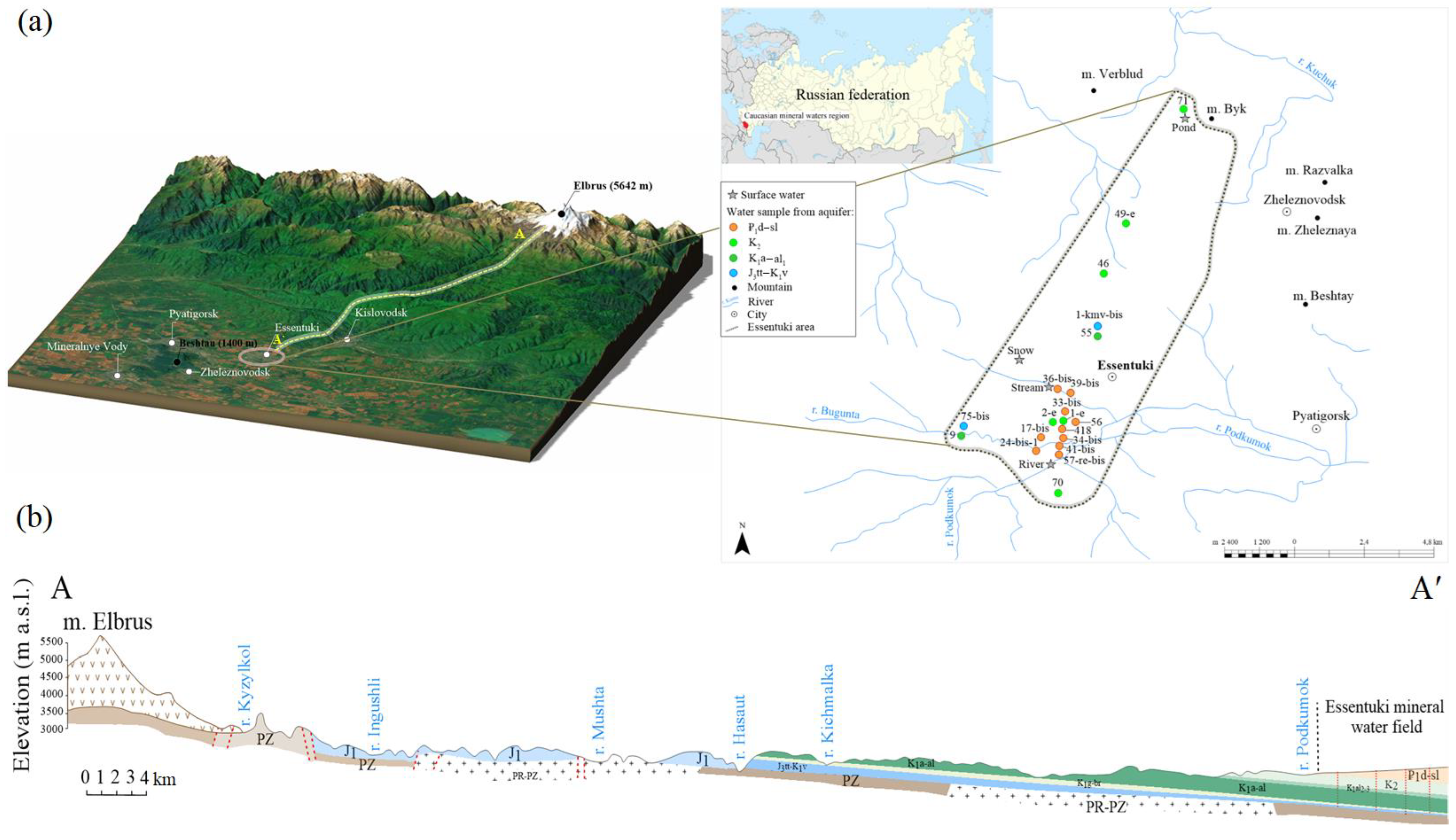
4.1. Hydrogeochemical Characteristics
Surface water streams in the region have low salinity of about 0.6 g/L and a various chemical composition; among anions, bicarbonate predominates followed by sulfate and chloride (
Table 2,
Figure 3). Calcium is predominant in the Podkumok river waters, although sodium prevails in a stream.
