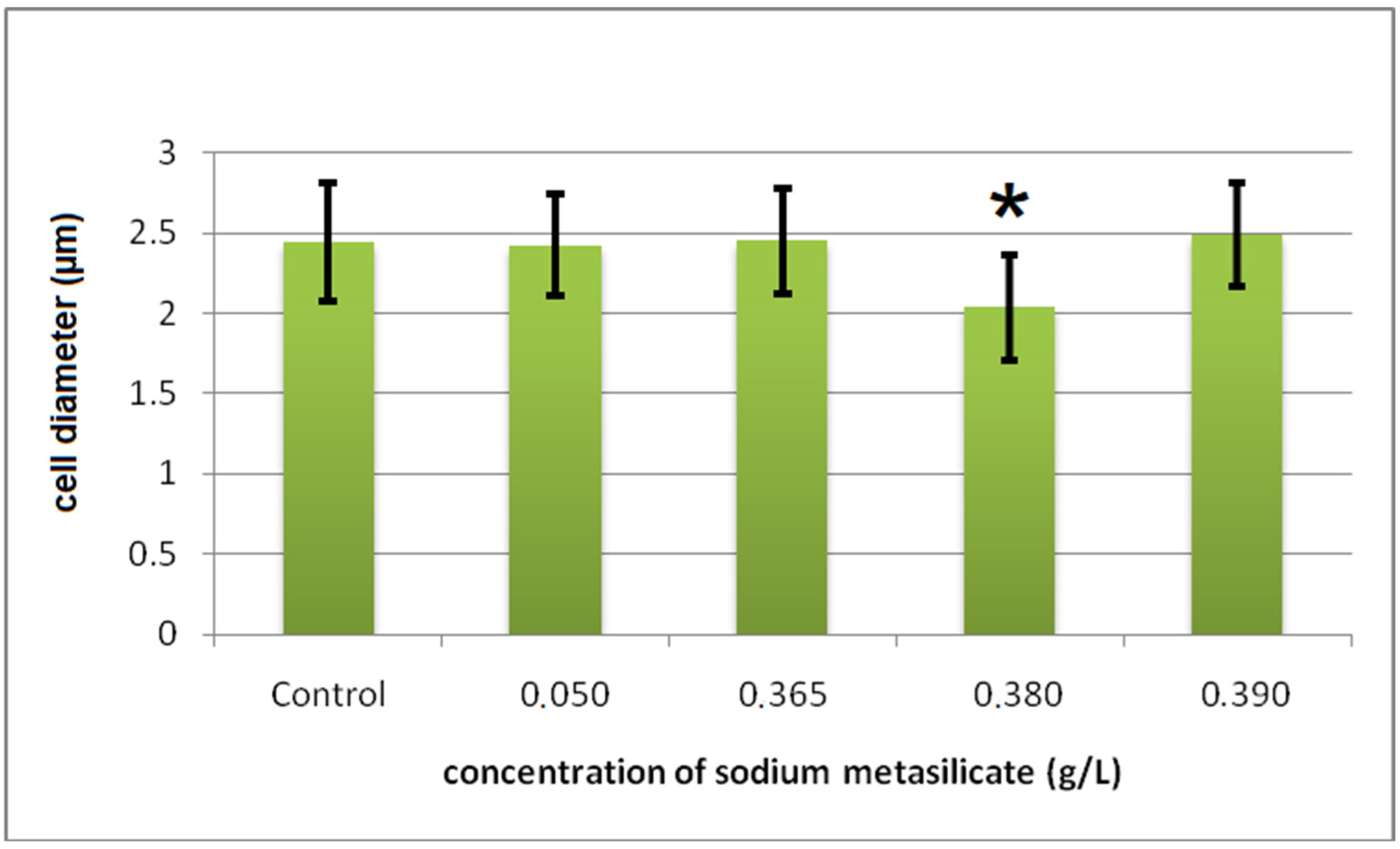
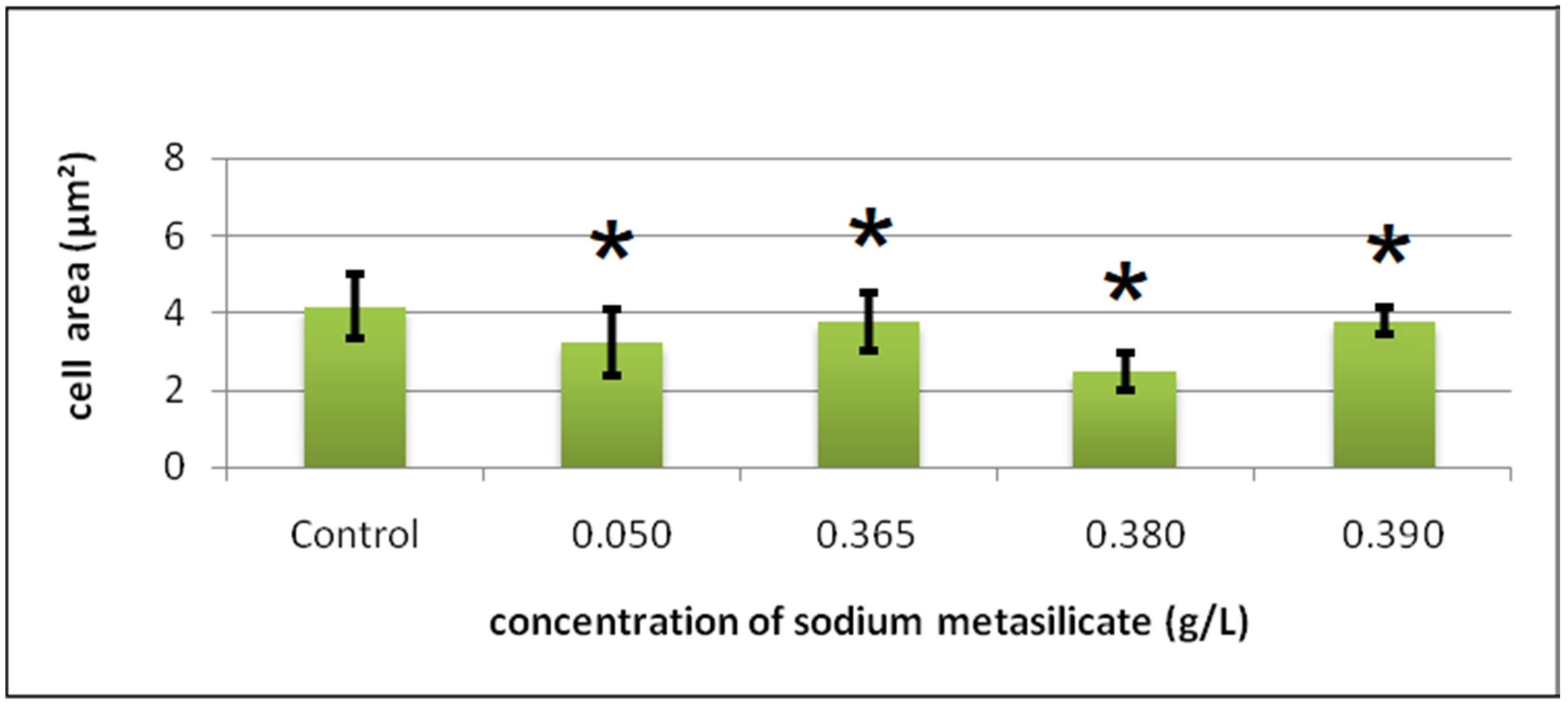
Cyto-Histological Changes in Green Hydra Caused by SM
3.2. Cytological Changes in Endosymbiotic Algae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Singh, B.K.; Nathanail, C.P.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef] [PubMed]
- Wharfe, J. Historical perspective and overview. In Environmental Toxicity Testing; Thompson, K.C., Wadhia, K., Loibner, A.P., Eds.; Blackwell Publishers Ltd.: Oxford, UK, 2005. [Google Scholar]
- Fawer, M.; Concannon, M.; Rieber, W. Life cycle inventories for production of sodium silicates. Int. J. Life Cycle Assess. 1999, 4, 207–212. [Google Scholar] [CrossRef]
- Elmore, A.R. Final Report on Safety Assessment of Potassium Silicate, Sodium Metasilicate, and Sodium Silicate. Int. J. Toxicol. 2005, 24, 103–117. [Google Scholar] [PubMed]
- Wills, J.H. A short history of the manufacture of soluble silicates in the United States. ACS Symp. Ser. 1982, 194, 3–15. [Google Scholar]
- HERA. Soluble Silicates (CAS No.: 1344-09-8, 6834-92-0, 10213-79-3, 13517-24-3, 1312-76-1): Human & Environmental Risk Assessment on Ingredients of European Household Cleaning Products; Draft for Public Comment; HERA Substance Team: Wendelsheim, Germany, 2005; pp. 1–64. [Google Scholar]
- Baehr, C.H.; Koehl, W. Soluble Silicates–Highly Versatile and Safe. SÖFW-J.–Int. J. Appl. Sci. 2007, 133, 88–94. [Google Scholar]
- EC 648/2004. Regulation (EC) No 648/2004 of the European Parliament and of the Council of 31 March 2004 on detergents. Off. J. L 2004, 104, 1–35. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32004R0648 (accessed on 17 May 2020).
- EC 907/2006. Commission Regulation (EC) No 907/2006 of 20 June 2006 amending Regulation (EC) No 648/2004 of the European Parliament and of the 10. Council on detergents, in order to adapt Annexes III and VII thereto. Off. J. L 2006, 168, 5–10. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006R0907 (accessed on 17 May 2020).
- EC 1272/2008. Regulation (EC) No1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006. Off. J. L 2008, 353, 1–1355. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed on 17 May 2020).
