Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Mud and Chemicals
2.2. Synthesis and Characterization of Mn-RM
2.3. Batch Adsorption Experiments
2.4. RSM Design
2.5. Environmental Risk Assessment
3. Results and Discussion
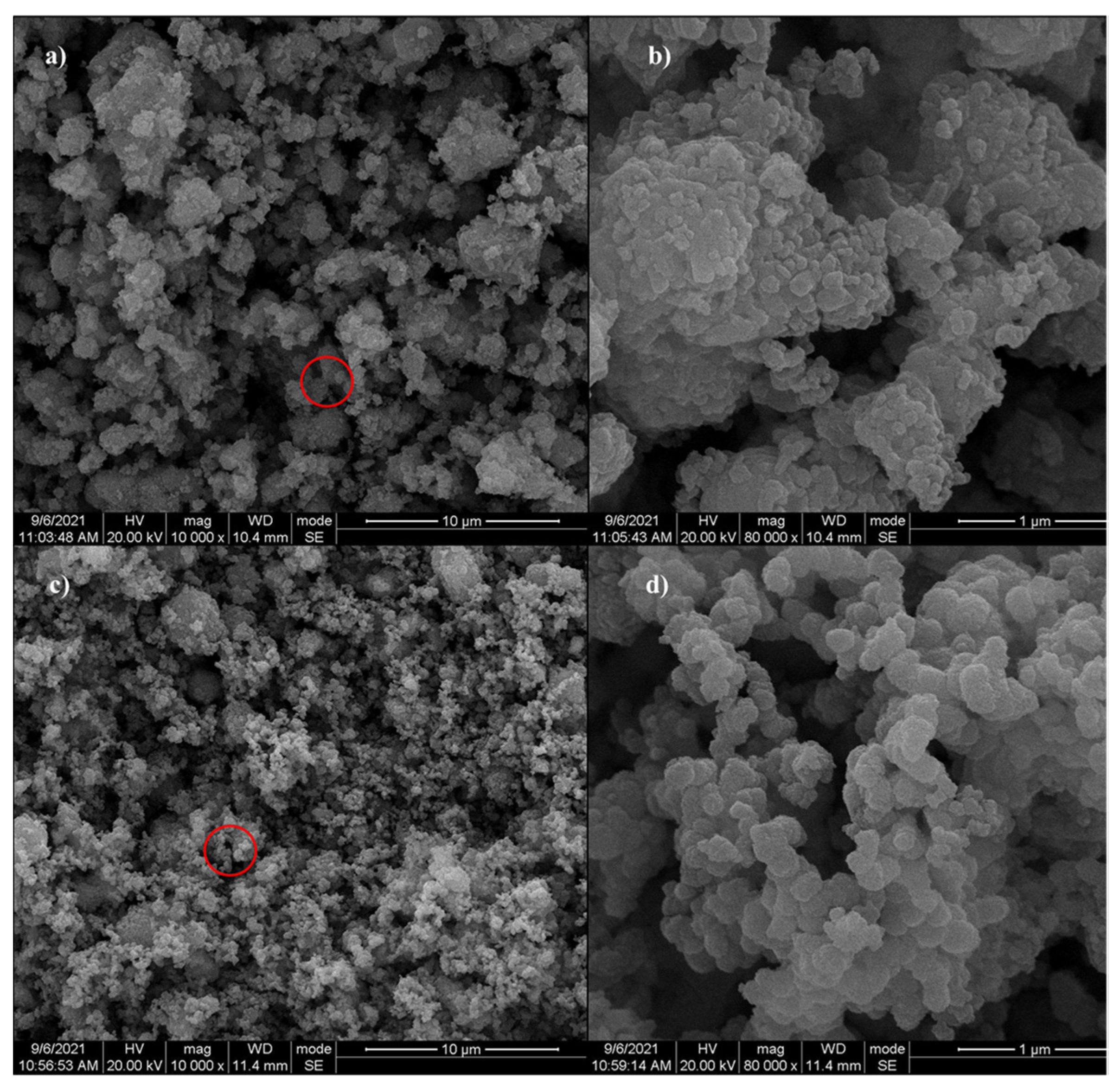
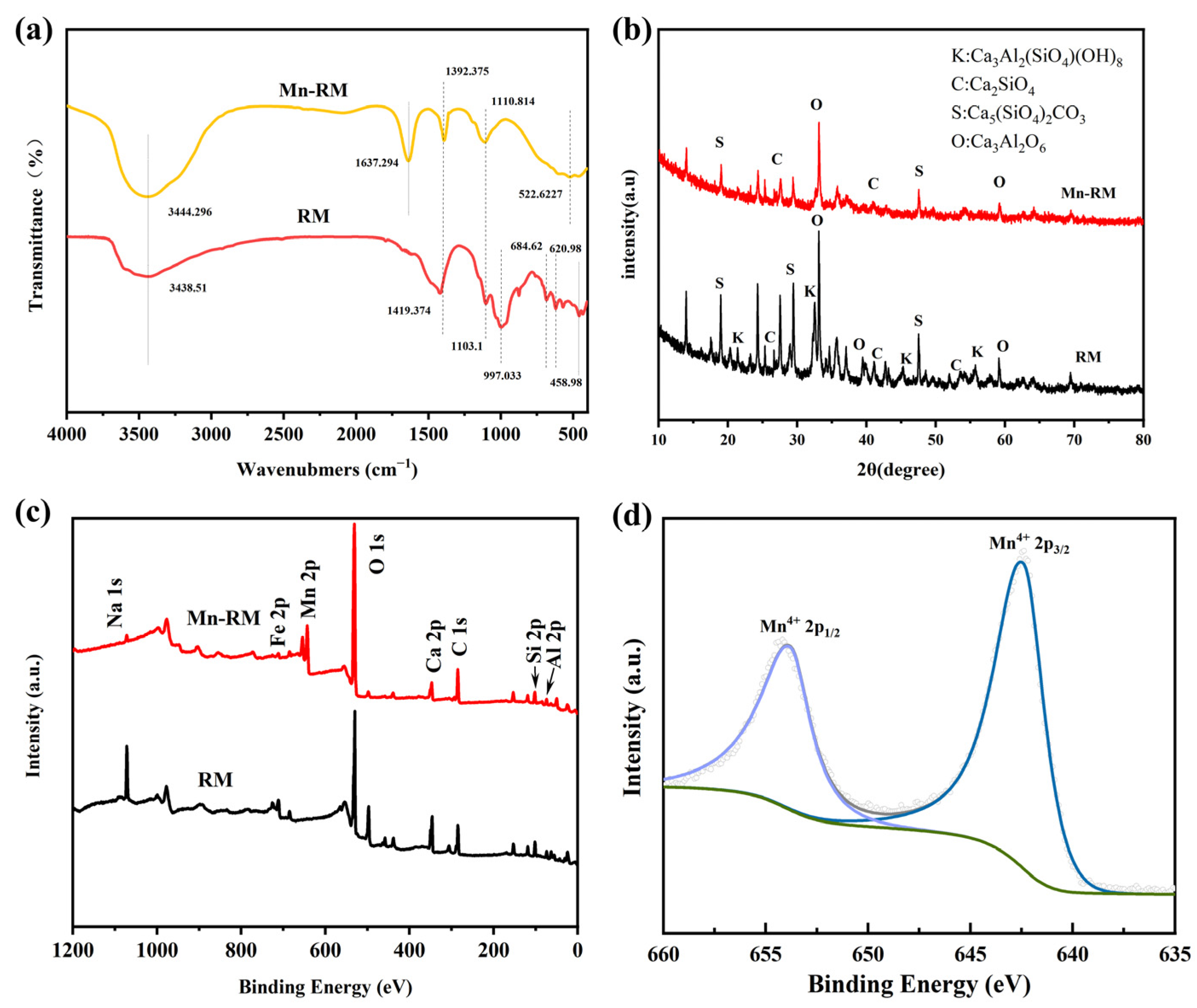