Pond waters are characterized by a significantly higher TDS (about 4.0 g/L) compared to surface water courses. Among anions in these waters sulfate ion prevails (2.25 g/L) followed by chlorine ion (0.4 g/L), and among cations sodium is predominant (0.63 g/L) followed by calcium (0.44 g/L) and magnesium (0.13 g/L). Atmospheric precipitation (snow) has a rather low salinity (not more than 0.02 g/L) and a mixed chemical composition (
Table 2).
All studied groundwater is weakly alkaline with pH varies from 6.3 to 8.5. While surface waters in Podkumok River and in the stream are characterized by more acidic pH values ranging from 5.7 to 6.1 (
Table 2,
Figure 4).
TDS of groundwater in the Essentuki field varies in a wide range from 0.6 to 12.8 g/L highly depending on the aquifers (
Figure 4). The waters from the Paleogene aquifer ₽
1d–sl are characterized by fairly high TDS values within 8.1–12.8 g/L (an average of 9.6). Groundwater in the Cenomanian-Maastrichtian aquifer (K
2) has a wider range of TDS values, from 0.6 to 9.8 g/L (an average of 5.3). Water sampled from wells in the Aptian-Lower Albian aquifer (K
1a–al
1) is characterized by low TDS within a narrow range of values 0.6–0.7 g/L, while mineral groundwater circulating deeper in the Titon-Valanginian aquifer (J
3tt–K
1v) has a higher salinity ranging from 2.5 to 6.5 g/L (an average of 4.5). A clear hydrogeochemical zoning is observed in the vertical section: groundwater circulating in the intermediate Aptian-Albian aquifer has significantly lower TDS than groundwater localized in rocks of the Paleogene (₽
1d–sl) and Upper Cretaceous (K
2) aquifers located structurally higher in the section, and also than groundwater from the Titon-Valanginian horizon (J
3tt–K
1v) laying lower in the section (
Figure 4). The lowest TDS (0.6 g/L) is observed for groundwater from wells 70 (K
2 aquifer) and 9 (K
1a–al
1 aquifer), located in the south part of the study area which located near in the infiltration recharge zone.
Average values main components in the studied waters according to the results of the 2019–2020 sampling are presented in
Table 2.
Bicarbonate ion is always predominant in the anion composition of mineral groundwaters from productive aquifers of the Essentuki field (
Table 2,
Figure 5). The content of sulfate ion and chlorine ion varies depending on the groundwater circulation zone, i.e., its occurrence in an aquifer (
Figure 3). In the Elburg aquifer water (₽
1d–sl), the content of HCO
3 ion among anions varies from 4360 to 6202 mg/L, Cl ion from 1376 to 2515 mg/L, and SO
4 ion is present in trace amounts except for well 24-bis-1 where the concentration of sulfate ion reaches 77.1 mg/L. The average content of dissolved carbon dioxide was 1.78 g/L. Despite the difference in TDS, all studied waters from this aquifer share the same chemical water type and genesis (
Table 2).
In the Cenomanian-Maastrichtian aquifer waters (K2), the content of HCO3 ion among anions varies from 370 to 4934 mg/L, Cl ion from 22 to 1801 mg/L, and SO4 ion was found in follow wells: 1-e and 2-e (H2S-bearing water) and 70 (fresh water) located close to the recharge zone. The content of sulfate-ion was 26.38, 23.11 and 47.21 mg/L accordingly. The concentration of dissolved CO2 gas varies very significantly from well to well and strongly depends on their location. The highest content of CO2 was found in the wells 46 (0.8 g/L) and the lowest in well 70 (0.05 mg/L).
In waters of the Aptian-Lower Albian aquifer (K1a–al1), the content of HCO3 ion (216 to 276 mg/L) is followed by SO4 ion (125 to 159 mg/L), and then by Cl ion (43 to 59 mg/L). The content of dissolved CO2 is low ranging from 0.14 to 0.41 g/L.