- EC 1336/2008. Regulation (EC) No 1336/2008 of the European Parliament and of the Council of 16 December 2008 Amending Regulation (EC) No 648/2004 in order to adapt it to Regulation (EC) No 1272/2008 on Classification, Labelling and Packaging of Substances and Mixtures. Off.J. L 2008, 354, 60–61. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2008.354.01.0060.01.ENG (accessed on 17 May 2020).
- Haneke, K.E. Sodium Metasilicate, Anhydrous [6834-92-0], sodium metasilicate pentahydrate [10213-79-3], and sodium metasilicate nonahydrate [13517-24-3]. In Review of toxicological literature; Prepared by Integrated Laboratory Systems, Inc., for National Institute of Environmental Health Sciences: Research Triangle Park, NC, USA, 2002. [Google Scholar]
- Richterich, K.; Mühlberg, B. Silicic Acid, Potassium Salt. Daphnia Magna, Acute Toxicity; Final Report, R-0100925; Henkel KGaA: Dusseldorf, Germany, 2001. [Google Scholar]
- Richterich, K.; Mühlberg, B. Silicic Acid, Potassium Salt. Fish, Acute Toxicity; Final Report, R-0100924; Henkel KGaA: Dusseldorf, Germany, 2001. [Google Scholar]
- Richterich, K.; Mühlberg, B. Silicic Acid (H2SiO3), Disodium Salt. Acute Bacterial Toxicity (Pseudomonas Oxygen Consumption Inhibition Test, DIN 38412-27); Final Report, R-0100923; Henkel KGaA: Dusseldorf, Germany, 2001. [Google Scholar]
- Richterich, K.; Mühlberg, B. Silicic Acid (H2SiO3), Disodium Salt. Fish, Acute Toxicity; Final Report, R-0100922; Henkel KGaA: Dusseldorf, Germany, 2001. [Google Scholar]
- Wallen, I.E.; Greer, W.C.; Lasater, R. Toxicity to “Gambusia Affinis” of Certain Pure Chemicals in Turbid Waters. Sew. Ind. Wastes 1957, 29, 695–711. [Google Scholar]
- Holt, E.A.; Miller, S.W. Bioindicators: Using organisms to measure environmental impacts. Nat. Educ. Knowl. 2011, 2, 1–10. [Google Scholar]
- Kalafatić, M. Regeneration and asexual reproduction of Hydra oligactis treated with different pesticides. Biologia 1997, 52, 475–480. [Google Scholar]
- Kalafatić, M.; Kopjar, N. Response of Green Hydra to the Treatment with Different Pesticides under Laboratory Conditions. Zeitsch. Angewan. Zool. 1994, 2, 213–223. [Google Scholar]
- Beach, M.J.; Pascoe, D. The role of Hydra vulgaris (Pallas) in assessing the toxicity of freshwater pollutants. Wat. Res. 1998, 32, 101–106. [Google Scholar] [CrossRef]
- Kovačević, G.; Kalafatić, M.; Ljubešić, N.; Šunjić, H. The effect of chloramphenicol on the symbiosis between alga and hydra. Biologia 2021, 56, 605–610. [Google Scholar]
