Abstract
The aim of this study is to evaluate the effects of ferrate (VI)-based treatment on surface water collected from the Rímac River as an irrigation water treatment model for bean (Phaseolus vulgaris), lettuce (Lactuca sativa), and radish (Raphanus sativus) plant species irrigated with treated water in the experimental field. The experimental field was divided into eight 625 m2 plots (50 m × 12.5 m) with sandy loam soil (sand 51%, silt 30%, clay 19%). The treatment system operated uninterrupted for three and a half months without deterioration in production, demonstrating that it can function continuously to improve water quality even when the effects on the parameters evaluated here did not reveal significant differences, presumably due to the prevailing effect from metal concentrations already found in the soil. This study also seeks to validate the effect of treatment on the concentration of plant tissue bacteria.
1. Introduction
The consumption of fresh foods, such as salads prepared with raw vegetables, has increased in recent years due to the promotion of healthy food options. However, the number of outbreaks of diseases transmitted by the consumption of fresh produce has concomitantly increased [1], and the presence of bacteria resistant to several antibiotics in fresh produce can only exacerbate the problem [2]. Disease outbreaks related to the consumption of fresh vegetables are commonly associated with agricultural irrigation water [3,4]. For example, in the Cajamarca valley, Peru, vegetable crops are irrigated with water from rivers containing untreated wastewater. These vegetables are then sold in local markets and consumed raw by the urban and rural population [5]. Similar scenarios have also been reported in countries such as Brazil [6], England [7], and Ghana [8].
Irrigation is the controlled use of water sources in a timely manner to increase or sustained crop production [9]; irrigation includes the water that is applied by an irrigation system during the growing season, the water applied during field preparation, preirrigation, weed control, harvesting, and for leaching salts from the root zone [10].
Colorless and foamless water with minimum turbidity, total dissolved solids (TDS) below 1000 mg/L at circumneutral pH, and specific conductance below 15 mmhos/m [11] is generally considered of good quality. However, Park et al. considers an electric conductivity of up to 0.75 mmhos/cm (750 mS/cm) not to be a problem [12]. Being that irrigation is the highest consumptive use of freshwater water [13] and since agricultural production of food needs to be increased by around 60% by 2050 to meet the demands and provide food security [14], irrigation will have to attend the demand for water because of water scarcity. Water scarcity is the condition where water demands from agriculture and other sectors cannot be met due to low water availability [15]. Because food supply is closely related to freshwater supply and water availability is in a critical state, unconventional and substandard sources will be required [16,17,18,19] including, for example, domestic wastewater, industrial wastewater, and agricultural wastewater [20]. Surface water from rivers and lakes is considerably different from reclaimed water because elevated levels of N and P, disinfection by-products, and bacterial pathogens can remain in reclaimed water even after extensive treatment [21,22]. However, the quality of surface waters has great variation, and this occurs in waters subject to intermittent contamination events such as runoff, livestock upstream use, and uncontrolled discharges. This pollution in irrigation systems increases the risk of food crop contamination [23].
Some experiences using reclaimed water for irrigation have been positive. For example, table grapes irrigated with municipal wastewater were free of bacterial contamination [24]. A similar scenario was reported for lettuce crops [25]. However, another study considered that reclaimed water introduced contaminants in hydroponic tomatoes [26]. An additional risk associated with irrigation using reclaimed water is the introduction of heavy metals or metalloids that can accumulate in plant tissues. Studies claim that long-term irrigation with treated wastewater can cause accumulations of heavy metals at several concentrations in different aerial parts of lemon [27], rice [28], mango, and banana [29] plants.
The water used for irrigation could be polluted with biological and inorganic contaminants. Pathogens (bacteria, viruses, and protozoans) pose the greatest acute risk to human health and are a concern in freshly eaten produce; pathogen contamination is related to surface water sources [30]. The presence of heavy metals is an issue due to its potential impact on food quality and human health. Heavy metals have bioaccumulation properties; therefore, organisms will accumulate then and even convert them into more toxic substances [31].
Agricultural water treatments seek to reduce the risk of contamination of fresh produce with pathogenic organisms [32] and remove heavy metals [33] and pathogens [34] from plants. Improvement in the safety of fresh produce could require soil remediation, water remediation or both.
Soil remediation techniques include containment (surface capping, encapsulation, and landfilling), removal (soil washing, soil flushing, electrokinetic extraction, and phytoremediation) and stabilization (solidification, vitrification, and chemical stabilization) [35]. Among these methods, adsorption has been considered one of the most effective methods due to its low cost and high efficiency when in use [36], the simultaneous adsorption of inorganic and organic pollutants such as Cr (VI) and phenol [37], or even incorporate a heterogeneous catalyst for the removal of atrazine (ATZ) from soil [38].
Irrigation water treatment technologies have been sought to decontaminate irrigation waters with food-borne bacterial pathogens: slow-bed sand filtration, membrane filtration, ultraviolet (UV) radiation, ozone disinfection, peroxyacetic acid treatment, chlorine dioxide treatment, and chlorination with sodium hypochlorite [39]. Potential irrigation water treatment techniques include hydrodynamic cavitation, electrolyzed oxidizing water (EO), and electrochemical treatment [32].
One of the water treatment agents used is ferrate(VI); it is an iron species with a high oxidation state that acts as a powerful oxidizing agent and whose remnants are harmless ferric cations; therefore, it is deemed a green treatment agent [40,41]. Proposals to use ferrate as a multivalent agent have been described to remove inorganic contaminants, pathogens, and endocrine disruptors without the formation of chlorinated by-products [42]. The ferrate removal efficiency of copper, manganese, zinc, and natural organic matter (NOM) from river water with ferrate reached 86% for NOM, 99% for copper, 73% for Mn, and 100% for Zn [43]. The use of a low dose of ferrate improved the reduction of the chemical oxygen demand measured with permanganate (CODMn) by iron-manganese co-oxide films. Using 0.1 mg/L potassium ferrate, the removal of a CODMn of 20.0 mg/L reached a removal efficiency of 92.5% [44]. Ferrate has also been applied effectively to reduce human fecal pollution indicators in sewage by removing DNA, damaging viral capsids and bacterial cell membranes [45], and also removing traces of micropollutants in natural waters [46].
In this study an irrigation water treatment plant was built based on ferrate and ferric ions ability to improve the water quality through oxidation and coagulation processes. Sodium ferrate was produced electrochemically in situ and applied together with ferric ions to treat surface water collected from the Rímac River as an irrigation water treatment model for bean, lettuce, and radish crop production. The Rímac River receives domestic and industrial discharges, which increase its concentrations of metal and microorganisms, and considering the effectiveness of a continuous treatment system for irrigation water, an automated system for the continuous production of ferrate is designed and developed on site to continuously produce ferrate to improve irrigation water quality. The study also seeks to validate the effects of treatment on the concentration of metals in leafy vegetables (lettuce), legumes (beans), and plant tissue bacteria (radishes).
2. Materials and Methods
2.1. Ferrate(VI) Production
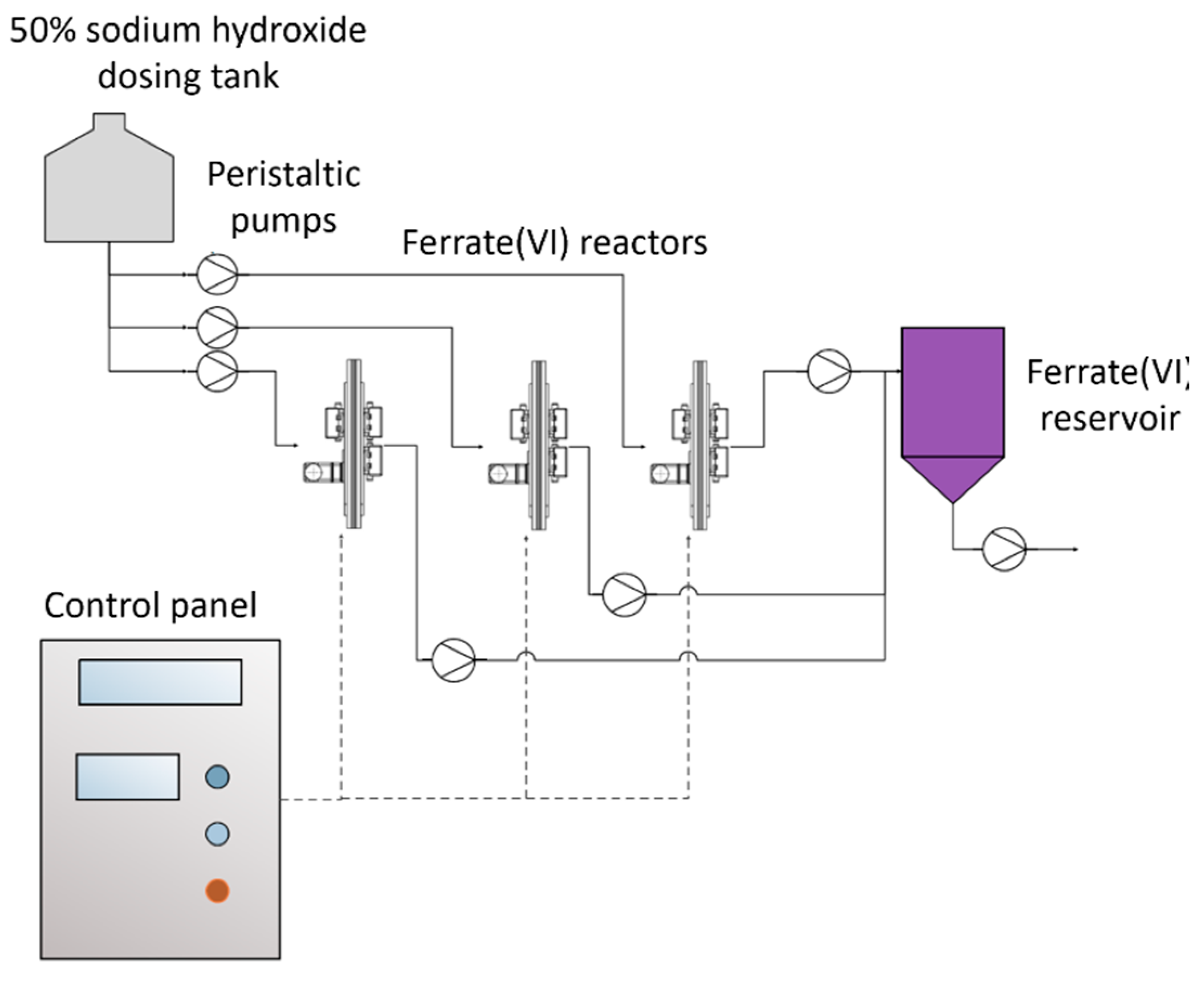
The ferrate(VI) ion is produced through electrochemical oxidation of a 20 cm × 20 cm iron anode and a 20 cm × 20 cm stainless steel cathode separated by a cation exchange membrane (2.3 w cm2; CTIEM-1, Zibo Cantian, Shandong, China), which jointly form a reactor. Three reactors of the same dimensions connected to a 5 V and 0.72 A power source for a current density of 80 A/m2 worked alternately during the 5 h electrolysis. A system of BT101S peristaltic pumps (LeadFluid, Baoding, China) controlled by a S7 1200 programmable logic controller (PLC) (Siemens, Berlin, Germany) filled each reactor chamber with 50% NaOH (Quimpac, Callao, Perú) and discharged the ferrate once the synthesis was complete. Each reactor produced 123.5 mL of 0.224 mol/L solution (26.9 g ferrate/L) in 5 h. Ferrate production was automated by the sequential working of the three reactors. The resulting ferrate was stored in a tank and later used for daily treatments. Ferrate concentrations were measured spectrophotometrically at 505 nm using a UV2600 spectrophotometer (Shimadzu, Kyoto, Japan) and a molar extinction coefficient of 1050 L/mol cm [47]. The ferrate(VI) production diagram is shown in Figure 1, and the production unit is shown in Figure 2.
