Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Photoreactor
2.3. Analytical Methodologies and Experimental Procedures for AO10 Degradation Tests
3. Results and Discussion
3.1. Photo-Decomposition of Periodate Ions
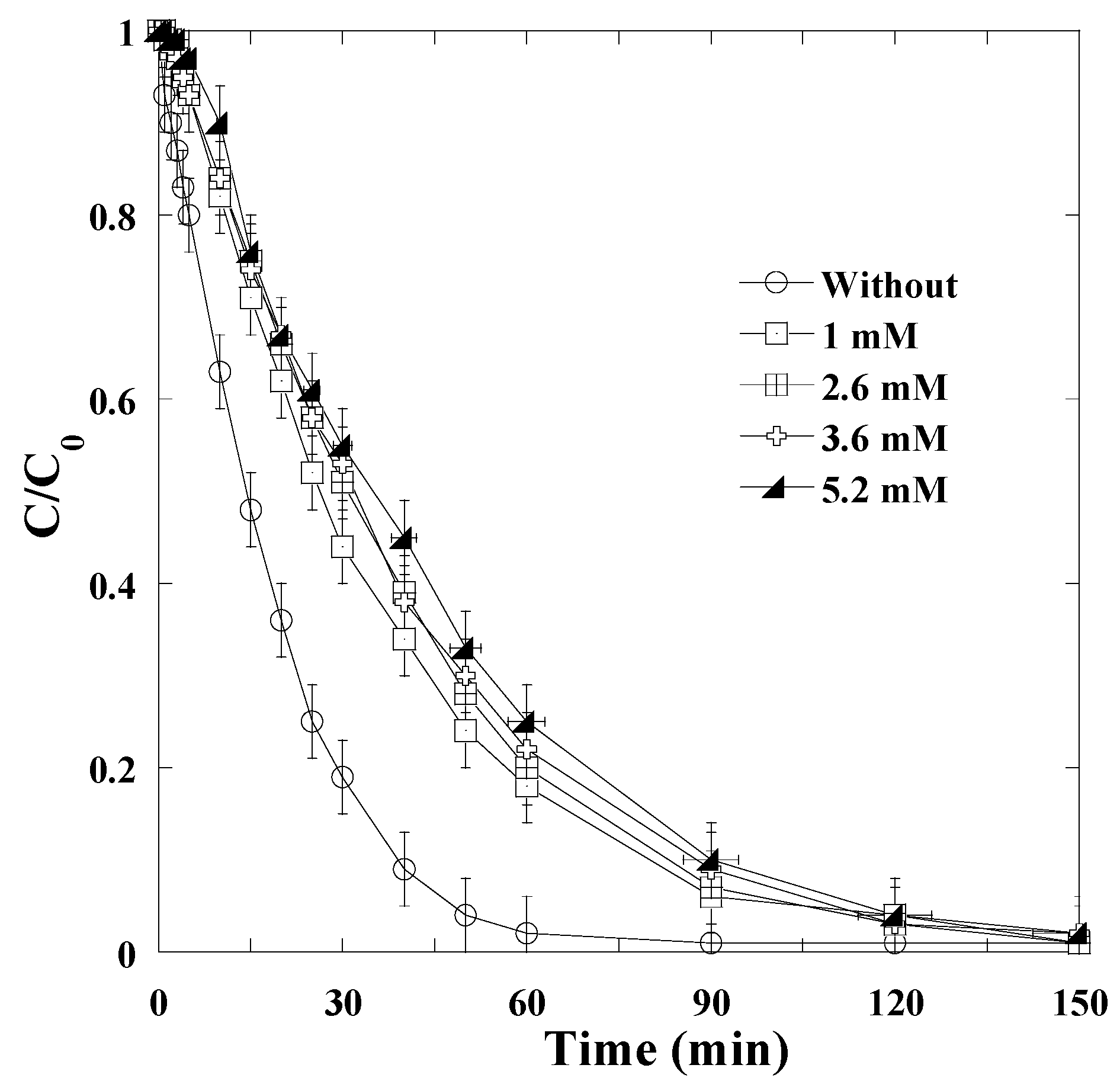
3.2. Effect of Periodate Concentration
3.3. Effect of Initial pH
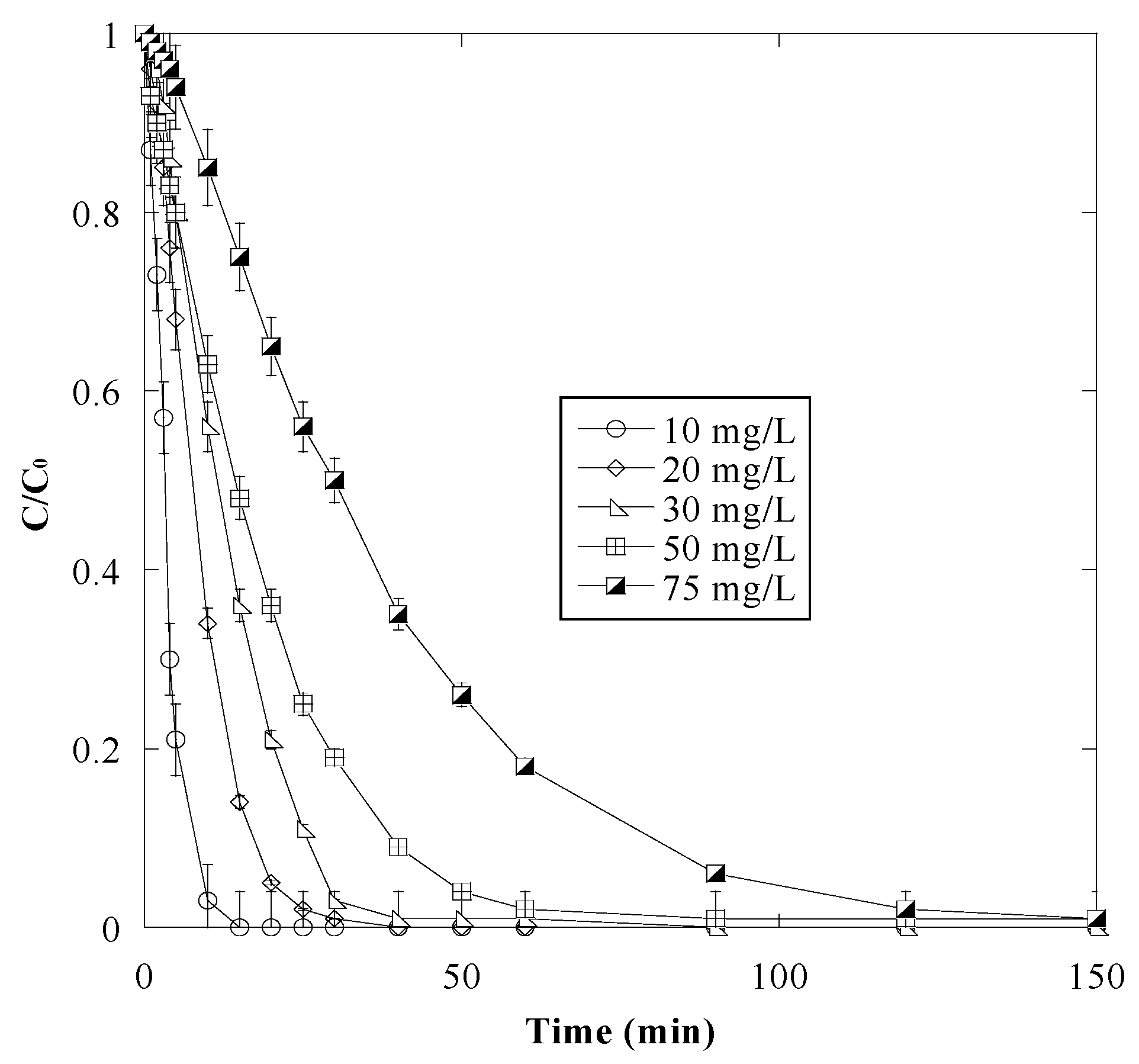
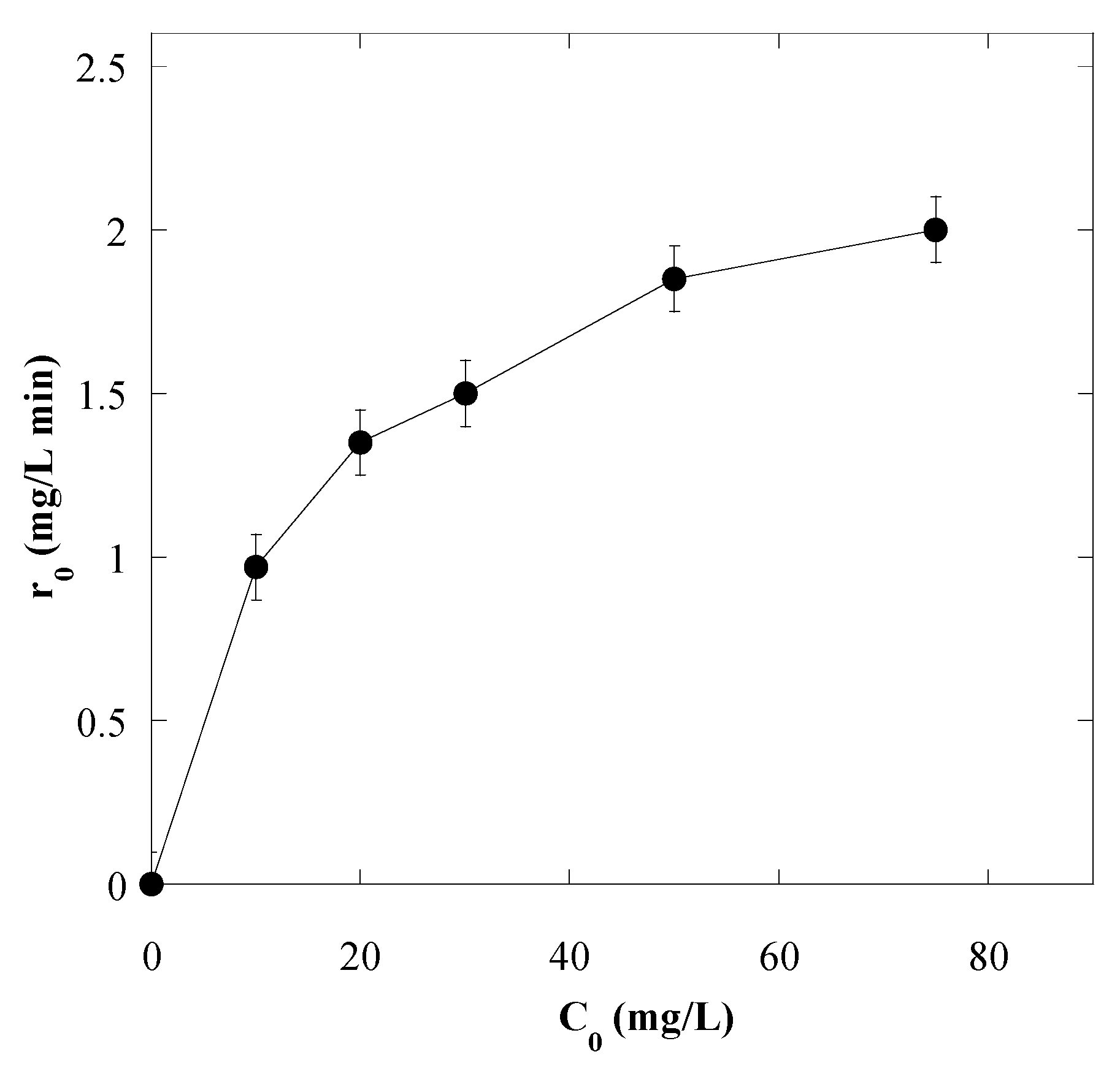
3.4. Effect of Initial Dye Concentration
3.5. Effect of Inorganic Anions