In the Titon-Valanginian aquifer waters (J3tt–K1v), the average concentration of HCO3− reaches 3064 mg/L, Cl−—56 mg/L, and SO42−—829 mg/L. The average content of dissolved carbon dioxide is high (1.46 g/L), close to that in waters of the Elburg aquifer. A very high gas factor of 17.5–31.5 m3/m3 is observed in groundwater from this aquifer.
An increased concentration of sulfate ion in water produced by the wells from the Aptian-Lower Albian (K1a–al1) and Titon-Valanginian (J3tt–K1v) aquifers compared to water from other productive horizons of the Essentuki field reflects the lithological composition of water-bearing rocks. It is discussed further in determining the isotopic composition of sulfate sulfur in groundwater.
The maximum concentration of HCO
3− is typical for waters of the Cenomanian-Maastrichtian aquifer (K
2), and the minimum concentration for the Aptian-Lower Albian aquifer (K
1a–al
1). The direct dependence of the studied waters’ TDS on HCO
3− content (
Figure 6) indicates that TDS of CO
2-rich waters is controlled mainly by the amount of bicarbonate ion, which is formed by the dissolution in water of CO
2 gas migrating from subsurface through open faults and tectonic deformation zones.
Chloride ion content also significantly affects the value of TDS, which is confirmed by their direct correlation (
Figure 6). The elevated Cl
− concentration in mineral waters of the Essentuki field is associated with the salt complex of sedimentation pore waters of marine genesis, which is partially preserved in weakly permeable blocks of terrigenous-carbonate rocks of the Cenomanian- Maastrichtian aquifer (K
2) [
9,
10].
Na
+ is predominant among the cations in all studied samples, while other cations have sharply inferior concentrations (
Figure 5).
Figure 6 shows a direct dependence of water salinity on sodium ion content at R
2 = 0.96.
The Gibbs diagram in the
Figure 7 shows that all samples of the studied natural mineral waters of different aquifers are located in the zone of rock dominance. This indicates the process of interaction between water and rocks, which is one of the main mechanisms controlling the chemical composition of studied groundwaters.
Calculations of the saturation index (SI) have shown that almost all the studied waters from different aquifers are oversaturated with quartz, hematite, goethite, dolomite, chalcedony, calcite, aragonite and albite (
Figure 8).
4.4. Isotopic Composition of Groundwater
The content of stable oxygen and hydrogen isotopes (δ
18O
SMOW and δD
SMOW) varies greatly in studied mineral waters: from −13.75 to −9.69‰, and from −101.08 to −74.34‰, respectively. Isotopic composition of surface waters ranges smaller: from −10.00 to −7.10‰ for δ
18O
SMOW, and from −72.30 to −61.00‰ for δD
SMOW (
Figure 10,
Table 4). Isotopic composition of the snow has very light values for both oxygen −29.00‰ and hydrogen −225.30‰, which indicates that it was formed at a high altitude.
The mineral waters of the Elburg (₽
1d–sl) and Cenomanian-Maastrichtian (K
2) aquifers are close to each other, as well as to atmospheric precipitation and surface waters of the region by their δ
18O
SMOW and δD
SMOW data (
Table 4). The binary diagram (
Figure 10) shows that the majority of figurative points of mineral waters in these productive aquifers are located along the global meteoric water line (GMWL) indicating their infiltration genesis. Also, the recharge area of the Elburg and Cenomanian-Maastrichtian aquifers is very compact, forming a cloud, suggesting a similar genesis for groundwater of both aquifers. The absence of a significant «right shift δ
18O
SMOW» that could indicate rock-water oxygen isotope exchange (Δ
18O
water-rock) under increased temperatures points to an active groundwater hydrodynamic regime in the aforementioned horizons and a weak interaction between groundwater and host-rocks. A slight deviation of some figurative points of groundwater in the Elburg aquifer and the pond water sample from the GMWL apparently reflects the initial evaporative concentration processes in the surface water (
Figure 10). Well-known that heavy isotope of hydrogen and oxygen accumulate in water during evaporation.
Mineral waters of the Aptian-Lower Albian (K1a–al1) and Titon-Valanginian (J3tt–K1v) aquifers differ from waters in the overlying horizons by much lighter values of δ18OSMOW and δDSMOW, while still being located on the GMWL, which indicates their infiltration genesis. The most light values of δ18OSMOW (from −13.75 to −12.91‰) and δDSMOW (from −101.08 to −93.49‰) registered for the Aptian-Lower Albian aquifer waters are probably caused either by a greater share of winter precipitation in the aquifer groundwater recharge, or by the location of their recharge area on structurally higher relief levels.