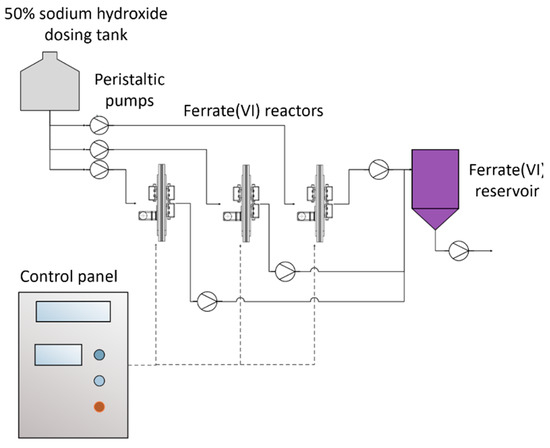
Figure 1.
Ferrate(VI) production system.

Figure 2.
The ferrate(VI) reactor installed in the treatment plant.
The supervisory control and data acquisition (SCADA) system manages and monitors ferrate production in the reactors. When a ferrate (VI) dose of 1.12 mg/L is used, under the same operating conditions, the ferrate(VI) electrosynthesis pilot plant can treat up to 288 m3/day.
2.2. Irrigation Water Treatment
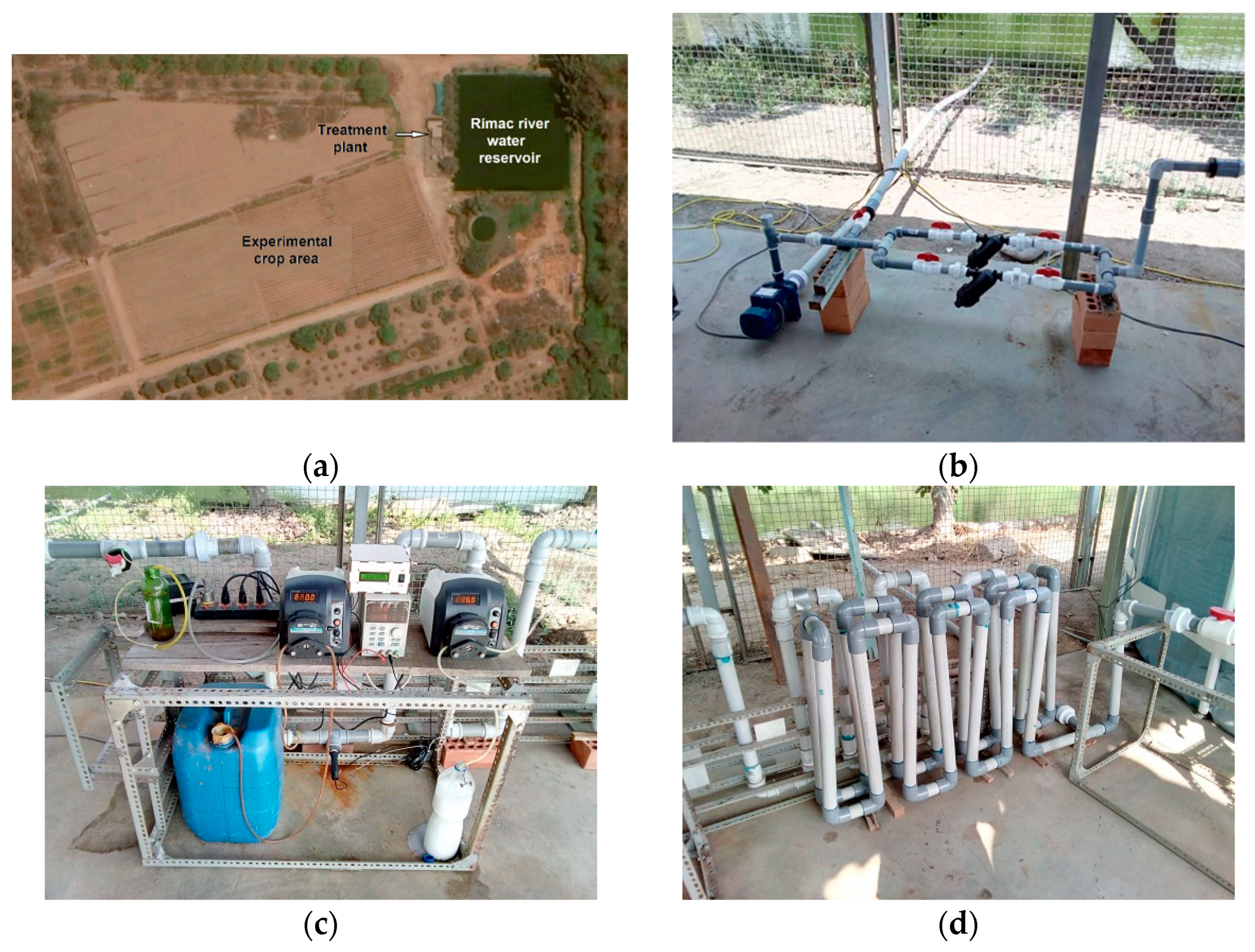
Our pilot water treatment plant was installed at the La Molina Experimental Center of the National Institute of Agricultural Innovation (INIA) (Figure 3) in Lima, Peru. The center comprises agricultural land dedicated to research by the Peruvian government. Crop irrigation water is extracted from the Rímac River through an irrigation canal and stored in a reservoir. The river water deviation point is approximately 7 km from the experimental crop area and receives domestic effluents and industrial discharges upstream. The reservoir is filled twice a week and its water is used to irrigate a 14-hectare plot of crop owned by the center. The experimental ferrate(VI)-based treatment plant is designed to treat 24 L/min for a daily total of 15 m3, and it is used to irrigate a half-hectare crop plot for this study.
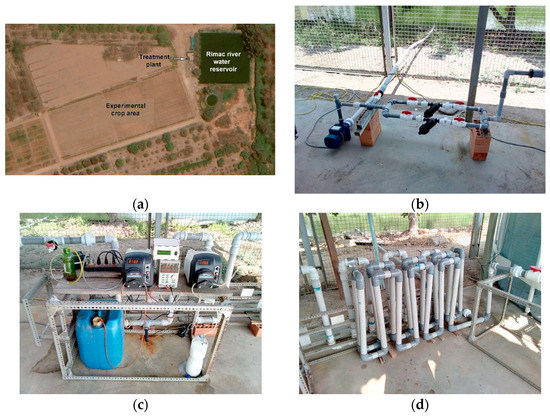

Figure 3.
(a) Aerial image of the experimental area at INIA. The color of the reservoir water is an indicator of the concentration of algae. Water treatment plant images: (b) pumping system of the Rímac River water reservoir; (c) dosing pumps, (d) serpentine mixer, (e) treated water reservoir, (f) geotube.
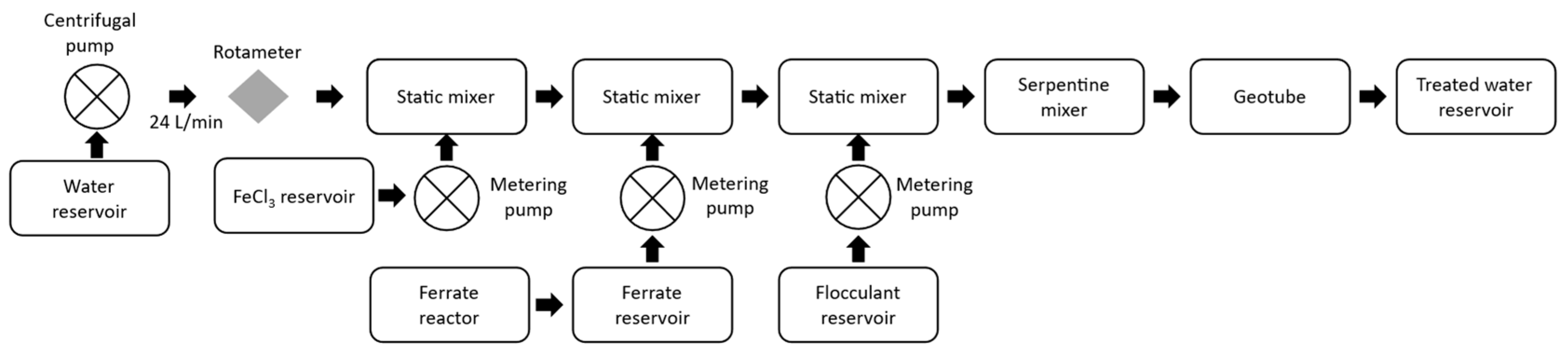
The pilot plant (Figure 4) consists of a centrifugal pump that drives the water for treatment, a water return system that manages the incoming water flow of the treatment plant, a rotameter, two static mixers, and a built-in serpentine mixer with 1.5-inch PVC pipes with a total length of 22 m.