- Arkhipchuk, W.; Blaise, C.; Malinovskaya, M.V. Use of hydra for chronic toxicity assessment of waters intended for human consumption. Environ. Pollut. 2005, 142, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kalafatić, M.; Žnidarić, D.; Lui, A.; Wrischer, M. Effects of insecticides (Dimiline WP 25, Torak EC 24 and Gamacide 20) on hydra (Hydra vulgaris Pallas). Int. J. Dev. Biol. 1991, 35, 335–340. [Google Scholar] [PubMed]
- Kalafatić, M.; Žnidarić, D.; Kopjar, N. The effect of insecticides upon green hydra. Period. Biol. 1994, 1, 129–130. [Google Scholar]
- Kalafatić, M.; Kopjar, N. Response of green hydra to pirimicarb. Biologia 1995, 50, 289–292. [Google Scholar]
- Kessler, E.; Kauer, G.; Rahat, M. Excretion of Sugars by Chlorella Species Capable and Incapable of Symbiosis with Hydra viridis. Bot. Acta. 1991, 104, 58–63. [Google Scholar] [CrossRef]
- Müller-Parker, G.; Pardy, R.L. Response of green hydra to feeding and starvation at four irradiances. Biol. Bull. 1987, 172, 46–60. [Google Scholar] [CrossRef]
- Žnidarić, D.; Kalafatić, M.; Kopjar, N. The survival of Hydra oligactis Pallas in unpleasant conditions. Zeitsch Angewan Zool. 1995, 2, 157–163. [Google Scholar]
- Burnett, A.L. Biology of Hydra; Academic Press: New York, NY, USA; London, UK, 1973. [Google Scholar]
- Habetha, M.; Anton-Erksleben, F.; Neumann, K.; Bosch, T.C.G. The Hydra viridis/Chlorella symbiosis. Growth and sexual differentiation in polyps without symbionts. Zoology 2003, 106, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fradkin, M.; Kakis, H.; Campbell, R. Effects of irradiation on hydra. Elimination of interstitial cells from viable hydra. Radiat. Res. 1978, 76, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Gagne, F.; Blaise, C. Hydra, a model system for environmental studies. Int. J. Dev. Biol. 2012, 56, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Karntanut, W.; Pascoe, D. The toxicity of copper, cadmium and zinc to four different Hydra (Cnidaria: Hydrozoa). Chemosphere 2002, 47, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.G. What Hydra Has to Say About the Role and Origin of Symbiotic Interactions. Biol. Bull. 2012, 223, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Wolfe, L.M. Sexual and asexual reproduction in a natural population of Hydra pseudoligactis. Can. J. Zool. 1984, 63, 851–856. [Google Scholar] [CrossRef]