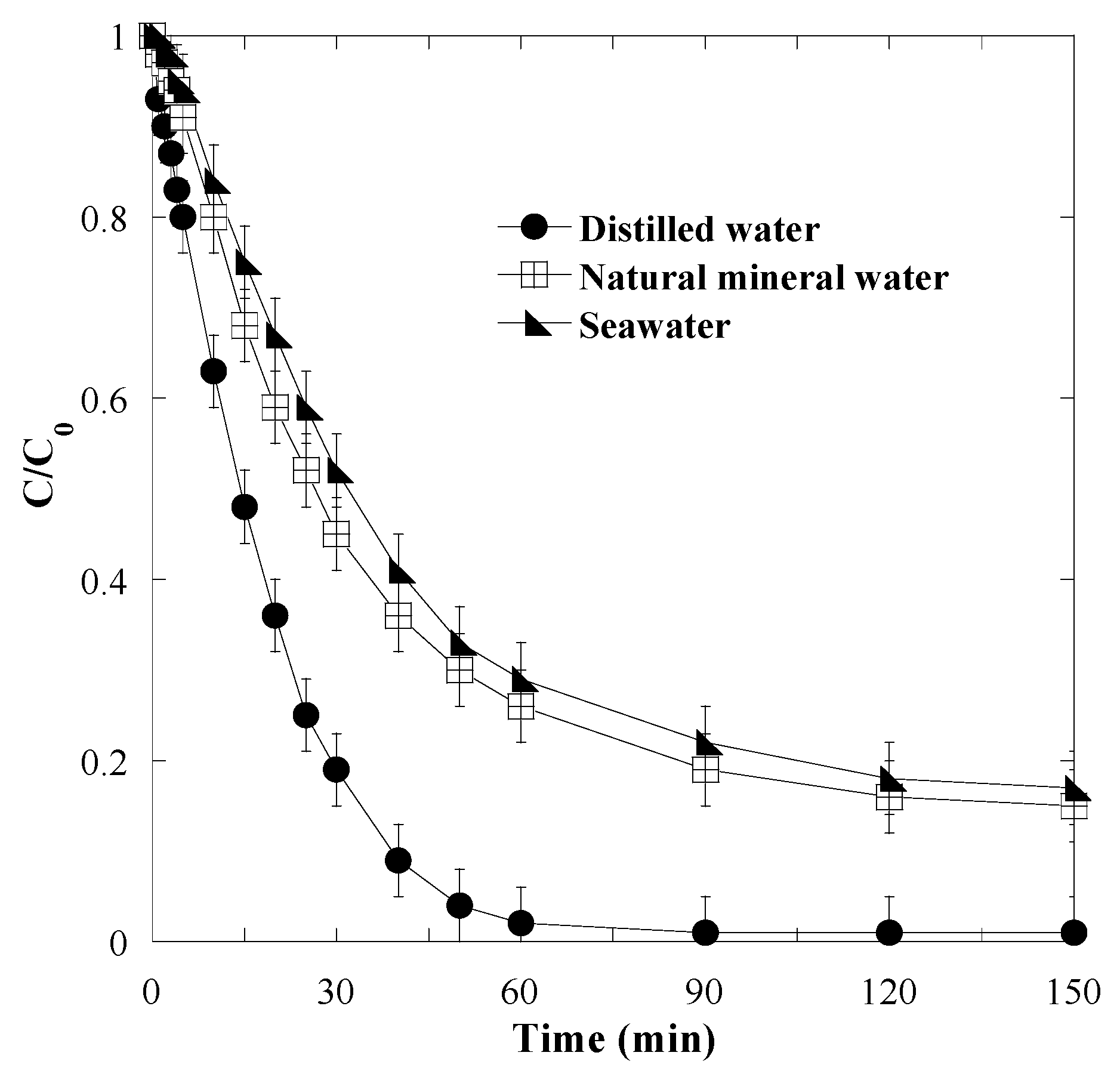
3.6. Effect of Water Matrix Components
- Natural mineral water: pH 7.2, Ca2+ = 81 mg L−1, Mg2+ = 24 mgL−1, Na+ = 15.8 mg L−1, Cl− = 72 mg L−1, SO42− = 53 mg L−1, HCO3− = 265 mg L−1.