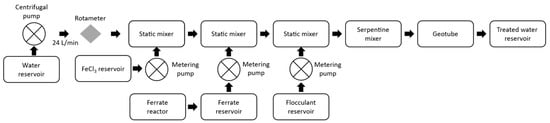
Figure 4.
Water treatment plant diagram.
Ferric chloride 40% (Quimpac, Callao, Perú) was dosed at the inlet of the serpentine mixer at a rate of 29 mg/L using a peristaltic pump; a couple of meters further on, ferrate(VI) was dosed at a rate of 1.12 mg/L using a diaphragm dosing pump; and at two more meters, the SIFLOC 13,980 flocculant (MERCK, Barcelona, Spain) was injected through another peristaltic pump at a rate of 1.0 mg/L. The outgoing flocs from the serpentine mixer were retained in a 3 m × 2 m Tencate GT500 geotube (Tencate Geosynthetics, Pendergrass, GA, USA), and the outlet water was pumped into a reservoir to irrigate the experimental area. The dosing of the ferrate(VI) alkaline solution increased the pH to a value close to the target value of 6.50.
After starting the operation of the treatment plant and controlling both the pH values and the floc production, the quality of treated and untreated water was evaluated for 13 consecutive days to verify the correct operation of the treatment plant. The inlet water was sampled once at the beginning of the day and after the reservoir was filled on the days it was refilled previously to the beginning of the treatment. An hourly composite of 5 samples of 1 L of water was made in the morning and afternoon for a total of two samples analyzed.
At this stage, pH, turbidity, biochemical oxygen demand (BOD5), conductivity, and total metal values were recorded.
On a daily basis, the treatment plant consumes an average of 2.25 L of 40% ferric chloride, 0.625 L of 26.9 g/L ferrate(VI) solution, and 15 L of flocculant 1000 mg/L.
2.3. Experimental Crop Fields
The evaluation of treated water in crops was carried out on an experimental half-hectare area with sandy loam soil (sand 51%, silt 30%, clay 19%), which was divided into eight 625 m2 plots (50 m × 12.5 m). The land was prepared for planting, and soil samples were analyzed to determine their initial metal content. Finally, irrigation tapes were installed, spaced 75 cm apart.
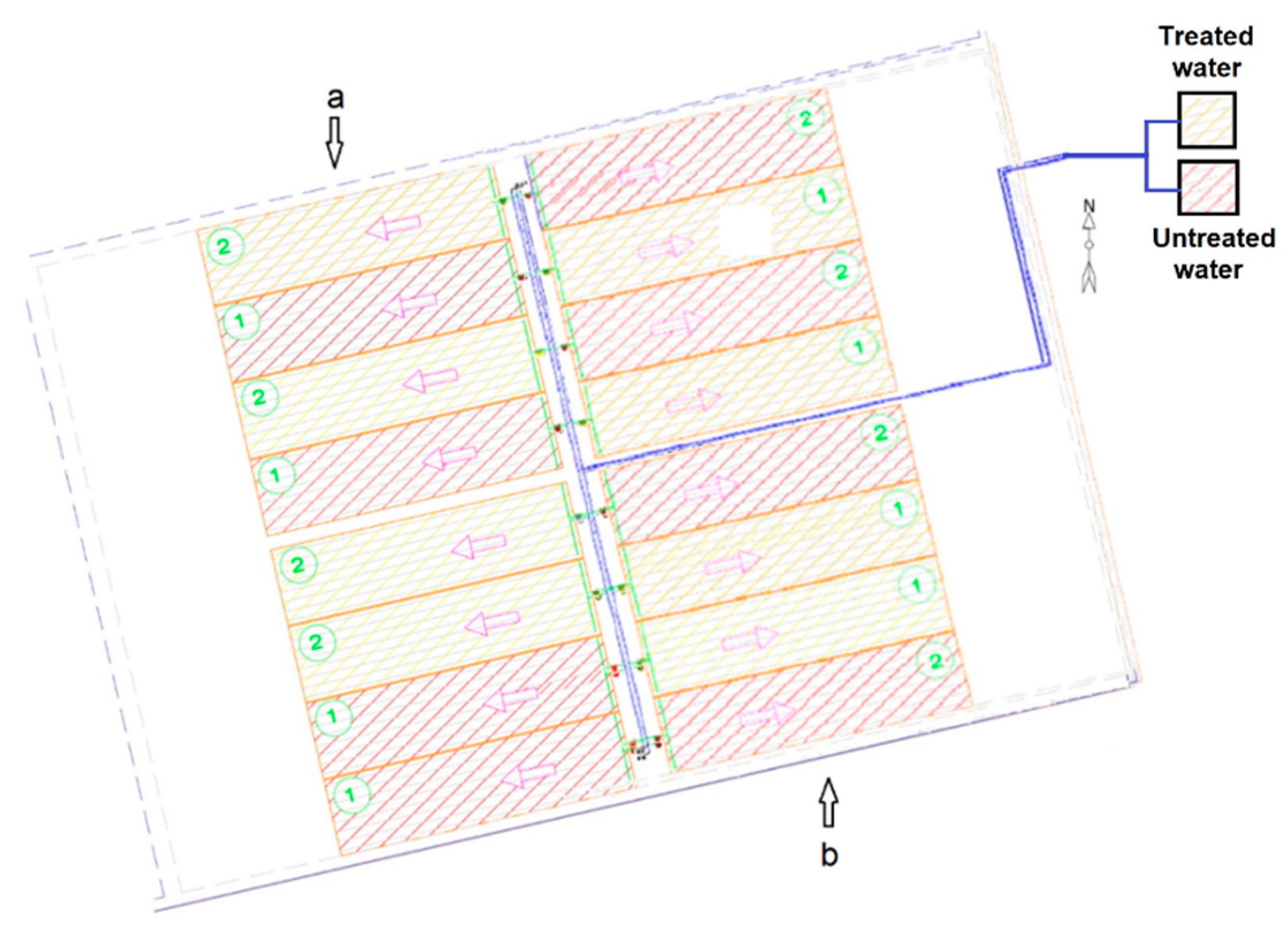
In this experimental field, we planted beans (Phaseolus vulgaris), lettuce (Lactuca sativa), and radishes (Raphanus sativus) in three different campaigns. Four plots were irrigated with treated water and the other four with untreated water extracted directly from the reservoir. The plot layouts were randomly selected, as shown in Figure 5. The plots were irrigated in 3 months for beans (green pod harvest), 1 month for lettuce, and 1 month for radishes.
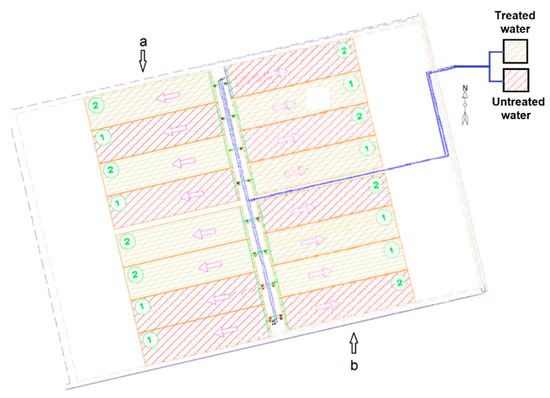
Figure 5.
Distribution of (a) lettuce and (b) radish crops in the experimental plots. Water flows in the direction of arrows, piping in blue. Yellow plots: irrigation with treated water; purple plots: irrigation with untreated water.
2.4. Water Quality Analysis
pH levels, electrical conductivity, BOD5, turbidity and concentrations of metals and metalloids (As, Al, Cd, Co, Fe, Hg, Pb, Na, and Zn) measured by a Shimadzu 2030 (Shimdazu Corporation, Kyoto, Japan) inductively coupled plasma mass spectrometer (ICP-MS) were recorded as previously described [48]. pH, electrical conductivity, and turbidity were recorded on-site, pH and electrical conductivity (EC) using Hach HQ40D (Hach, Loveland, CO, USA) multiparameter equipment, and turbidity using a Lovibond TB-211 IR turbidimeter (Lovibond, Schleefstraße, Dormund, Germany). BOD5 was determined using a Hach BODTrak II instrument in the laboratory using samples collected less than an hour before measurement.
2.5. Soil Analysis
Soil sampling was performed randomly in each of the 16 plots of the experimental crop area. These samples were analyzed by a certified laboratory (General Analytical Services (SAG), Lima, Peru) using the EPA/SW-846 method. The results are listed in Table A1 of Appendix A.
2.6. Microbiological Analysis for Escherichia coli Detection in Radishes
Fresh items without damage were rinsed with water and decontaminated by immersion in 95% ethanol and rubbed for 60 s; after this step they were immersed and rubbed for 60 s in 2.5% sodium hypochlorite, washed three times with sterile water, and allowed to dry in a clean bench for 1 h. Ten-gram samples were transferred to sterile 0.5 L stomacher bags containing 100 mL of 0.1% buffered peptone and homogenized for 60 s at 250 rpm [49]. Serial dilutions were performed, and one milliliter of each dilution was placed in a petri dish adding 20 mL of molten RAPID’E.coli 2 medium (BioRad, Hercules, CA, USA). Plates were incubated at 37 °C for 24 h and the results were expressed as CFU/g.