- Campbell, R.D. Transmission of symbiotic algae through sexual reproduction in Hydra: Movement of algae into the oocyte. J. Tissue Cell 1990, 22, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S. Ecotoxicology and impact on biodiversity. J. Pharmacogn. Phytochem. 2013, 2, 1–19. [Google Scholar]
- Slobodkin, L.B. The connection between single species and ecosystems. In Water Quality & Stress Indicators in Marine and Freshwater Systems: Linking Levels of Organization; Sutcliffe, D.W., Ed.; Freshwater Biological Association, FBA Special Publications: Cumbria, UK, 1994; Volume 4, pp. 75–87. [Google Scholar]
- Kovačević, G.; Gregorović, G.; Matijević, A.; Kalafatić, M. Toxic effects of iron on green and brown hydra. Curr. Sci. 2015, 110, 502–504. [Google Scholar]
- Klimovich, A.V.; Bosch, T.C.G. Rethinking the Role of the Nervous System: Lessons from the Hydra Holobiont. Bioessays 2018, 40, e1800060. [Google Scholar] [CrossRef] [PubMed]
- McFall-Ngai, M.; Bosch, T.C.G. Animal development in the microbial world: The power of experimental model systems. Curr. Top. Dev. Biol. 2021, 141, 371–397. [Google Scholar] [PubMed]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Paus, R.; Bosch, T.C.G. Hydra and the Hair Follicle—An Unconventional Comparative Biology Approach to Exploring the Human Holobiont. BioEssays 2022, 44, e2100233. [Google Scholar] [CrossRef] [PubMed]
- Franzenburg, S.; Fraune, S.; Altrock, P.M.; Künzel, S.; Baines, J.F.; Traulsen, A.; Bosch, T.C.G. Bacterial colonization of Hydra hatchlings follows a robust temporal pattern. ISME J. 2013, 7, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Brucker, R.M.; Bordenstein, S.R. The hologenomic basis of speciation: Gut bacteria cause hybrid lethality in the genus Nasonia. Science 2013, 341, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Fraune, S.; Bosch, T.C.G. Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra. Proc. Natl. Acad. Sci. USA 2007, 104, 13146–13151. [Google Scholar] [CrossRef] [PubMed]
- Bathia, J.; Schröder, K.; Fraune, S.; Lachnit, T.; Rosenstiel, P.; Bosch, T.C.G. Symbiotic algae of Hydra viridissima play a key role in maintaining homeostatic bacteria colonization. Front. Microbiol. 2022, 13, 869666. [Google Scholar] [CrossRef]
- Bosch, T.C.G. Beyond Lynn Margulis’ green hydra. Symbiosis 2022, 87, 11–17. [Google Scholar] [CrossRef]