3.1. Characterization of As-Prepared Mn-RM
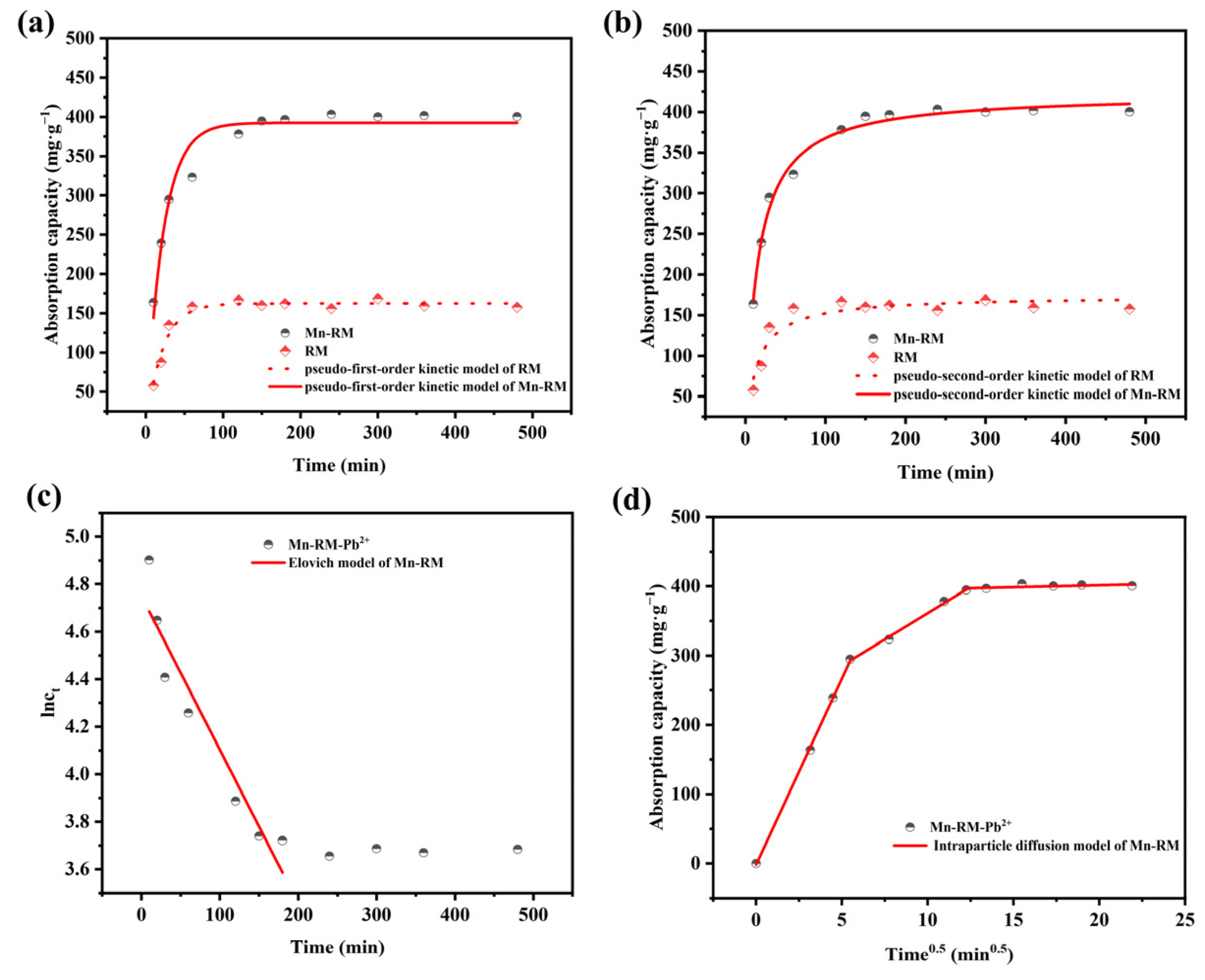
3.2. Adsorption Kinetics
3.3. Adsorption Isotherms and Thermodynamics
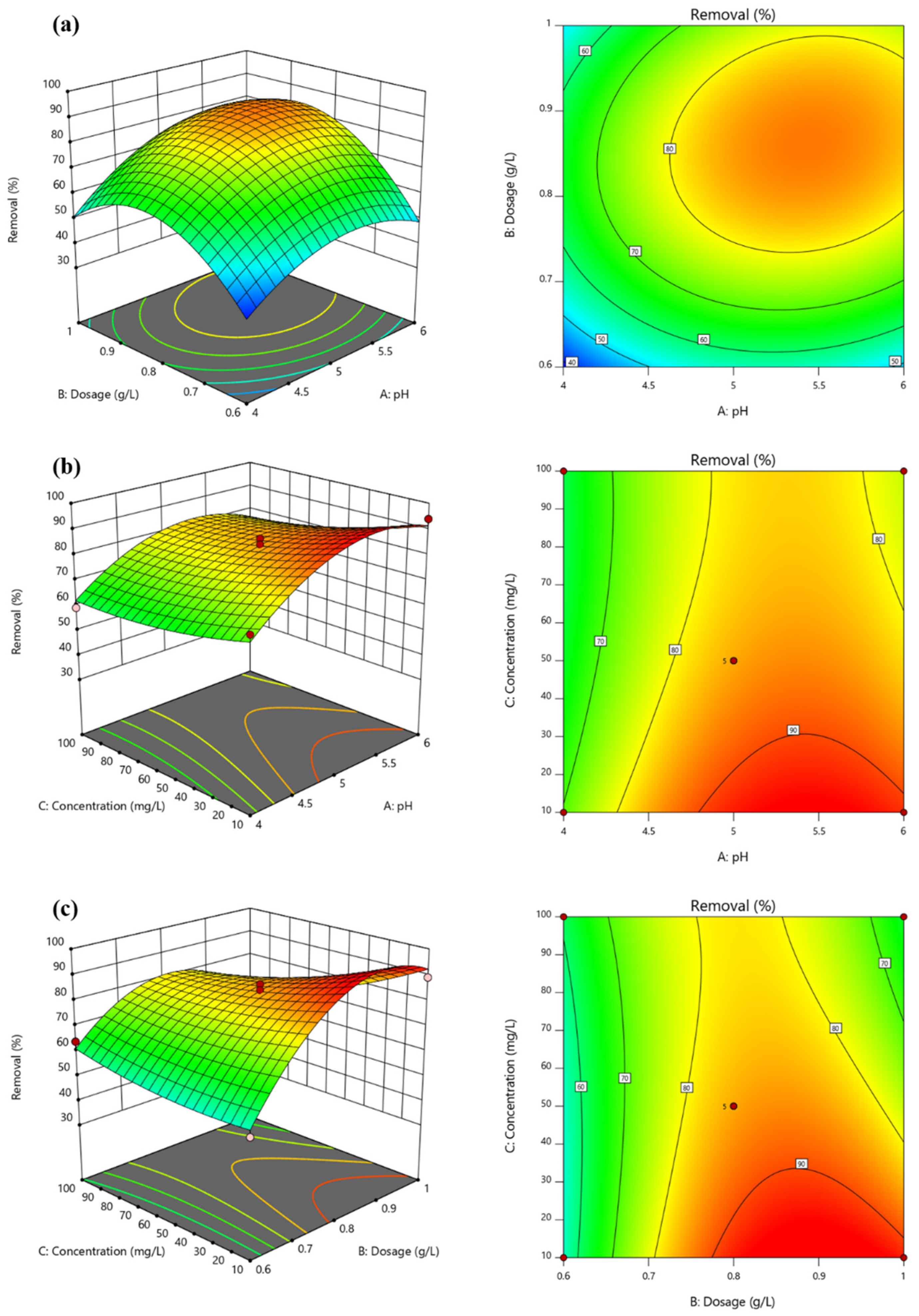
3.4. Optimization of Reaction Conditions for Pb2+ Adsorption using Mn-RM
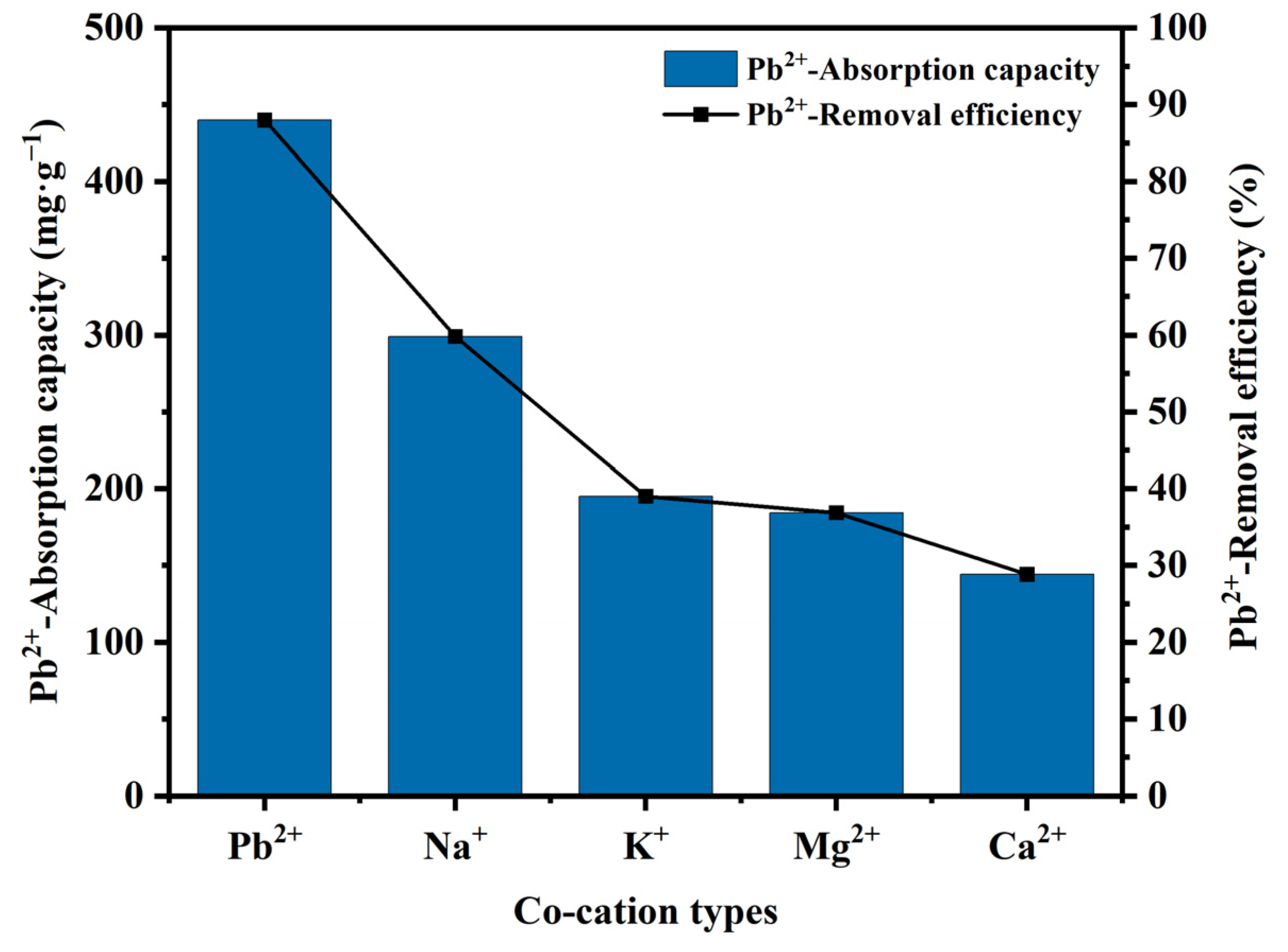