2.7. Plant Tissue Metal Analysis
Elemental analysis of the samples were performed by ICP-MS. The samples were oven dried at 60 °C for 24 h. Then, to determine metal concentrations by ICP- MS, 0.50 g of these samples were digested using 3 mL of a nitric acid–perchloric acid mixture in a 3:1 ratio [50].
The bioaccumulation factor (BAF) of the metals was calculated as follows according to [51].
where Cplant is the concentration in the edible part of the vegetable and Csoil represents the metal concentration in the soil (Table A1 of Appendix A), both expressed in mg/kg of dry matter.
2.8. Statistical Analysis
Comparison of Escherichia coli CFU per gram of plant tissue was performed with a Welch two-sample t-test using R [52].
3. Results
The results of the combined treatment of ferrate(VI) and Fe(III) irrigation water are as follows:
3.1. Variation in pH, Electrical Conductivity, BOD, and Turbidity
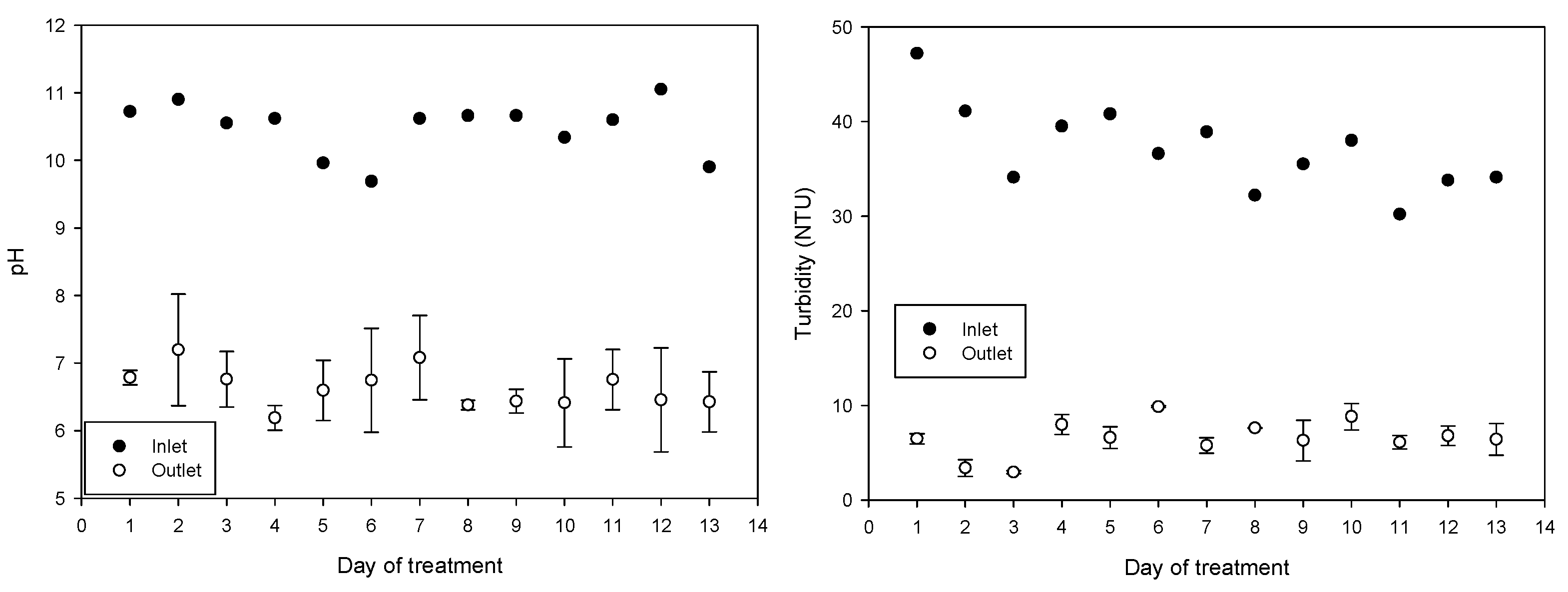
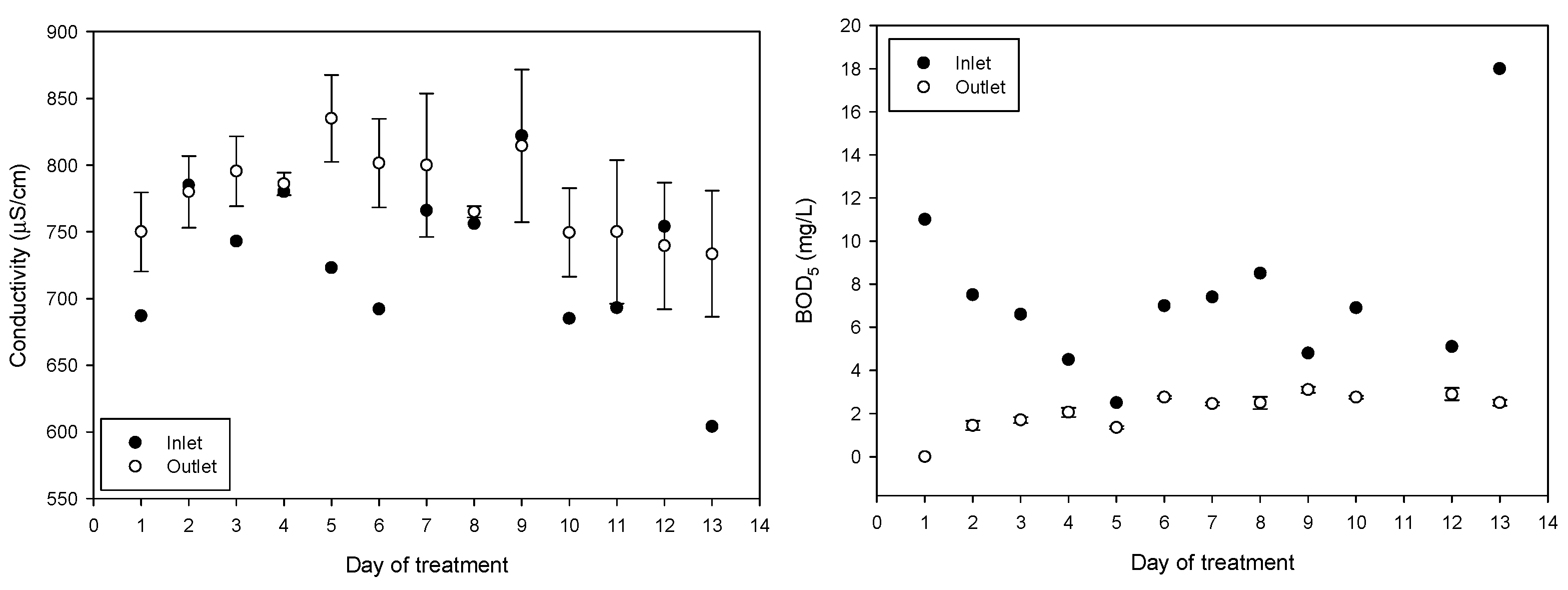
The average incoming pH during the 13 treatment days was 10.48 ± 0.39 (average ± SD); water has diurnal variations due to the drawing of the water to irrigate the other fields and because the reservoir is replenished with water. After treatment, the pH of the water decreased to an average of 6.63 ± 0.47, achieving a significant reduction in BOD from 7.5 ± 4 to 2.1 ± 0.87 and a significant reduction in turbidity from 37 ± 4.5 to 6.5 ± 2.0. The average incoming electrical conductivity of 730 ± 55 μs/cm increased to 777 ± 42 μs/cm after treatment. The increase in conductivity is attributed to the combined treatment of ferric chloride and NaOH solution containing the synthesized ferrate(VI). The daily values obtained of pH, electrical conductivity, BOD, and turbidity are shown in Figure 6.
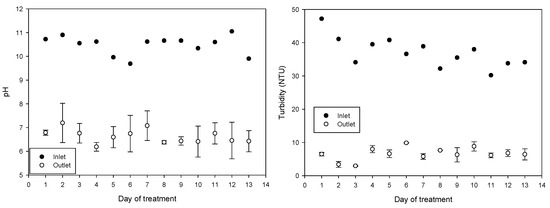
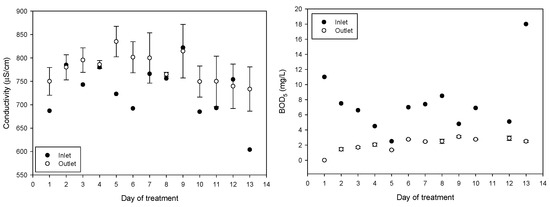
Figure 6.
pH, turbidity, electrical conductivity, and BOD5 variations throughout the 13 days of treatment (mean values with standard deviations).