- Fraune, S.; Anton-Erxleben, F.; Augustin, R.; Franzenburg, S.; Knop, M.; Schröder, K.; Willoweit-Ohl, D.; Bosch, T.C.G. Bacteria–bacteria interactions within the microbiota of the ancestral metazoan Hydra contribute to fungal resistance. ISME J. 2015, 9, 1543–1556. [Google Scholar] [CrossRef]
- Murillo-Rincon, A.P.; Klimovich, A.; Pemöller, E.; Taubenheim, J.; Mortzfeld, B.; Augustin, R.; Bosch, T.C.G. Spontaneous body contractions are modulated by the microbiome of Hydra. Sci. Rep. 2017, 7, 15937. [Google Scholar] [CrossRef] [PubMed]
- Taubenheim, J.; Willoweit-Ohl, D.; Knop, M.; Franzenburg, S.; He, J.; Bosch, T.C.; Fraune, S. Bacteria- and temperature-regulated peptides modulate β-catenin signaling in Hydra. Proc. Natl. Acad. Sci. USA 2020, 117, 35, 21459–21468. [Google Scholar] [CrossRef] [PubMed]
- Glauber, K.M.; Dana, C.E.; Steele, R.E. Hydra. Curr. Biol. 2010, 22, R964–R965. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, G.; Želježić, D.; Horvatin, K.; Kalafatić, M. Morphological features and comet assay of green and brown hydra treated with aluminium. Symbiosis 2007, 44, 145–152. [Google Scholar]
- Ambrosone, A.; Mattera, L.; Marchesano, V.; Quarta, A.; Susha, A.S.; Tino, A.; Rogach, A.L.; Tortiglione, C. Mechanisms underlying toxicity induced by CdTe quantum dots determined in an invertebrate model organism. Biomaterials 2012, 33, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Murugadas, A.; Zeeshan, M.; Thamaraiselvi, K.; Ghaskadbi, S.; Akbarsha, M.A. Hydra as a model organism to decipher the toxic effects of copper oxide nanorod: Eco-toxicogenomics approach. Sci. Rep. 2016, 6, 29663. [Google Scholar] [CrossRef] [PubMed]
- Cera, A.; Cesarini, G.; Spani, F.; Scalici, M. Hydra vulgaris assay as environmental assessment tool for ecotoxicology in freshwaters: A review. Mar. Freshw. Res. 2020, 72, 745–753. [Google Scholar] [CrossRef]
- Boxall, A.B.A. The environmental side effects of medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic chemicals as agents of global change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Kopjar, N.; Kalafatić, M.; Žnidarić, D. Combined effect of Gamacide 20 and UV irradiation on green hydra (Hydra viridissima Pallas). Biologia 1994, 49, 371–375. [Google Scholar]
- Vimalkumar, K.; Sangeetha, S.; Felix, L.; Kay, P.; Pugazhendhi, A. A systematic review on toxicity assessment of persistent emerging pollutants (EPs) and associated microplastics (MPs) in the environment using the Hydra animal model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109320. [Google Scholar] [CrossRef]