- Seawater: pH 7.6, Ca2+ = 416 mg L−1, Mg2+ = 1295 mg L−1, Na+ = 11,600 mg L−1, Cl− = 21,400 mgL−1, SO42− = 3060 mg L−1, Br− = 66 mg L−1, Sr2+ = 27 mg L−1, B3+ = 13 mg L−1, F− = 1 mg L−1.
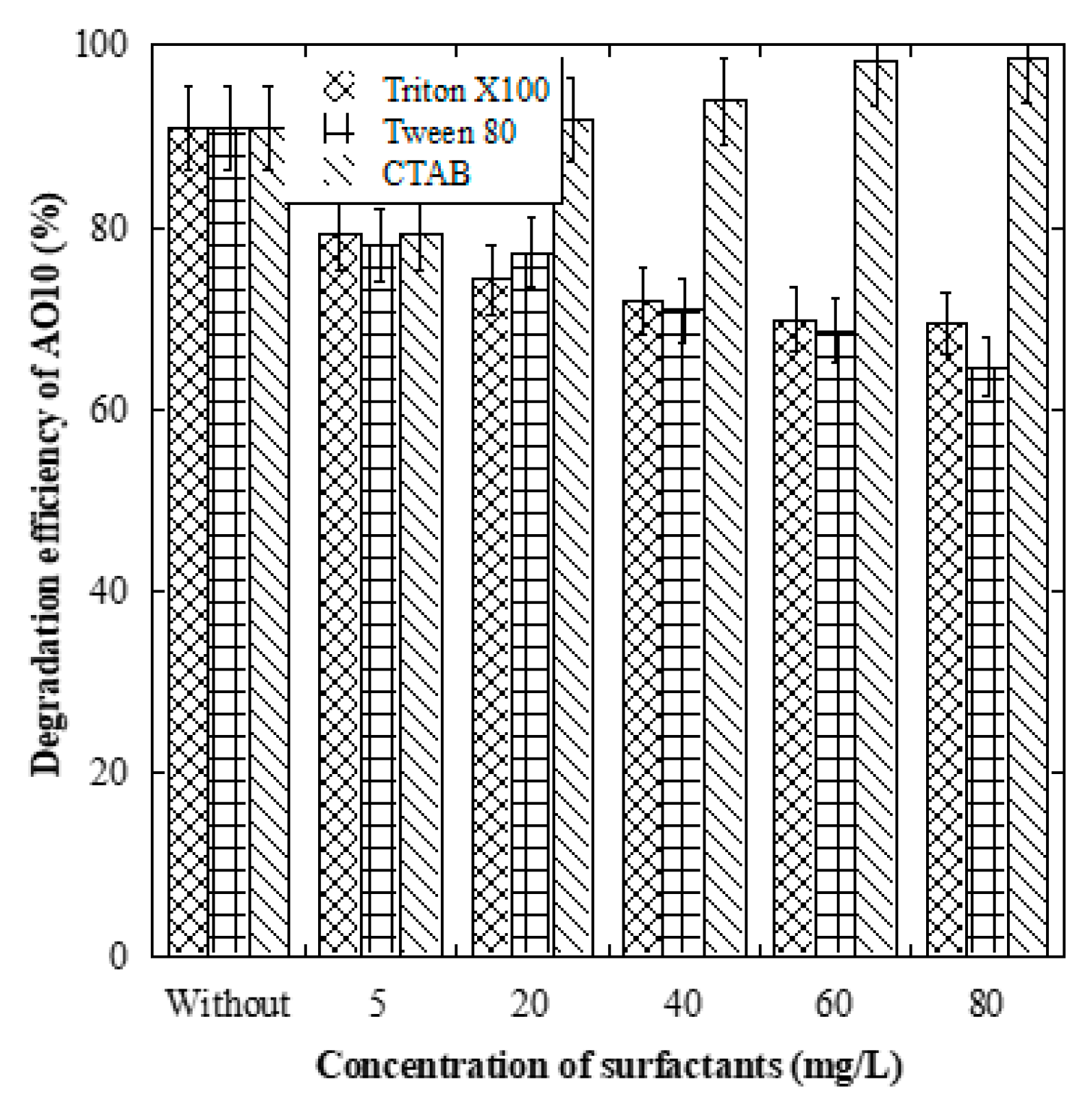
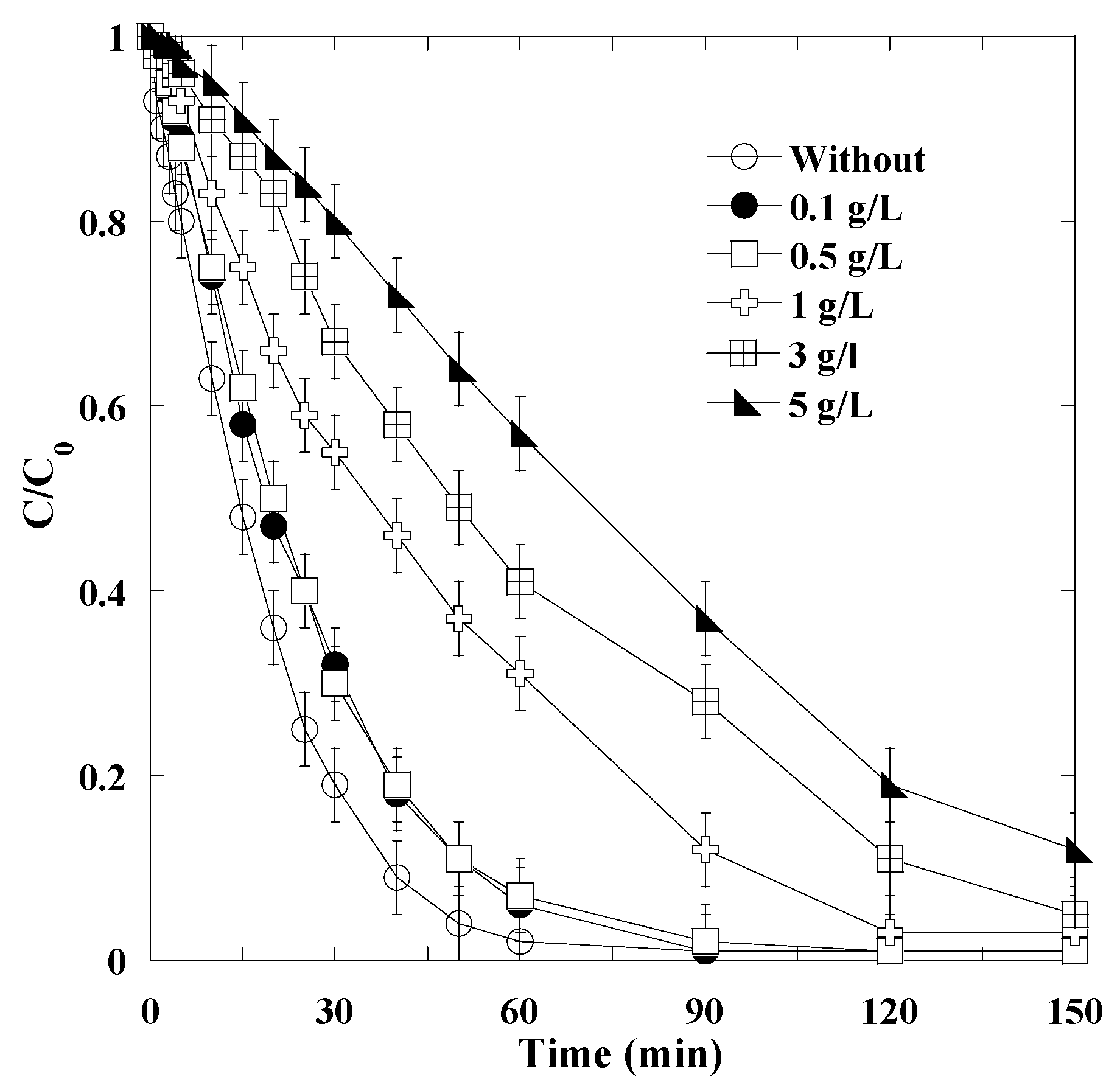
3.7. Effect of Surfactants on the Degradation of AO10
3.8. Effect of Sucrose Addition
3.9. Effect of Tert-Butanol Addition
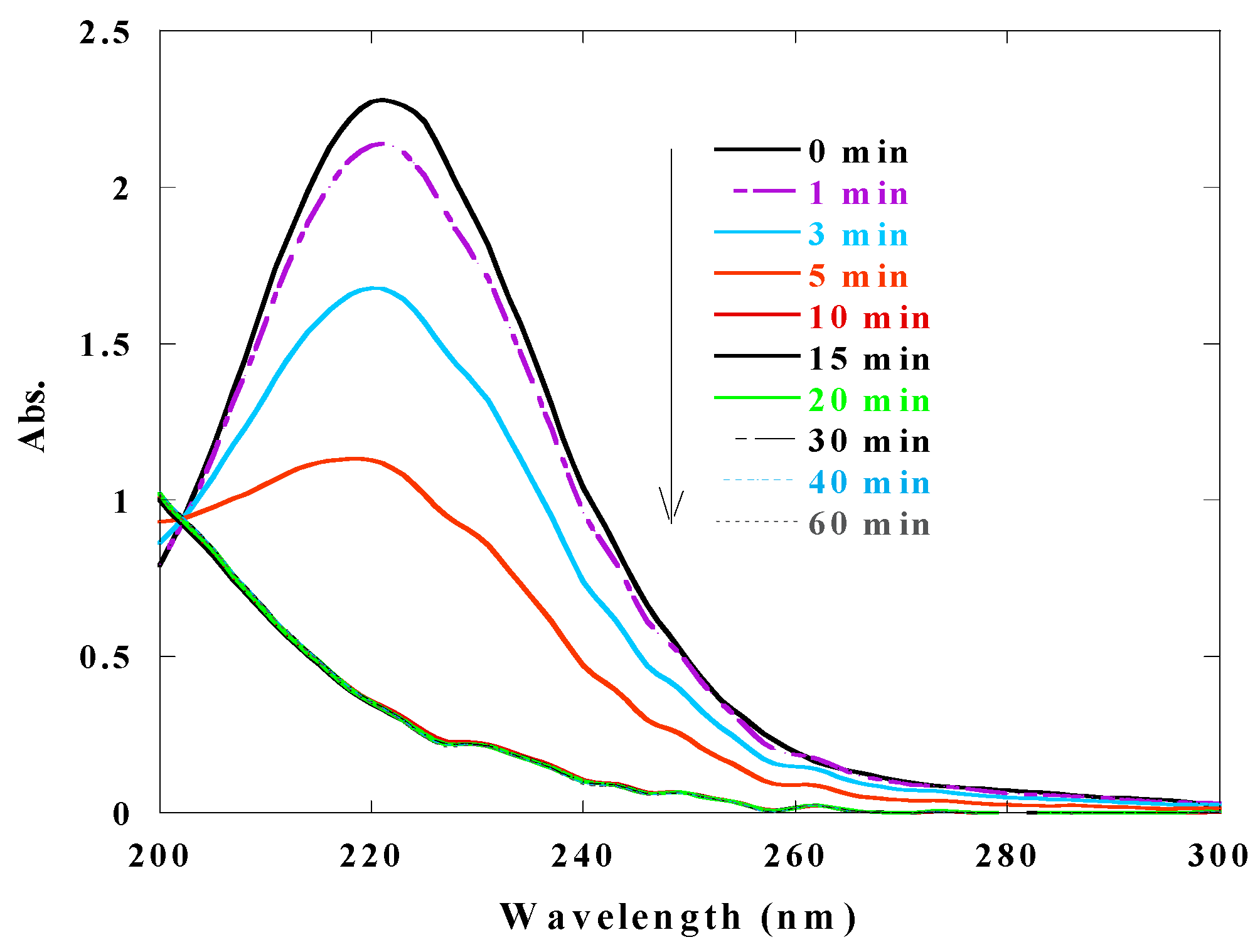
3.10. Evolution of the Spectrum of the Reaction Mixture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Govindaraju, M.; Senthamilselvi, S.; Premkumar, K. Photocatalytic degradation of methyl orange dye using silver (Ag) nanoparticles synthesized from Ulva lactuca. Colloids Surf. B Biointerfaces 2013, 103, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Nezamzadeh-Ejhieh, A.; Khorsandi, M. Heterogeneous photodecolorization of Eriochrome Black T using Ni/P zeolite catalyst. Desalination 2010, 262, 79–85. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigments 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Nachiyar, C.V.; Rajkumar, G.S. Degradation of a tannery and textile dye, Navitan Fast Blue S5R by Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2003, 19, 609–614. [Google Scholar] [CrossRef]
- Kalyani, D.C.; Patil, P.S.; Jadhav, J.P.; Govindwar, S.P. Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1. Bioresour. Technol. 2008, 99, 4635–4641. [Google Scholar] [CrossRef]
- Aquino, J.M.; Rocha-Filho, R.C.; Ruotolo, L.A.M.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of a real textile wastewater using β-PbO2 and DSA® anodes. Chem. Eng. J. 2014, 251, 138–145. [Google Scholar] [CrossRef]
- Khatri, J.; Nidheesh, P.V.; Singh, T.S.A.; Kumar, M.S. Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 2018, 348, 67–73. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Sukhatskiy, Y.; Shepida, M.; Sozanskyi, M.; Znak, Z.; Gogate, P.R. Periodate-based advanced oxidation processes for wastewater treatment: A review. Sep. Purif. Technol. 2023, 304, 122305. [Google Scholar] [CrossRef]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/Periodate (IO4−): A novel advanced oxidation technology for the degradation of refractory organic pollutants. Environ. Sci. Water Res. Technol. 2019, 5, 1113–1123. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Qi, C.; Lin, C. Activation of periodate by granular activated carbon for acid orange 7 decolorization. J. Taiwan Inst. Chem. Eng. 2016, 68, 211–217. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Qi, C.; Zhang, H.; Ma, J. Enhanced activation of periodate by iodine-doped granular activated carbon for organic contaminant degradation. Chemosphere 2017, 181, 609–618. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Shi, J.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Saien, J.; Shafiei, H.; Amisama, A. Photo-activated periodate in homogeneous degradation and mineralization of Quinoline: Optimization, kinetic, and energy consumption. Environ. Prog. Sustain. Energy 2017, 36, 1621–1627. [Google Scholar] [CrossRef]
- Barat, F.; Gilles, L.; Hickel, B.; Lesigne, B. Transient species in the pulse radiolysis of periodate ion in neutral aquesous solutions. Chem. Commun. 1971, 847, 847–848. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Bardhan, D.K. Radiolysis of aqueous solution of potassium periodate. Bull. Chem. Soc. Jpn. 1970, 43, 2808–2811. [Google Scholar] [CrossRef]
- Kläning, U.K.; Sehested, K.; Wolff, T. Laser flash photolysis and pulse radiolysis of iodate and periodate in aqueous solution. Properties of iodine (VI). J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1981, 77, 1707–1718. [Google Scholar] [CrossRef]
- Patil, S.F.; Patil, R.M.; Mudaliar, M. Effect of iso-propanol on the radiolysis of aqueous solutions of periodate. J. Radioanal. Nucl. Chem. 1990, 139, 323–329. [Google Scholar] [CrossRef]
- Wagner, I.; Strehlow, H. Flash Photolysis in Aqueous Periodate-Solutions. Ber. Bunsenges. Phys. Chem. 1982, 86, 297–301. [Google Scholar] [CrossRef]
- Chia, L.-H.; Tang, X.; Weavers, L.K. Kinetics and mechanism of photoactivated periodate reaction with 4-chlorophenol in acidic solution. Environ. Sci. Technol. 2004, 38, 6875–6880. [Google Scholar] [CrossRef]
- Ghodbane, H.; Hamdaoui, O. Degradation of anthraquinonic dye in water by photoactivated periodate. Desalin. Water Treat. 2016, 57, 4100–4109. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Merouani, S. Improvement of sonochemical degradation of brilliant blue R in water using periodate ions: Implication of iodine radicals in the oxidation process. Ultrason. Sonochem. 