3.2. Variations in Metal and Metalloid Concentrations
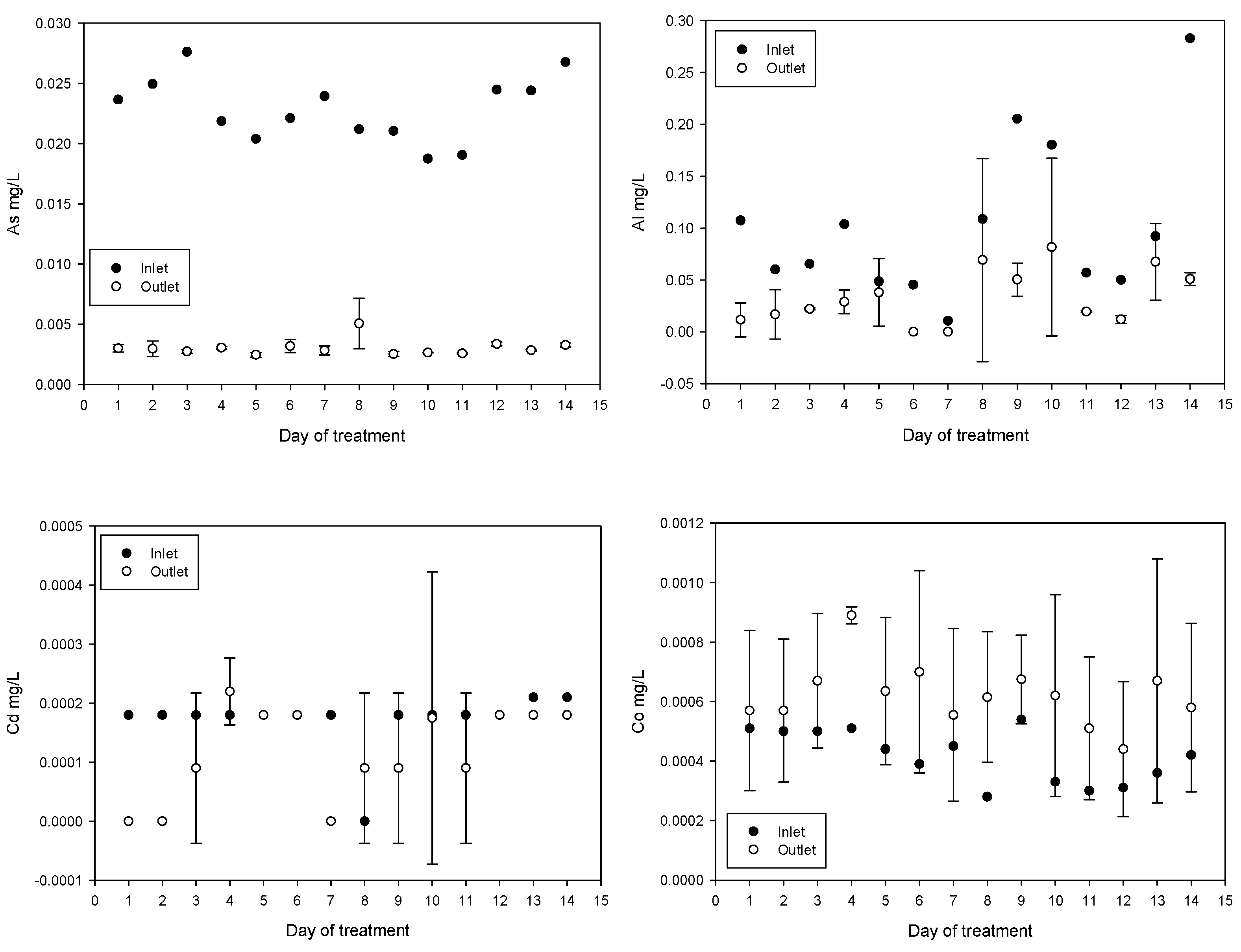
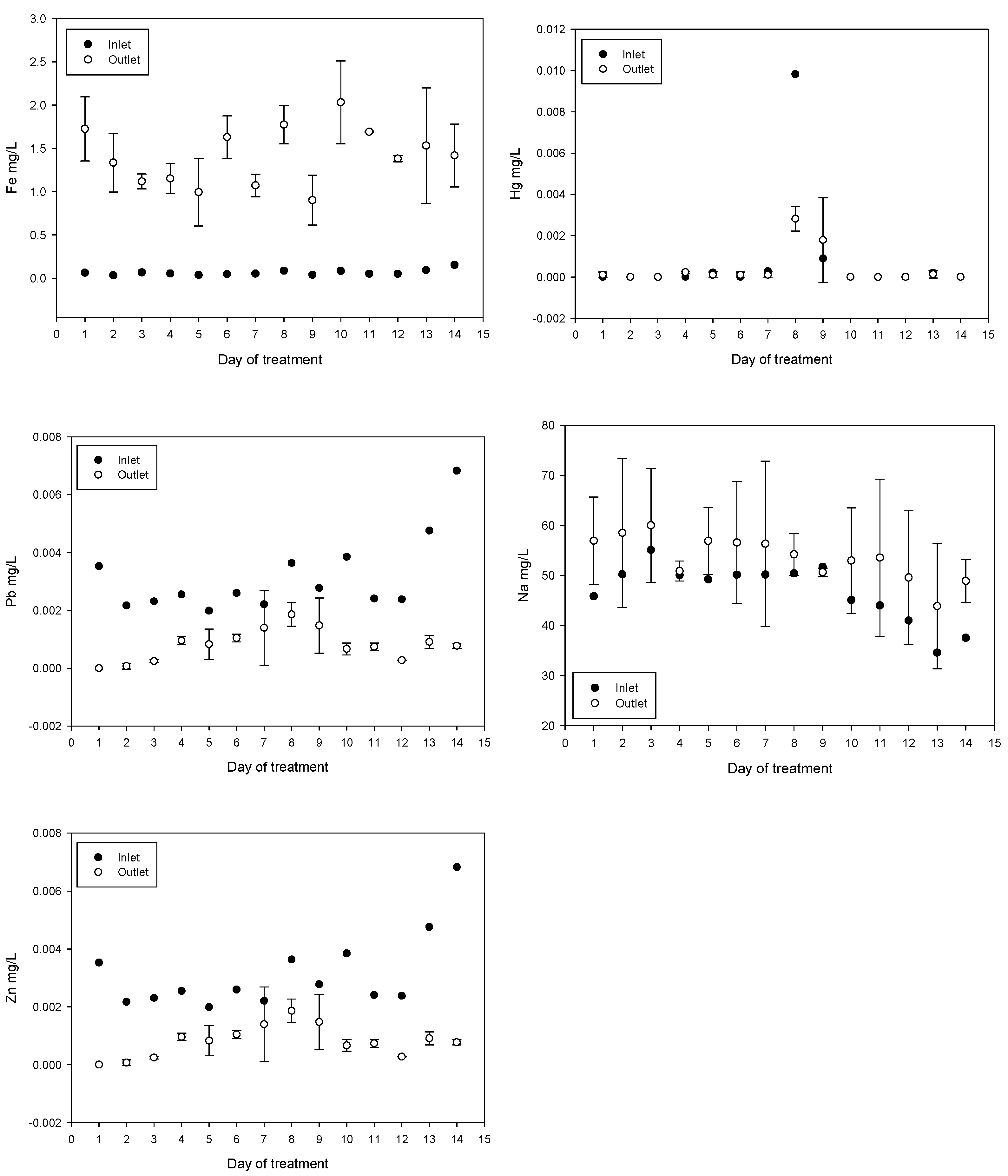
Treatment reduced the concentrations of As, Al, Pb, and Zn. In contrast, the concentrations of Fe, Co, and Na increased. The increase in Fe can be explained by the addition of ferrate and ferric chloride. The increase in Co may be related to the composition of the iron anode used for ferrate generation. The increase in Na is due to iron anode oxidation in a 20 mol/L NaOH solution, for ferrate(VI) synthesis, injected directly into the inlet. The results obtained for Cd and Hg were not conclusive. Daily values of metal and metalloid concentrations obtained are shown in Figure 7.
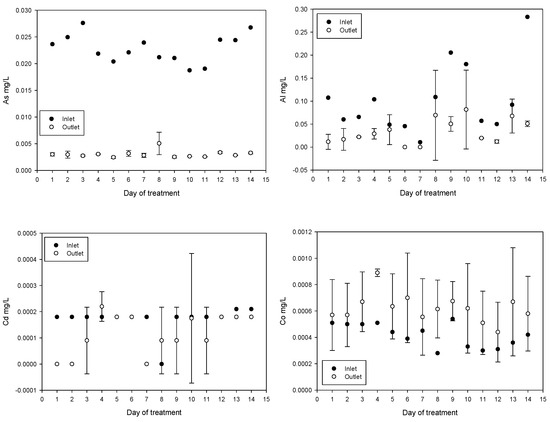
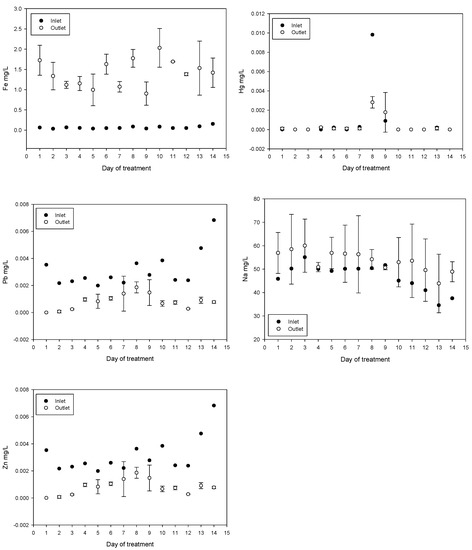
Figure 7.
Metal and metalloid concentration variations (mean values with standard deviations).
3.3. Heavy Metal Contents in Beans and Lettuce
The results for beans and lettuce were obtained from an average of eight samples each and are listed in Table 1 and Table 2, respectively.

Table 1.
Heavy metal content in beans (Phaseolus vulgaris).

Table 2.
Heavy metal content in lettuce (Lactuca sativa).