3.5. Effect of Co-Cation Types on Pb2+ Adsorption
3.6. Adsorption Mechanisms
3.7. Comparison of Mn-RM with Other Adsorbents for Pb2+ Removal
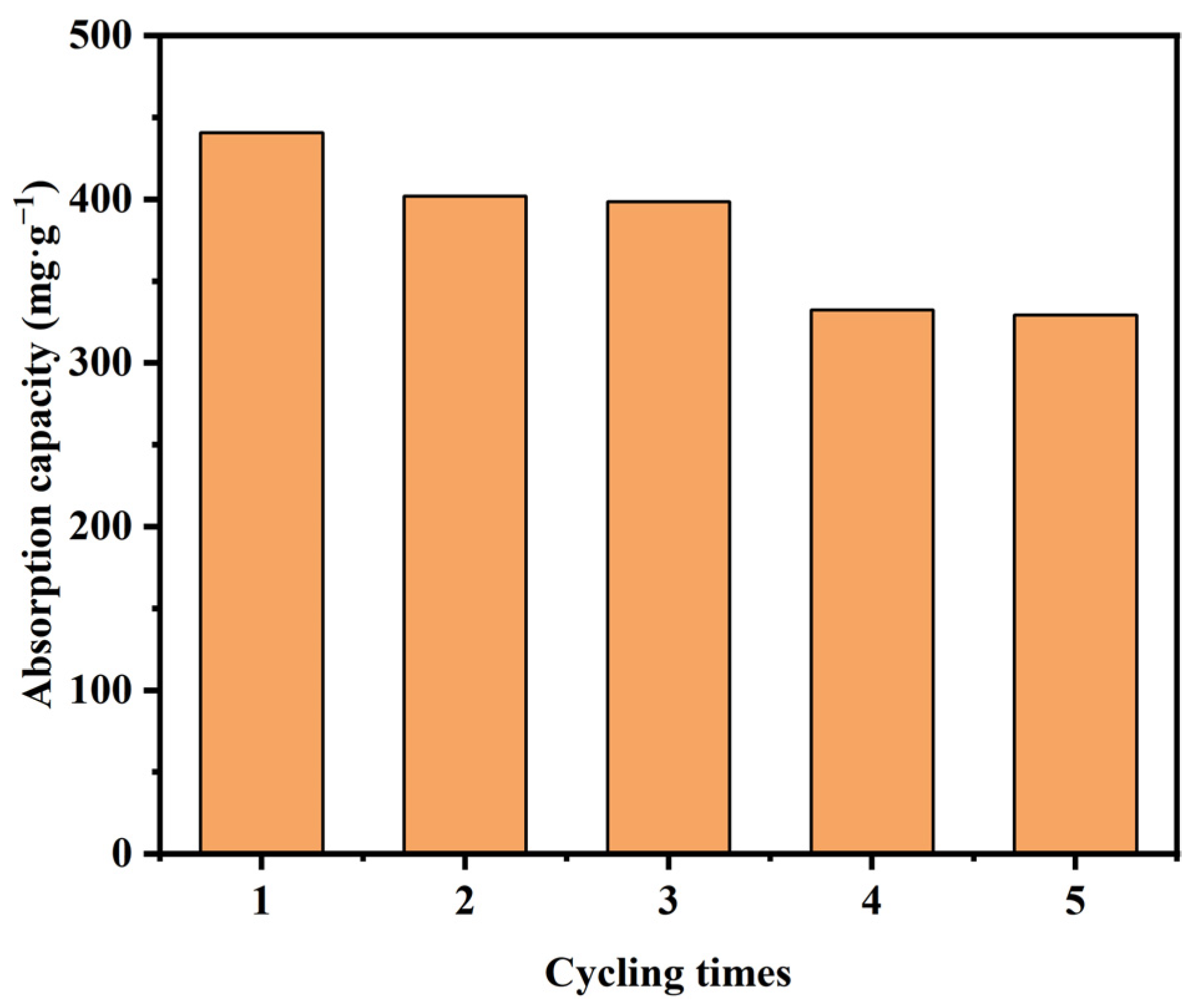
3.8. Reusability and Regeneration of Mn-RM
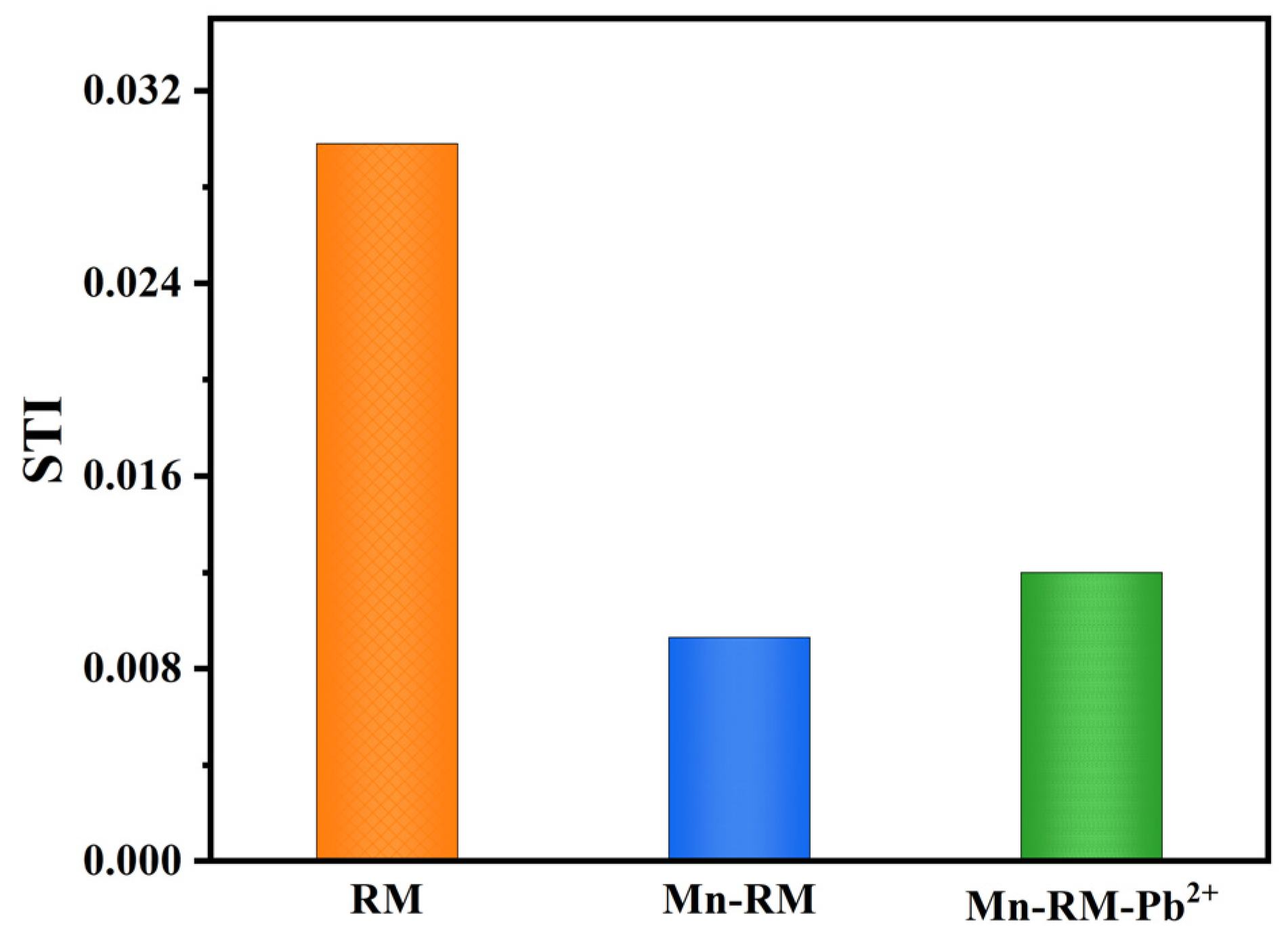
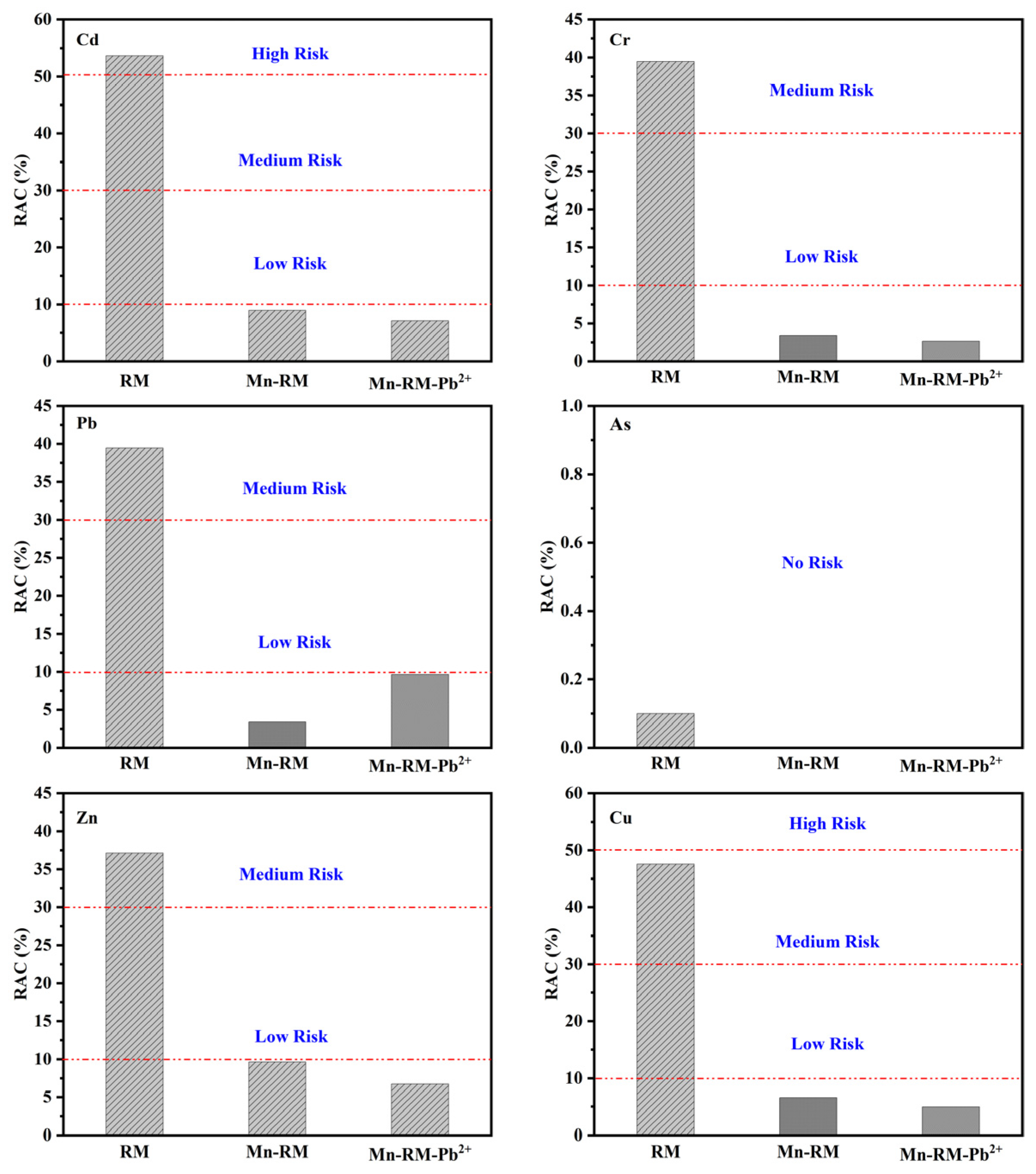
3.9. Environmental Risk Assessment
3.9.1. Leaching of Heavy Metals
3.9.2. Fractionation of Heavy Metals
3.9.3. Environmental Risk Assessment
4. Conclusions
- (1)
- The characterization results indicate that compared to raw red mud, the surface of Mn-RM is rougher and contains many spherical and flocculent particles. The specific surface area and the pore volume of Mn-RM increases by approximately eight times, and the loaded manganese dioxide exists in an amorphous phase structure. Mn-RM exhibits superior adsorption stability and regeneration ability, with a 25% decrease in Pb2+ adsorption capacity after five adsorption–desorption cycles.