2017, 37, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient degradation method of emerging organic pollutants in marine environment using UV/periodate process: Case of chlorazol black. Mar. Pollut. Bull. 2018, 126, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yoon, J. Application of photoactivated periodate to the decolorization of reactive dye: Reaction parameters and mechanism. J. Photochem. Photobiol. A Chem. 2004, 165, 35–41. [Google Scholar] [CrossRef]
- Tang, X.; Weavers, L.K. Using photoactivated periodate to decompose TOC from hydrolysates of chemical warfare agents. J. Photochem. Photobiol. A Chem. 2008, 194, 212–219. [Google Scholar] [CrossRef]
- Tang, X.; Weavers, L.K. Decomposition of hydrolysates of chemical warfare agents using photoactivated periodate. J. Photochem. Photobiol. A Chem. 2007, 187, 311–318. [Google Scholar] [CrossRef]
- Weavers, L.K.; Hua, I.; Hoffmann, M.R. Degradation of triethanolamine and chemical oxygen demand reduction in wastewater by photoactivated periodate. Water Environ. Res. 1997, 69, 1112–1119. [Google Scholar] [CrossRef]
- Saien, J.; Fallah Vahed Bazkiaei, M. Homogenous UV/periodate process in treatment of p-nitrophenol aqueous solutions under mild operating conditions. Environ. Technol. 2018, 39, 1823–1832. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Yu, X.; Kamali, M.; Appels, L.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Efficiency and mechanism of 2, 4-dichlorophenol degradation by the UV/IO4−process. Sci. Total Environ. 2021, 782, 146781. [Google Scholar] [CrossRef]
- Tian, F.-X.; Hu, X.-J.; Xu, B.; Zhang, T.-Y.; Gao, Y.-Q. Phototransformation of iodate by UV irradiation: Kinetics and iodinated trihalomethane formation during subsequent chlor (am) ination. J. Hazard. Mater. 2017, 326, 138–144. [Google Scholar] [CrossRef]
- Filip, J.; Cajthaml, T.; Najmanová, P.; Černík, M.; Zbořil, R. Advanced Nano-Bio Technologies for Water and Soil Treatment; Springer Nature: Cham, Switzerland, 2020; ISBN 3030298396. [Google Scholar]
- Ilin, A.; Nersesyan, A. Toxicology of iodine: A mini review. Arch. Oncol. 2013, 21, 65–71. [Google Scholar] [CrossRef]