- Kovačević, G.; Matulić, A. Effects of Querzetin on the Green Hydra (Hydra viridissima Pallas). Int. J. Biol. 2013, 5, 57–63. [Google Scholar] [CrossRef]
- Kovačević, G.; Kalafatić, M.; Ljubešić, N. Effects of Norflurazon on Green and Brown Hydra. Folia Biol. 2009, 57, 91–96. [Google Scholar] [CrossRef]
- Patwardhan, V.; Ghaskadbi, S. Invertebrate alternatives for toxicity testing: Hydra stakes its claim. Altex Proc. 2013, 2, 69–76. [Google Scholar]
- Bosch, T.C.; Miller, D.J. The Hydra Holobiont: A Tale of Several Symbiotic Lineages. In The Holobiont Imperative: Perspectives from Early Emerging Animals; Bosch, T.C., Miller, D.J., Eds.; Springer: Vienna, Austria, 2016; pp. 79–97. [Google Scholar]
- Tortiglione, C. An ancient model organism to test in vivo novel functional nanocrystals. In Biomedical Engineering—From Theory to Applications; Fazel, R., Ed.; InTechOpen: Rijeka, Croatia, 2011; pp. 225–252. [Google Scholar]
- Bode, H.R. The interstitial cell lineage of hydra: A stem cell system that arose early in the evolution. J. Cell Sci. 1996, 109, 1155–1164. [Google Scholar] [CrossRef]
- Buzgariu, W.; Haddad, H.A.; Tomczyk, S.; Wenger, Y.; Galliot, B. Multi-functionality and plasticity characterise epithelial cells in Hydra. Tissue Barriers 2015, 3, e1068908. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovačević, G.; Gračan, R.; Gottstein, S. Ecotoxicological Effects of Sodium Metasilicate on Two Hydra Species, Hydra viridissima Pallas, 1766 and Hydra oligactis Pallas, 1766. Water 2023, 15, 4228. https://doi.org/10.3390/w15244228
Kovačević G, Gračan R, Gottstein S. Ecotoxicological Effects of Sodium Metasilicate on Two Hydra Species, Hydra viridissima Pallas, 1766 and Hydra oligactis Pallas, 1766. Water. 2023; 15(24):4228. https://doi.org/10.3390/w15244228
Chicago/Turabian StyleKovačević, Goran, Romana Gračan, and Sanja Gottstein. 2023. "Ecotoxicological Effects of Sodium Metasilicate on Two Hydra Species, Hydra viridissima Pallas, 1766 and Hydra oligactis Pallas, 1766" Water 15, no. 24: 4228. https://doi.org/10.3390/w15244228
APA StyleKovačević, G., Gračan, R., & Gottstein, S. (2023). Ecotoxicological Effects of Sodium Metasilicate on Two Hydra Species, Hydra viridissima Pallas, 1766 and Hydra oligactis Pallas, 1766. Water, 15(24), 4228. https://doi.org/10.3390/w15244228