- (2)
- Adsorption isotherms of Pb2+ on Mn-RM are better explained by the Langmuir isotherm model, and adsorption kinetics are better explained by a pseudo-second-order kinetic model. According to the thermodynamic analysis, it can be concluded that the adsorption of Pb2+ using Mn-RM is a process that absorbs heat and leads to an increase in entropy. The theoretically calculated maximum saturation adsorption capacity is 721.35 mg·g−1. Based on FTIR and XPS characterization, the adsorption of Pb2+ using Mn-RM mainly involves electrostatic attraction, ion exchange, and chemical adsorption.
- (3)
- The response surface analysis demonstrates that the removal of Pb2+ is mainly influenced by the interaction between the initial concentration and solid–liquid ratio, and between the solid–liquid ratio and pH value. The response surface model calculates the optimum treatment conditions as pH = 5.21, dosage = 0.83 g·L−1, and initial concentration = 301.04 mg·L−1, resulting in the highest Pb2+ removal efficiency of 87.45%.
- (4)
- The manganese modification of red mud effectively reduces the leaching of heavy metal components, and the leaching contents are within the specified range. Heavy metal speciation analysis reveals that manganese modification transforms heavy metals which were originally present in an unstable form in RM into a relatively stable state. The risk assessment code (RAC) values of each heavy metal in Mn-RM are less than 1%, and the synthesis toxicity index (STI) values decrease significantly. The RAC and STI values of Pb increase slightly after adsorption, but remain within the low-risk range.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A Mini-Review on Functional Nucleic Acids-Based Heavy Metal Ion Detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) Ions from Aqueous Solutions by Biochars Derived from Potassium-Rich Biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, T.; Gao, L.; Cui, L.; Hu, L.; Yan, L.; Du, B.; Wei, Q. Magnetic Hydroxypropyl Chitosan Functionalized Graphene Oxide as Adsorbent for the Removal of Lead Ions from Aqueous Solution. Desalin. Water Treat. 2016, 57, 3975–3984. [Google Scholar] [CrossRef]
- Senthil Kumar, P. Adsorption of Lead(II) Ions from Simulated Wastewater Using Natural Waste: A Kinetic, Thermodynamic and Equilibrium Study. Environ. Prog. Sustain. Energy 2014, 33, 55–64. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Abhinaya, R.V.; Thiruvengadaravi, K.V.; Baskaralingam, P.; Sivanesan, S. Lead(II) Adsorption onto Sulphuric Acid Treated Cashew Nut Shell. Sep. Sci. Technol. 2011, 46, 2436–2449. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced Removal of Heavy Metal Ions from Aqueous Solution Using Manganese Dioxide-Loaded Biochar: Behavior and Mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhou, P.; Wang, J.; Ma, Y.; Wu, J.; Su, C. Groundwater Pollution Model and Diffusion Law in Ordovician Limestone Aquifer Owe to Abandoned Red Mud Tailing Pit. Water. 2022, 14, 1472. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A Review of the Use of Red Mud as Adsorbent for the Removal of Toxic Pollutants from Water and Wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Tsamo, C.; Djomou Djonga, P.N.; Dangwang Dikdim, J.M.; Kamga, R. Kinetic and Equilibrium Studies of Cr(VI), Cu(II), and Pb(II) Removal from Aqueous Solution Using Red Mud, a Low-Cost Adsorbent. Arab. J. Sci. Eng. 2018, 43, 2353–2368. [Google Scholar] [CrossRef]
- Almeida, A.C.M.; do Nascimento, R.A.; Amador, I.C.B.; de Sousa Santos, T.C.; Martelli, M.C.; de Faria, L.J.G.; da Paixão Ribeiro, N.F. Chemically Activated Red Mud: Assessing Structural Modifications and Optimizing Adsorption Properties for Hexavalent Chromium. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127325. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd (II) Sorption by Red Mud with Heat Treatment: Performance and Mechanisms of Sorption. J. Environ. Manag. 2020, 255, 109866. [Google Scholar] [CrossRef] [PubMed]
- Grudić, V.V.; Perić, Đ.; Blagojević, N.Z.; Vukašinović-Pešić, V.L.; Brašanac, S.; Mugoša, B. Pb (II) and Cu (II) Sorption from Aqueous Solutions Using Activated Red Mud--Evaluation of Kinetic, Equilibrium, and Thermodynamic Models. Polish J. Environ. Stud. 2013, 22, 377–385. [Google Scholar]
- He, C.; Xie, F. Adsorption Behavior of Manganese Dioxide towards Heavy Metal Ions: Surface Zeta Potential Effect. Water Air Soil Pollut. 2018, 229, 77. [Google Scholar] [CrossRef]
- Su, Q.; Pan, B.; Wan, S.; Zhang, W.; Lv, L. Use of Hydrous Manganese Dioxide as a Potential Sorbent for Selective Removal of Lead, Cadmium, and Zinc Ions from Water. J. Colloid Interface Sci. 2010, 349, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gu, L.; Zhang, L.; Zheng, S.; Wan, H.; Sun, J.; Zhu, D.; Xu, Z. Removal of Aqueous Pb (II) by Adsorption on Al2O3-Pillared Layered MnO2. Appl. Surf. Sci. 2017, 406, 330–338. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Z. A Facile One-Step Synthesized Epsilon-MnO2 Nanoflowers for Effective Removal of Lead Ions from Wastewater. Chemosphere 2020, 250, 126329. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Liu, J.; Hu, P.; Meng, K.; Lv, F.; Tong, W.; Chu, P.K. Dealkalization of Red Mud by Carbide Slag and Flue Gas. CLEAN-Soil Air Water 2018, 46, 1700634. [Google Scholar] [CrossRef]