- Thomas, O.; Mazas, N. La mesure de la demande chimique en oxygène dans les milieux faiblement pollués. Analusis 1986, 14, 300–302. [Google Scholar]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Using photoactivated acetone for the degradation of Chlorazol Black in aqueous solutions: Impact of mineral and organic additives. Sci. Total Environ. 2019, 653, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Barat, F.; Gilles, L.; Hickel, B.; Lesigne, B. Pulsed radiolysis and flash photolysis of iodates in aqueous solution. J. Phys. Chem. 1972, 76, 302–307. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Singlet-Oxygen generation in alkaline periodate solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Klaning, U.K.; Sehested, K. Photolysis of periodate and periodic acid in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1978, 74, 2818–2838. [Google Scholar] [CrossRef]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Acetone photoactivated process: Application to the degradation of refractory organic pollutants in very saline waters. Water Environ. J. 2019, 34, 1–8. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Merouani, S. Ultrasonic destruction of acid Orange 7: Effect of humic acid, surfactants and complex matrices. Water Environ. Res. 2017, 89, 250–259. [Google Scholar] [CrossRef]
- Belghit, A.A.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Alghyamah, A.; Bouhelassa, M. Influence of processing conditions on the synergism between UV irradiation and chlorine toward the degradation of refractory organic pollutants in UV/chlorine advanced oxidation system. Sci. Total Environ. 2020, 736, 139623. [Google Scholar] [CrossRef]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. Influence of mineral water constituents, organic matter and water matrices on the performance of the H2O2/IO4−-Advanced oxidation process. Environ. Sci. Water Res. Technol. 2019, 5, 1985–1992. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017. [Google Scholar]
- Gu, X.; Lu, S.; Li, L.; Qiu, Z.; Sui, Q.; Lin, K.; Luo, Q. Oxidation of 1, 1, 1-Trichloroethane Stimulated by Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2011, 50, 11029–11036. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, R.; Guo, Y.; Xu, L.; Liu, J. Effects of chloride ions on bleaching of azo dyes by Co2+/oxone regent: Kinetic analysis. J. Hazard. Mater. 2011, 190, 1083–1087. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Ugazio, E.; Carlotti, M.E.; Sapino, S.; Trotta, M.; Vione, D.; Minero, C. Photodegradation of cinnamic acid in different media. J. Dispers. Sci. Technol. 2008, 29, 641–652. [Google Scholar] [CrossRef]
- Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient photocatalytic degradation of Safranin O by integrating solar-UV/TiO2/persulfate treatment: Implication of sulfate radical in the oxidation process and effect of various water matrix components. J. Photochem. Photobiol. A Chem. 2017, 345, 80–91. [Google Scholar] [CrossRef]
- Almarhabi, S.; Ashokkumar, M. Sonochemical degradation of p-toluenesulfonic acid in aqueous environment. Energy Environ. Focus 2015, 4, 239–244. [Google Scholar] [CrossRef]
- Destaillats, H.; Alderson, T.W.; Hoffmann, M.R. Applications of ultrasound in NAPL remediation: Sonochemical degradation of TCE in aqueous surfactant solutions. Environ. Sci. Technol. 2001, 35, 3019–3024. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravind, U.K.; Aravindakumar, C.T. Sonochemical degradation of Coomassie Brilliant Blue: Effect of frequency, power density, pH and various additives. Chemosphere 2015, 119, 848–855. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Morelli, R.; Russo-Volpe, S.; Bruno, N.; Lo Scalzo, R. Fenton-dependent damage to carbohydrates: Free radical scavenging activity of some simple sugars. J. Agric. Food Chem. 2003, 51, 7418–7425. [Google Scholar] [CrossRef]








| Structure | 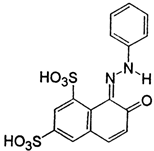 |
|---|---|
| Formula: | C16H10N2Na2O7S2 |
| Mw = 452.36 g/mol; λmax = 477 nm | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nessaibia, M.; Ghodbane, H.; Ferkous, H.; Merouani, S.; Alam, M.; Balsamo, M.; Benguerba, Y.; Erto, A. Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water. Water 2023, 15, 758. https://doi.org/10.3390/w15040758
Nessaibia M, Ghodbane H, Ferkous H, Merouani S, Alam M, Balsamo M, Benguerba Y, Erto A. Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water. Water. 2023; 15(4):758. https://doi.org/10.3390/w15040758
Chicago/Turabian StyleNessaibia, Maroua, Houria Ghodbane, Hana Ferkous, Slimane Merouani, Manawwer Alam, Marco Balsamo, Yacine Benguerba, and Alessandro Erto. 2023. "Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water" Water 15, no. 4: 758. https://doi.org/10.3390/w15040758
APA StyleNessaibia, M., Ghodbane, H., Ferkous, H., Merouani, S., Alam, M., Balsamo, M., Benguerba, Y., & Erto, A. (2023). Homogenous UV/Periodate Process for the Treatment of Acid Orange 10 Polluted Water. Water, 15(4), 758. https://doi.org/10.3390/w15040758