3.4. Coliforms in Radishes
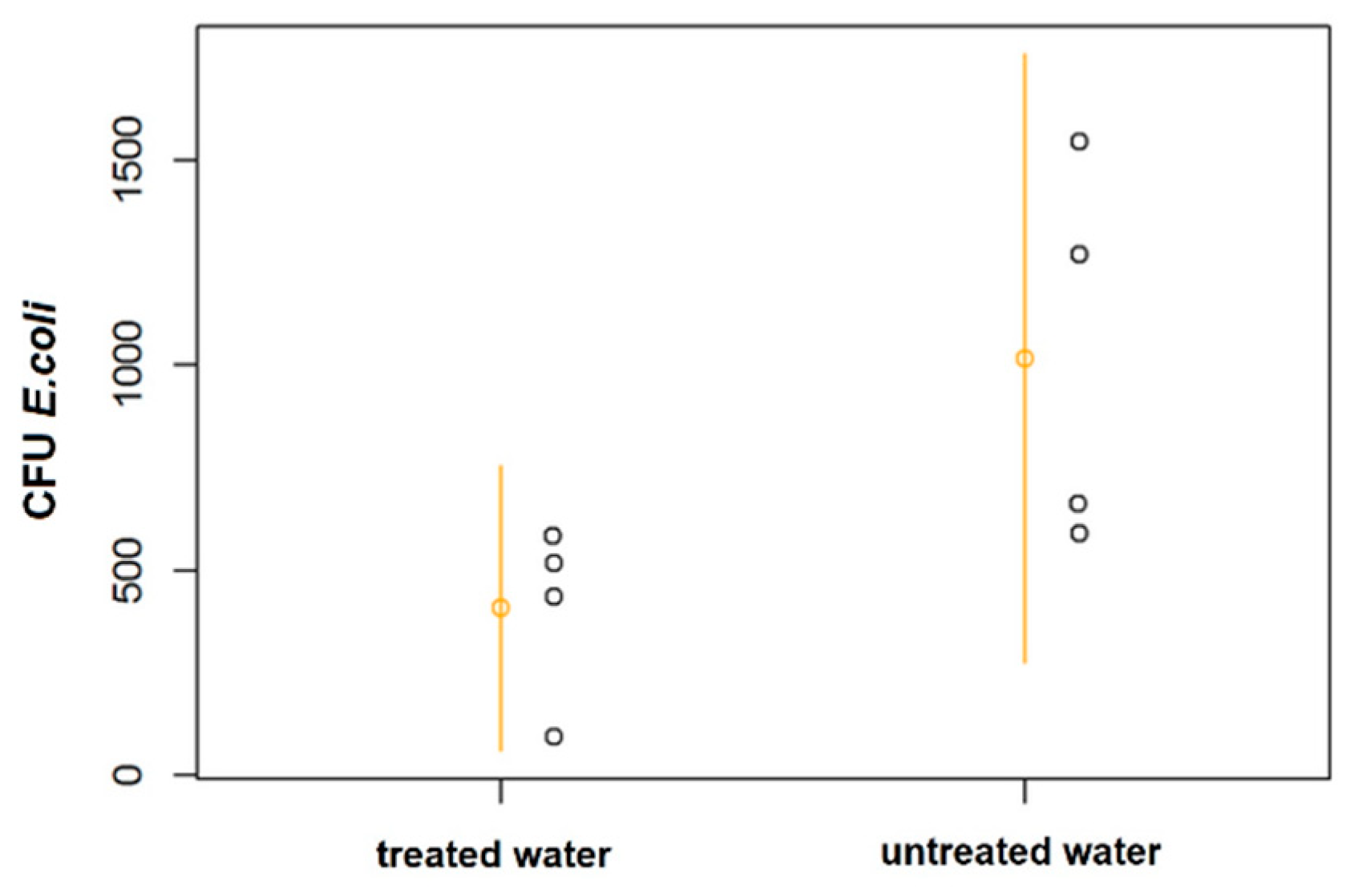
Figure 8 shows a dot plot chart with the results of coliform CFU per gram of plant tissue in radish crops. In the comparison, no significant differences (p = 0.07) in the number of Escherichia coli in radishes (4 samples per experimental plot, 16 total samples); however, in a larger sample size, a significant difference should be detected.
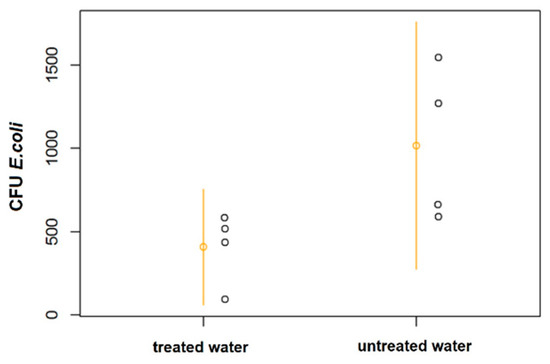
Figure 8.
CFU of Escherichia coli per gram of plant tissue, dotplot with means and 95% confidence intervals. Each point represents the average of four samples per plot grouped as a single experimental unit.
4. Discussion
Ferrate(VI) is used as an advanced oxidation agent for the removal of multiple pollutants from water, such as metals, microorganisms, and organic contaminants, and has previously been used for water treatment [53]. Ferrate(VI) solutions have been proven to be effective in removing algae and turbidity from water [54,55]. In this study, ferrate(VI) solutions were able to successfully remove both turbidity and BOD5.
As expected, the electrical conductivity increased from 730 ± 57 to 801 ± 33 mS/cm (p < 0.001) because ferrate production by electrosynthesis requires a concentrated electrolyte solution and its dosing increases the electrical conductivity (EC) of the treated water. Increase in EC in irrigation water has negative effects on production; a 25–30% yield reduction was found in soilless grown strawberry plants irrigated to 4.5 mS/cm compared with 2.5 mS/cm [56] and a reduction in vegetative growth of lettuce while increasing EC (1.0, 1.5 and 1.8 mS/cm) [57], the water produced in the treatment plant reached 0.8 mS/cm and can still be considered freshwater [58]. Potential concerns about negative changes in soil conductivity [59] because of sodium addition can be addressed by using potassium hydroxide as an electrolyte instead of sodium hydroxide for ferrate synthesis.
Untreated water exceeded pH 9 (10.48 ± 0.40), pH influences the reduction of saturate hydraulic conductivity by altering the surface charge of clay particles, effects that are more noticeable at pH higher than 9 and in acid soils [60]. The normal pH range for irrigation water is from 6.5 to 8.4; pH values above 8.5 are often caused by high alkalinity and can affect drip or microarray irrigation systems when calcite or scales accumulate, reducing flow [61]. Combining Fe (VI)/Fe (III) treatment reduced pH to an average of 6.63 ± 0.47 and reduced the frequency of obstructions reported on irrigation tapes as observed in the field (results not shown).
Turbidity levels were reduced from 37.1 ± 4.5 NTU for incoming water to 6.5 ± 2.0 NTU after treatment. The removal of turbidity in irrigation water has been associated with a general improvement in the microbiological quality of the water because turbidity could be related to greater protection of microorganisms from environmental stress [62]. A study observed a correlation (0.52, p < 0.01) between Norovirus (NoV) genogroup I and water turbidity [63], and a strong correlation was shown between turbidity and helminth eggs in turbid water and wastewater [64].
The concentration of As, Al, Pb, and Zn was reduced while the concentration of Fe increased; this is related with the Fe added during the treatment. An increase in Co concentration, which is related to anode composition, was subtle (from an average of 0.000417 to 0.000621 mg/L after treatment), below the maximum permissible value of 0.05 mg/L and well below the concentrations of 0.46 to 1.24 mg/L in irrigation samples tested for plant toxicity and health risks [65]. On the days eight and nine during plant testing a spike in mercury levels were measured however, these events appear to be isolated.
Both the bean seed and lettuce leaf analyses did not reveal significant differences in the content of the metals evaluated or in bioaccumulation, despite the reduction in the concentrations reported for some elements. Hence, the effects of soil composition may prevail. Count of E. coli CFU was reduced compared with untreated water (Figure 8) and the microbiological analysis of the radish tissue did not reveal significant differences (p = 0.07); however, the resulting p value suggests that the effect is likely to be significant with a larger sample.
At a ferrate (VI) dose of 0.2 mg/L, the ferrate(VI) electrosynthesis pilot plant was able to treat 288 m3/day:
The ferrate treatment system operated uninterrupted for three and a half months without deterioration in production, demonstrating that it can function continuously to improve water quality even when the effects on the parameters evaluated here did not reveal significant differences, presumably due to the prevailing effect from metal concentrations already found in the soil or a small microbiology sample.
5. Conclusions
Automatization of a ferrate(VI) electrochemical reactor in a pilot water treatment plant could generate this oxidizing agent on site in sufficient quantities to guarantee continuous water flow treatment for three and a half months; thus, the feasibility of producing ferrate for water treatment purposes was demonstrated. Using ferrate (VI)-based treatment on water from the Rímac River, we were able to adjust pH to appropriate levels while reducing water turbidity, simultaneously removing BOD5, As, Al, Pb, and Zn, thus improving the quality of the water quality to be used for crop irrigation. However, for bean (Phaseolus vulgaris), lettuce (Lactuca sativa), and radish (Raphanus sativus) plant species irrigated with treated water in the experimental field, no significant differences were observed in heavy metal content or BAF, presumably because soil effect was higher. The significance (p < 0.07) differences in Escherichia coli numbers in vegetal tissue justifies a new assessment with a large sample size.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, F.P.L., E.S.O. and J.Q.-F.; resources, M.E.R.M. and K.A.V.G.; writing—original draft preparation, F.P.L., E.S.O. and J.Q.-F.; writing—review and editing, F.P.L., E.S.O. and J.Q.-F.; project administration, M.E.R.M. and K.A.V.G.; funding acquisition, M.E.R.M. and K.A.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Programa Nacional de Innovación Agraria (PNIA) grant number 093-PI.
Data Availability Statement
Data available in https://doi.org/10.6084/m9.figshare.22083239 (accessed on 12 January 2023).
Acknowledgments
Support from Instituto Nacional de Innovación Agraria (INIA), Facultad de Ingeniería y Arquitectura Universidad de Lima and, Instituto de Investigación Científica de la Universidad de Lima—IDIC are greatly acknowledged.
Conflicts of Interest
Authors declare no conflict of interest.
Appendix A

Table A1.
Soil element content.
Table A1.