- Lyu, F.; Hu, Y.; Wang, L.; Sun, W. Dealkalization Processes of Bauxite Residue: A Comprehensive Review. J. Hazard. Mater. 2021, 403, 123671. [Google Scholar] [CrossRef]
- Fayazi, M.; Afzali, D.; Ghanei-Motlagh, R.; Iraji, A. Synthesis of Novel Sepiolite–Iron Oxide–Manganese Dioxide Nanocomposite and Application for Lead(II) Removal from Aqueous Solutions. Environ. Sci. Pollut. Res. 2019, 26, 18893–18903. [Google Scholar] [CrossRef]
- Afzali, D.; Fayazi, M. Deposition of MnO2 Nanoparticles on the Magnetic Halloysite Nanotubes by Hydrothermal Method for Lead(II) Removal from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2016, 63, 421–429. [Google Scholar] [CrossRef]
- HJ/T299-2007; Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method. State Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.; Wang, L.; Liu, R.; Sun, W.; He, D. Efficient Removal of Pb (II) Ions from Aqueous Solution by Modified Red Mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Nejadshafiee, V.; Islami, M.R. Intelligent-Activated Carbon Prepared from Pistachio Shells Precursor for Effective Adsorption of Heavy Metals from Industrial Waste of Copper Mine. Environ. Sci. Pollut. Res. 2020, 27, 1625–1639. [Google Scholar] [CrossRef] [PubMed]
- Pagnanelli, F.; Sambenedetto, C.; Furlani, G.; Vegliò, F.; Toro, L. Preparation and Characterisation of Chemical Manganese Dioxide: Effect of the Operating Conditions. J. Power Sources 2007, 166, 567–577. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Q.; Tie, B.; Lei, M.; Yang, J.; Luo, S.; Song, Z. Manganese Dioxide Nanosheet Suspension: A Novel Absorbent for Cadmium (II) Contamination in Waterbody. J. Colloid Interface Sci. 2015, 456, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Narayanan, S.; Venkatesan, G.; Vetha Potheher, I. Equilibrium Studies on Removal of Lead (II) Ions from Aqueous Solution by Adsorption Using Modified Red Mud. Int. J. Environ. Sci. Technol. 2018, 15, 1687–1698. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium Uptake, Isotherm and Kinetic Studies of Cd(II) Adsorption onto Iron Oxide Activated Red Mud from Aqueous Solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Antonović, D. The Influence of Citrate Anion on Ni(II) Removal by Raw Red Mud from Aluminum Industry. Chem. Eng. J. 2013, 214, 327–335. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.; Zeng, G.; Huang, B.; Dong, H.; Huang, J.; Yang, Z.; Wei, J.; Hu, L.; Zhang, Q. Phase Transformation of Crystalline Iron Oxides and Their Adsorption Abilities for Pb and Cd. Chem. Eng. J. 2016, 284, 247–259. [Google Scholar] [CrossRef]
- Liu, D.-H.; Guo, Y.; Zhang, L.-H.; Li, W.-C.; Sun, T.; Lu, A.-H. Switchable Transport Strategy to Deposit Active Fe/Fe3C Cores into Hollow Microporous Carbons for Efficient Chromium Removal. Small 2013, 9, 3852–3857. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F.C. Kinetics of Adsorption and Desorption and the Elovich Equation. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 1970; Volume 21, pp. 1–49. [Google Scholar]
- Wang, Z.; Su, J.; Hu, X.; Ali, A.; Wu, Z. Isolation of Biosynthetic Crystals by Microbially Induced Calcium Carbonate Precipitation and Their Utilization for Fluoride Removal from Groundwater. J. Hazard. Mater. 2021, 406, 124748. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe--N Modification on the Properties of Biochars and Their Adsorption Behavior on Tetracycline Removal from Aqueous Solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Equilibrium, Kinetics and Thermodynamics Study on the Adsorption of Cr(VI) and As(III) by Diatomite-Modified MnO2. J. Dispers. Sci. Technol. 2020, 43, 859–872. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Ren, Z.; Chen, F.; Wang, B.; Song, Z.; Zhou, Z.; Ren, D. Magnetic Biochar from Alkali-Activated Rice Straw for Removal of Rhodamine B from Aqueous Solution. Environ. Eng. Res. 2020, 25, 536–544. [Google Scholar] [CrossRef]
- Yang, T.; Xu, Y.; Huang, Q.; Sun, Y.; Liang, X.; Wang, L.; Qin, X.; Zhao, L. Adsorption Characteristics and the Removal Mechanism of Two Novel Fe-Zn Composite Modified Biochar for Cd (II) in Water. Bioresour. Technol. 2021, 333, 125078. [Google Scholar] [CrossRef]
- Nethaji, S.; Sivasamy, A. Removal of Hexavalent Chromium from Aqueous Solution Using Activated Carbon Prepared from Walnut Shell Biomass through Alkali Impregnation Processes. Clean Technol. Environ. Policy 2014, 16, 361–368. [Google Scholar] [CrossRef]
- Wu, S.; Xie, F.; Chen, S.; Fu, B. The Removal of Pb (II) and Cd (II) with Hydrous Manganese Dioxide: Mechanism on Zeta Potential and Adsorption Behavior. Environ. Technol. 2020, 41, 3219–3232. [Google Scholar] [CrossRef]
- Xiyili, H.; Çetintaş, S.; Bingöl, D. Removal of Some Heavy Metals onto Mechanically Activated Fly Ash: Modeling Approach for Optimization, Isotherms, Kinetics and Thermodynamics. Process Saf. Environ. Prot. 2017, 109, 288–300. [Google Scholar] [CrossRef]
- Huang, X.; Hao, H.; Zhang, G.; Li, J.; Ji, P. Adsorption of Cd2+ from Wastewater by Modified Fly Ash. Chin. J. Appl. Ecol. 2019, 30, 3215–3223. [Google Scholar]
- Sari, A.; Tuzen, M. Cd (II) Adsorption from Aqueous Solution by Raw and Modified Kaolinite. Appl. Clay Sci. 2014, 88, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, X.; Villalobos, M.; Tan, W.; Liu, F. Sorption Behavior of Heavy Metals on Birnessite: Relationship with Its Mn Average Oxidation State and Implications for Types of Sorption Sites. Chem. Geol. 2012, 292, 25–34. [Google Scholar] [CrossRef]
- Meng, K.; Wu, X.; Zhang, X.; Su, S.; Huang, Z.; Min, X.; Liu, Y.; Fang, M. Efficient Adsorption of the Cd (II) and As (V) Using Novel Adsorbent Ferrihydrite/Manganese Dioxide Composites. ACS Omega 2019, 4, 18627–18636. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, Q.; Gu, K.; Sun, Y.; Wang, Y.; Zhang, P.; Zheng, Z.; Guo, Y.; Xin, M.; Bian, R. Stability Evaluation of Potentially Toxic Elements in MSWI Fly Ash during Carbonation in View of Two Leaching Scenarios. Sci. Total Environ. 2022, 803, 150135. [Google Scholar] [CrossRef] [PubMed]
- GB5085.3-2007; Hazardous Waste Identification Criteria: Leaching Toxicity Identification Standards. State Environmental Protection Administration: Beijing, China, 2007. (In Chinese)
- Qi, X.; Wang, H.; Zhang, L.; Xu, B.; Shi, Q.; Li, F. Removal of Cr (III) from Aqueous Solution by Using Bauxite Residue (Red Mud): Identification of Active Components and Column Tests. Chemosphere 2020, 245, 125560. [Google Scholar] [CrossRef] [PubMed]
- Jalil, A.A.; Triwahyono, S.; Yaakob, M.R.; Azmi, Z.Z.A.; Sapawe, N.; Kamarudin, N.H.N.; Setiabudi, H.D.; Jaafar, N.F.; Sidik, S.M.; Adam, S.H.; et al. Utilization of Bivalve Shell-Treated Zea Mays L. (Maize) Husk Leaf as a Low-Cost Biosorbent for Enhanced Adsorption of Malachite Green. Bioresour. Technol. 2012, 120, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhu, F.; Zhao, L.; Jiang, H.; Zhang, Z. Heavy Metal Speciation in Various Types of Fly Ash from Municipal Solid Waste Incinerator. J. Mater. Cycles Waste Manag. 2014, 16, 608–615. [Google Scholar] [CrossRef]
- Izquierdo, M.; Querol, X. Leaching Behaviour of Elements from Coal Combustion Fly Ash: An Overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Z.; Zhou, J.; Zhao, J.; Ruan, X.; Liu, J.; Qian, G. Chemical Characteristics and Risk Assessment of Typical Municipal Solid Waste Incineration (MSWI) Fly Ash in China. J. Hazard. Mater. 2013, 261, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.; Huang, Y.; Shimaoka, T.; Wang, H.; Wang, Y.; Ma, L.; Zhang, D. Evaluation of Chemical Speciation and Environmental Risk Levels of Heavy Metals during Varied Acid Corrosion Conditions for Raw and Solidified/Stabilized MSWI Fly Ash. Waste Manag. 2019, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Chai, M.; Li, R. Heavy Metal Migration and Potential Environmental Risk Assessment during the Washing Process of MSW Incineration Fly Ash and Molten Slag. Procedia Environ. Sci. 2016, 31, 351–360. [Google Scholar] [CrossRef]


| Factors | Codes | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Initial pH | A | 4 | 5 | 6 |
| Solid–liquid ratio (g·L−1) | B | 0.6 | 0.8 | 1.0 |
| Initial Pb2+concentration (mg·L−1) | C | 300 | 450 | 600 |
| Kinetic Model | Parameters | Mn-RM | RM |
|---|---|---|---|
| Pseudo-first-order | k1 (g·mg−1·min−1) | 0.0456 | 0.0470 |
| qe (mg·g−1) | 392.6024 | 162.4999 | |
| Adj. R2 | 0.9520 | 0.96137 | |
| Pseudo-second-order | k2 (g·mg−1·min−1) | 0.0002 | 0.0004 |
| qe (mg·g−1) | 422.9852 | 173.9959 | |
| Adj. R2 | 0.9879 | 0.8826 | |
| Elovich | kext | 0.0065 | - |
| Adj. R2 | 0.8966 | - | |
| Intraparticle diffusion | kp,1 (mg·g−1·min1/2) | 53.7602 | - |
| Adj. R2 | 0.9992 | - | |
| kp,2 (mg·g−1·min1/2) | 15.1661 | - | |
| Adj. R2 | 0.9945 | - | |
| kp,3 (mg·g−1·min1/2) | 0.1620 | - | |
| Adj. R2 | 0.7896 | - | |
| Experiment | qe (mg·g−1) | 400.25 | 159.33 |
| Temperature °C | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L·mg−1) | qm (mg·g−1) | R2 | KF | 1/n | R2 | |
| 25 | 0.1111 | 730.3500 | 0.8944 | 140.7254 | 0.3660 | 0.8678 |
| 45 | 0.0687 | 867.0535 | 0.9507 | 163.2401 | 0.3452 | 0.8608 |
| 65 | 0.0870 | 905.5887 | 0.9315 | 209.1909 | 0.3105 | 0.8916 |
| Adsorbents | Adsorption Capacities (mg·g−1) |
|---|---|
| Active carbon | 103.69 |
| Zeolite | 98.55 |
| Chitosan | 57.31 |
| Mn-RM | 403.28 |
| Leaching Methods | Sample | Cd | Pb | Cr | As | Zn | Cu |
|---|---|---|---|---|---|---|---|
| HJ/T299-2007 | RM | 0.0081 | 0.28 | 0.291 | 2.267 | 0.43 | 0.132 |
| Mn-RM | 0.016 | 0.21 | 0.54 | ND | 0.07 | 0.126 | |
| Mn-RM-Pb2+ | 0.009 | 0.007 | 0.65 | ND | 0.052 | 0.037 | |
| TCLP | RM | 0.006 | 0.241 | 0.09 | 2 | 0.021 | 0.032 |
| Mn-RM | 0.002 | 0.153 | 0.56 | ND | 0.049 | 0.026 | |
| Mn-RM-Pb2+ | 0.007 | 2.62 | 0.45 | ND | 0.069 | 0.024 | |
| Standard | 1 | 5 | 15 | 5 | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y.; Pang, Y.; Wu, Z.; Li, X.; Jing, J.; Wang, H.; Zhou, Z. Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk. Water 2023, 15, 4314. https://doi.org/10.3390/w15244314
Bai Y, Pang Y, Wu Z, Li X, Jing J, Wang H, Zhou Z. Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk. Water. 2023; 15(24):4314. https://doi.org/10.3390/w15244314
Chicago/Turabian StyleBai, Yang, Yin Pang, Zheng Wu, Xi Li, Jiang Jing, Hongbin Wang, and Zheng Zhou. 2023. "Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk" Water 15, no. 24: 4314. https://doi.org/10.3390/w15244314
APA StyleBai, Y., Pang, Y., Wu, Z., Li, X., Jing, J., Wang, H., & Zhou, Z. (2023). Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk. Water, 15(24), 4314. https://doi.org/10.3390/w15244314