Soil element content.
| Metal | Detection Limit | Unit | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|---|
| Ag | 0.07 | mg/kg | 0.37 | 0.84 | <0.07 | 0.51 |
| Al | 1.4 | mg/kg | 11,015.0 | 10,690.2 | 11,127.9 | 10,855.5 |
| As | 0.1 | mg/kg | 56.3 | 72.2 | 51.1 | 63.4 |
| B | 0.2 | mg/kg | 4.6 | 4.2 | 4.8 | 4.4 |
| Ba | 0.2 | mg/kg | 106.6 | 134.2 | 89.0 | 114.1 |
| Be | 0.03 | mg/kg | 0.39 | 0.37 | 0.38 | 0.37 |
| Ca | 4.7 | mg/kg | 8054.7 | 8165.2 | 7467.5 | 7698.6 |
| Cd | 0.04 | mg/kg | 2.77 | 3.19 | 2.54 | 2.91 |
| Ce | 0.2 | mg/kg | 27.6 | 27.5 | 28.1 | 27.9 |
| Co | 0.05 | mg/kg | 8.26 | 8.17 | 8.40 | 8.26 |
| Cr | 0.04 | mg/kg | 14.07 | 22.33 | 11.41 | 18.69 |
| Cu | 0.1 | mg/kg | 63.8 | 74.6 | 53.9 | 63.9 |
| Fe | 0.2 | mg/kg | 16,099.9 | 16,300.3 | 16,353.5 | 16,290.1 |
| Hg | 0.1 | mg/kg | 1.0 | 1.1 | 0.5 | 0.9 |
| K | 4.3 | mg/kg | 1741.7 | 1728.6 | 1874.2 | 1735.3 |
| Li | 0.3 | mg/kg | 26.6 | 24.7 | 28.3 | 26.0 |
| Mg | 4.4 | mg/kg | 6600.4 | 6501.0 | 6873.3 | 6618.5 |
| Mn | 0.05 | mg/kg | 496.82 | 505.30 | 509.37 | 494.43 |
| Mo | 0.2 | mg/kg | 0.6 | 0.6 | 0.6 | 0.6 |
| Na | 2.3 | mg/kg | 507.3 | 496.0 | 516.2 | 500.9 |
| Ni | 0.06 | mg/kg | 4.46 | 4.59 | 4.60 | 4.61 |
| P | 0.3 | mg/kg | 1247.9 | 1272.0 | 1213.0 | 1197.3 |
| Pb | 0.06 | mg/kg | 132.80 | 164.79 | 94.35 | 135.36 |
| Sb | 0.2 | mg/kg | 2.4 | 2.6 | 1.7 | 2.4 |
| Se | 0.3 | mg/kg | <0.3 | <0.3 | <0.3 | <0.3 |
| Sn | 0.1 | mg/kg | 0.8 | 1.3 | 0.6 | 0.9 |
| Sr | 0.1 | mg/kg | 67.8 | 66.5 | 67.2 | 66.1 |
| Ti | 0.03 | mg/kg | 287.11 | 280.38 | 274.37 | 270.14 |
| Tl | 0.3 | mg/kg | <0.3 | <0.3 | <0.3 | <0.3 |
| V | 0.04 | mg/kg | 26.84 | 26.39 | 27.91 | 26.59 |
| Zn | 0.2 | mg/kg | 315.2 | 432.1 | 218.3 | 332.6 |
References
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Kayode, A.J.; Igere, B.E.; Okoh, A.I. High Levels of Multi Drug Resistant Escherichia coli Pathovars in Preharvest Environmental Samples: A Ticking Time Bomb for Fresh Produce Related Disease Outbreak. Front. Environ. Sci. 2022, 10, 218. Available online: https://www.frontiersin.org/article/10.3389/fenvs.2022.858964 (accessed on 3 July 2022). [CrossRef]
- Rodrigues, C.; da Silva, A.L.; Dunn, L.L. Factors Impacting the Prevalence of Foodborne Pathogens in Agricultural Water Sources in the Southeastern United States. Water 2020, 12, 51. [Google Scholar] [CrossRef]
- Hanning, I.B.; Nutt, J.D.; Ricke, S.C. Salmonellosis Outbreaks in the United States Due to Fresh Produce: Sources and Potential Intervention Measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef]
- Rivera-Jacinto, M.; Rodríguez-Ulloa, M.; López-Orbegoso, J. Contaminación fecal en hortalizas que se expenden en mercados de la ciudad de Cajamarca, Perú, [Fecal contamination in vegetables that are sold in markets of the city of Cajamarca, Peru]. Rev. Peru. Med. Exp. Salud Publica 2008, 26, 45–48. [Google Scholar]
- Bartz, F.W.; Teixeira, L.B.; Schroder, R.; Das Mercês Santos, A.F.; Trindade, P.; Tondo, E.C. First Fatal Cases due to Escherichia coli O157 and Campylobacter jejuni subsp. jejuni Outbreak Occurred in Southern Brazil. Foodborne Pathog. Dis. 2022, 19, 241–247. [Google Scholar] [CrossRef]
- Dallman, T.J.; Jalava, K.; Verlander, N.Q.; Gally, D.; Jenkins, C.; Godbole, G.; Gharbia, S. Identification of domestic reservoirs and common exposures in an emerging lineage of Shiga toxin-producing Escherichia coli O157:H7 in England: A genomic epidemiological analysis. Lancet Microbe 2022, 3, e606–e615. [Google Scholar] [CrossRef]
- Amuah, E.E.Y.; Amanin-Ennin, P.; Antwi, K. Irrigation water quality in Ghana and associated implications on vegetables and public health. A systematic review. J. Hydrol. 2022, 604, 127211. [Google Scholar] [CrossRef]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation Water Quality—A Contemporary Perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Dieter, C.A.; Maupin, M.A.; Caldwell, R.R.; Harris, M.A.; Ivahnenko, T.I.; Lovelace, J.K.; Barber, N.L.; Linsey, K.S. Estimated Use of Water in the United States in 2015; US Geological Survey: Reston, VA, USA, 2018.
- Frenkel, H. Reassessment of Water Quality for Irrigation. In Soil Salinity under Irrigation: Processes and Management; Shainberg, I., Shalhevet, J., Eds.; Springer: Berlin, Germany, 1984; pp. 143–165. [Google Scholar]
- Park, D.M.; White, S.A.; McCarty, L.B.; Menchyk, N.A. Interpreting Irrigation Water Quality Reports; CU-14-700; Clemson University Cooperative Extension: Clemson, SC, USA, 2014. [Google Scholar]
- Bruinsma, J. (Ed.) World Agriculture: Towards 2015/2030 an FAO Perspective; Earthscan Publications Ltd.: London, UK, 2003; ISBN 9251048355. [Google Scholar]
- FAO. How to Feed the World in 2050. Insights Expert Meet. In Insights from an Expert Meeting at FAO; FAO: Rome, Italy, 2009; Volume 2050, pp. 1–35. [Google Scholar]
- Oki, T.; Kanae, S. Global hydrological cycles and world water resources. Science 2006, 313, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, U.J.; Mara, D.D.; Peasey, A.; Ruiz-Palacios, G.; Stott, R. Guidelines for the microbiological quality of treated wastewater used in agriculture: Recommendations for revising WHO guidelines. Bull. World Health Organ. 2000, 78, 1104–1116. [Google Scholar] [PubMed]
- Rodriguez, C.; Van Buynder, P.; Lugg, R.; Blair, P.; Devine, B.; Cook, A.; Weinstein, P. Indirect Potable Reuse: A Sustainable Water Supply Alternative. Int. J. Environ. Res. Public Health 2009, 6, 1174–1203. [Google Scholar] [CrossRef] [PubMed]
- Falkenmark, M. Growing water scarcity in agriculture: Future challenge to global water security. Phil. Trans. R. Soc. A 2013, 371, 20120410. [Google Scholar] [CrossRef] [PubMed]
- Drechsel, P.; Qadir, M.; Baumann, J. Water reuse to free up freshwater for higher-value use and increase climate resilience and water productivity. Irrig. Drain. 2022, 71, 100–109. [Google Scholar] [CrossRef]
- Ungureanu, N.; Vlăduț, V.; Voicu, G. Water Scarcity and Wastewater Reuse in Crop Irrigation. Sustainability 2020, 12, 9055. [Google Scholar] [CrossRef]
- Zhang, J.; He, X.; Zhang, H.; Liao, Y.; Wang, Q.; Li, L.; Yu, J. Factors Driving Microbial Community Dynamics and Potential Health Effects of Bacterial Pathogen on Landscape Lakes with Reclaimed Water Replenishment in Beijing, PR China. Int. J. Environ. Res. Public Health 2022, 19, 5127. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, C.; Rosenwinkel, K.-H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef]
- Lothrop, N.; Bright, K.R.; Sexton, J.; Pearce-Walker, J.; Reynolds, K.A.; Verhougstraete, M.P. Optimal strategies for monitoring irrigation water quality. Agric. Water Manag. 2018, 199, 86–92. [Google Scholar] [CrossRef]
- Abi Saab, M.T.; Zaghrini, J.; Makhlouf, H.; Fahed, S.; Romanos, D.; Khairallah, Y.; Hajjar, C.; Abi Saad, R.; Sellami, M.H.; Todorovic, M. Table grapes irrigation with treated municipal wastewater in a Mediterranean environment. Water Environ. J. 2021, 35, 617–627. [Google Scholar] [CrossRef]
- Bichai, F.; Polo-López, M.I.; Fernández Ibañez, P. Solar disinfection of wastewater to reduce contamination of lettuce crops by Escherichia coli in reclaimed water irrigation. Water Res. 2012, 46, 6040–6050. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Galvez, F.; Allende, A.; Pedrero-Salcedo, F.; Alarcon, J.J.; Gil, M.I. Safety assessment of greenhouse hydroponic tomatoes irrigated with reclaimed and surface water. Int. J. Food Microbiol. 2014, 191, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Albdaiwi, R.N.; Al-Hawadi, J.S.; Al-Rawashdeh, Z.B.; Al-Habahbeh, K.A.; Ayad, J.Y.; Al-Sayaydeh, R.S. Effect of Treated Wastewater Irrigation on the Accumulation and Transfer of Heavy Metals in Lemon Trees Cultivated in Arid Environment. Horticulturae 2022, 8, 514. [Google Scholar] [CrossRef]
- Menghua, X.; Yuanyuan, L. Distribution Characteristics and Ecological Risk Assessment of Heavy Metals under Reclaimed Water Irrigation and Water Level Regulations in Paddy Field. Pol. J. Environ. Stud. 2022, 31, 2355–2365. [Google Scholar] [CrossRef]
- Ullah, S.; Shahbaz, A.; Aslam, M.Z. Impact Of Irrigation Water On the Quality Attributes of Selected Indigenous Plants. J. Turk. Chem. Soc. Sect. A Chem. 2022, 9, 639–650. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Preharvest Transmission Routes of Fresh Produce Associated Bacterial Pathogens with Outbreak Potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.; Liu, C.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Dandie, C.E.; Ogunniyi, A.D.; Ferro, S.; Hall, B.; Drigo, B.; Chow, C.W.K.; Venter, H.; Myers, B.; Deo, P.; Donner, E.; et al. Disinfection options for irrigation water: Reducing the risk of fresh produce contamination with human pathogens. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2144–2174. [Google Scholar] [CrossRef]
- Munyengabe, A.; Zvinowanda, C.; Ramontja, J.; Zvimba, J.N. Effective Desalination of Acid Mine Drainage Using an Advanced Oxidation Process: Sodium Ferrate (VI) Salt. Water 2021, 13, 2619. [Google Scholar] [CrossRef]
- Hong, C.X.; Moorman, G.W. Plant Pathogens in Irrigation Water: Challenges and Opportunities. Crit. Rev. Plant Sci. 2005, 24, 189–208. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Qian, W.; Liang, J.Y.; Zhang, W.X.; Huang, S.T.; Diao, Z.H. A porous biochar supported nanoscale zero-valent iron material highly efficient for the simultaneous remediation of cadmium and lead contaminated soil. J. Environ. Sci. 2022, 113, 231–241. [Google Scholar] [CrossRef]
- Dong, F.X.; Yan, L.; Zhou, X.H.; Huang, S.T.; Liang, J.Y.; Zhang, W.X.; Guo, Z.W.; Guo, P.R.; Qian, W.; Kong, L.J.; et al. Simultaneous adsorption of Cr(VI) and phenol by biochar-based iron oxide composites in water: Performance, kinetics and mechanism. J. Hazard. Mater. 2021, 416, 125930. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.H.; Zhang, W.X.; Liang, J.Y.; Huang, S.T.; Dong, F.X.; Yan, L.; Qian, W.; Chu, W. Removal of herbicide atrazine by a novel biochar based iron composite coupling with peroxymonosulfate process from soil: Synergistic effect and mechanism. Chem. Eng. J. 2021, 409, 127684. [Google Scholar] [CrossRef]
- Gurtler, J.B.; Gibson, K.E. Irrigation water and contamination of fresh produce with bacterial foodborne pathogens. In Current Opinion in Food Science; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; Volume 47, p. 100889. [Google Scholar] [CrossRef]
- Thomas, M.; Drzewicz, P.; Więckol-Ryk, A.; Panneerselvam, B. Effectiveness of potassium ferrate (VI) as a green agent in the treatment and disinfection of carwash wastewater. Environ. Sci. Pollut. Res. 2022, 29, 8514–8524. [Google Scholar] [CrossRef] [PubMed]
- Levia, L.; Lalhmunsiama, L.; Chhakchhuak, V.; Diwakar, T.; Soon, C.S.; Seung-Mok, L. Newer Insights on Ferrate(VI) Reactions with Various Water Pollutants: A Review. Appl. Chem. Eng. 2022, 33, 258–271. [Google Scholar] [CrossRef]
- Reimers, R.S.; Reinhart, D.R.; Sharma, V.K.; Austin, G.C. The Application of the Green Oxidant Ferrate for Wastewater Disinfection and Reuse to Be Utilized for Wetland Restoration, Irrigation and Groundwater Recharge. 2018. Available online: https://www.accesswater.org/?id=-294247 (accessed on 4 February 2023).
- Lim, M.; Kim, M.-J. Effectiveness of Potassium Ferrate (K2FeO4) for Simultaneous Removal of Heavy Metals and Natural Organic Matters from River Water. Water Air Soil Pollut. 2010, 211, 313–322. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, B.; Yuan, S.; Zhang, Y.; Yang, J.; Zhang, R.; Liu, L. Simultaneous Removal of CODMn and Ammonium from Water by Potassium Ferrate-Enhanced Iron-Manganese Co-Oxide Film. Water 2022, 14, 2651. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, W.; Wang, X.; Zhou, L.; Zheng, G. Spatial distribution of fecal pollution indicators in sewage sludge flocs and their removal and inactivation as revealed by qPCR/viability-qPCR during potassium ferrate treatment. J. Hazard. Mater. 2023, 443, 130262. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Zhang, S.; Petri, M.; Mosbach, C. Exploration of Ferrate(VI) Potential in Treating Lake Constance Water. Environments 2023, 10, 25. [Google Scholar] [CrossRef]
- Alsheyab, M.; Jiang, J.-Q.; Stanford, C. On-line production of ferrate with an electrochemical method and its potential application for wastewater treatment—A review. J. Environ. Manag. 2009, 90, 1350–1356. [Google Scholar] [CrossRef]
- Lipps, W.C.; Baxter, T.E.; Braun-Howland, E. (Eds.) 3125 Metals by inductively coupled plasma-mass spectrometry. In Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Heredia, N.; Solís-Soto, L.; Venegas, F.; Bartz, F.E.; de Aceituno Anna Fabiszewski Jaykus, L.-A.; Leon, J.S.; García, S. Validation of a Novel Rinse and Filtration Method for Efficient Processing of Fresh Produce Samples for Microbiological Indicator Enumeration. J. Food Prot. 2015, 78, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Caçador, I. Assessment of Extraction Methods of Trace Metallic Elements in Plants: Approval of a Common Method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- Abi Saab, M.T.; Jomaa, I.; Hage, R.E.; Skaf, S.; Fahed, S.; Rizk, Z.; Massaad, R.; Romanos, D.; Khairallah, Y.; Azzi, V.; et al. Are Fresh Water and Reclaimed Water Safe for Vegetable Irrigation? Empirical Evidence from Lebanon. Water 2022, 14, 1437. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical ## Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 9 January 2023).
- Li, N. Ferrate as a New Treatment Chemical for Removal of Effluent Organic Matter (EfOM) and Emerging Micro-Pollutants in Treated Municipal Wastewater for Water Reuse. Theses, Dissertations and Culminating Projects 37, 2017. Available online: https://digitalcommons.montclair.edu/etd/37 (accessed on 18 July 2022).
- Alshahri, A.H.; Giagnorio, M.; Dehwah, A.H.A.; Obaid, M.; Missimer, T.M.; Leiknes, T.; Ghaffour, N.; Fortunato, L. Advanced coagulation with liquid ferrate as SWRO desalination pretreatment during severe algal bloom. Process performance, environmental impact, and cost analysis. Desalination 2022, 537, 115864. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Xu, B.; Wang, L.; Ma, G.; Chen, S.; Tan, F.; Shao, Y.; Zhang, L.; Yang, Z.; et al. A novel technology using iron in a coupled process of moderate preoxidation–hybrid coagulation to remove cyanobacteria in drinking water treatment plants. J. Clean. Prod. 2022, 342, 130947. [Google Scholar] [CrossRef]
- D’Anna, F.; Incalcaterra, G.; Moncada, A.; Miceli, A. Effects of different levels of electrical conductivity on strawberry grown in soilless culture. ISHS Acta Hortic. 2003, 609, 355–360. [Google Scholar] [CrossRef]
- Abou-Hadid, A.F.; Abd-Elmoniem, E.M.; El-Shinawy, M.Z.; Abou-Elsoud, M. Effect of electrical conductivity on growth and mineral composition of lettuce plants in the hydroponic system. Acta Hortic. 1996, 434, 59–66. [Google Scholar] [CrossRef]
- Amer, K.H. Corn crop response under managing different irrigation and salinity levels. Agric. Water Manag. 2010, 97, 1553–1563. [Google Scholar] [CrossRef]
- Arienzo, M.; Christen, E.W.; Jayawardane, N.S.; Quayle, W.C. The relative effects of sodium and potassium on soil hydraulic conductivity and implications for winery wastewater management. Geoderma 2012, 173–174, 303–310. [Google Scholar] [CrossRef]
- Ali, A.; Biggs AJ, W.; Marchuk, A.; Bennett, J.M. Effect of Irrigation Water pH on Saturated Hydraulic Conductivity and Electrokinetic Properties of Acidic, Neutral, and Alkaline Soils. Soil Sci. Soc. Am. J. 2019, 83, 1672–1682. [Google Scholar] [CrossRef]
- Irrigation Water Quality Criteria-0.506. Extension. Available online: https://extension.colostate.edu/topic-areas/agriculture/irrigation-water-quality-criteria-0-506/ (accessed on 4 February 2023).
- Harris, L.J.; Berry, E.D.; Blessington, T.; Erickson, M.; Jay-Russell, M.; Jiang, X.; Killinger, K.; Michel, F.C., Jr.; Millner, P.A.T.; Schneider, K.; et al. A Framework for Developing Research Protocols for Evaluation of Microbial Hazards and Controls during Production That Pertain to the Application of Untreated Soil Amendments of Animal Origin on Land Used To Grow Produce That May Be Consumed Raw. J. Food Prot. 2013, 76, 1062–1084. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Truchado, P.; Sánchez, G.; Aznar, R.; Gil, M.I.; Allende, A. Occurrence of enteric viruses in reclaimed and surface irrigation water: Relationship with microbiological and physicochemical indicators. J. Appl. Microbiol. 2016, 121, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, M.E.; Keraita, B.; Olsen, A.; Boateng, O.K.; Thamsborg, S.M.; Pálsdóttir, G.R.; Dalsgaard, A. Use of Moringa oleifera seed extracts to reduce helminth egg numbers and turbidity in irrigation water. Water Res. 2012, 46, 3646–3656. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Khan, Z.I.; Zafar, A.; Ma, J.; Nadeem, M.; Ahmad, K.; Mahpara, S.; Wajid, K.; Bashir, H.; Munir, M.; et al. Evaluation of toxicity potential of cobalt in wheat irrigated with wastewater: Health risk implications for public. Environ. Sci. Pollut. Res. 2021, 28, 21119–21131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).