In summary, the water phase of mineral waters of the Essentuki field, undoubtedly, has meteoric genesis, and the ion-salt composition of the Essentuki type mineral water forms through its interaction with the layer of water-bearing carbonate-terrigenous rocks. Similar chemical composition and most importantly, identical distribution of the major components in mineral waters of Paleogene and Upper Cretaceous horizons indicate a common genesis of waters (
Figure 3). Therefore, all studied mineral waters are originally meteoric, and the current chemical composition is the result of a combination of hydrogeochemical and hydrobiochemical processes in the system groundwater-rock-gas-living matter. During the circulation originally meteoric water interacts with water-bearing rocks and the metamorphization of water occurs, in which the content of salts of marine genesis starts to increase and sodium predominates in the chemical composition of water. The recharge area of mineral waters of the field is located in the spurs of the Caucasus Mountains at 615–2100 m altitude, and the recharge area of waters circulating in the Aptian-Albian horizon is structurally higher than the recharge area of waters from the Titon-Valanginian horizon.
The obtained data are in agreement with the earlier studies of genesis and the circulation ways of the Essentuki field mineral waters [
1,
9,
12,
13,
14,
15]. Debatable idea about the genesis aqueous and gaseous phases from CO
2-rich mineral waters located within the Caucasus region are considered in the works [
9,
12,
13]. Works [
1,
15] are devoted to the geological structure and hydrogeological conditions of Caucasus region. Paper [
14] represents the results of the estimation of mineral water supply within the Essentuki field.
In addition, the analysis of the data obtained confirms the views that the Essentuki type CO
2-rich waters penetrate to the field from the northern part of the region in an already practically formed state, while the long-term circulation of waters in the Elburg marls does not change their chemical type [
7,
16]. The exact type of water (Essentuki-4 or Essentuki-17) depends on the mixing ratio of fresh groundwater coming from the southern recharge areas with high TDS mineral water moving from the north from the Nagut flexure.
H
2S-bearing mineral waters of the Essentuki field produced by wells 1-e and 2-e from the Cenomanian-Maastrichtian aquifer are similar to CO
2-rich waters by their δ
18O
SMOW and δD
SMOW data, which confirms their identical genesis (
Table 4,
Figure 10). Lower TDS values (1.0–3.0 g/L) are caused by a significant dilution of the «initial» Essentuki CO
2-rich mineral waters with high TDS by fresh groundwater from the local recharge area (e.g., well 70). The genesis of dissolved H
2S (up to 22 mg/L) in these waters is debatable. Most likely, the presence of dissolved H
2S in water is caused by anaerobic sulfate reduction reactions that occur during the interaction of local groundwater containing sulfate ion in significant concentrations with water-bearing rocks (limestone and marl) [
17].
4.5. Isotopic Composition of Carbon Bicarbonate Ion
Isotopic composition of carbon bicarbonate ion (δ
13C
DIC) clarifies the source of water dissolved carbon in the studied mineral waters. The values of δ
13C
DIC in the Essentuki mineral waters vary in a wide range from −14.43 to +8.59‰, revealing a polygenetic carbonate source (
Figure 11,
Table 4). The heaviest values of δ
13C
DIC were observed in water sample from the Titon-Valanginian aquifer (well 75-bis), which indicates that bicarbonate ion enters the water in the well mainly due to the dissolution of carbonate rocks. Waters of the same horizon sampled from well 1-kmv-bis are also characterized by positive values +2.97‰ which are typical for carbonate water-bearing strata. Lighter δ
13C
DIC values indicate a significant admixture of magmatic carbonic acid coming through deep faults and tectonic disturbance zones from the Proterozoic-Paleozoic basement.
The lightest isotopic composition of δ13CDIC (−14.43‰ and −10.95‰) is typical for samples taken from the Aptian-Lower Albian aquifer (wells 9 and 55). These low values of δ13CDIC indicate a mixed carbonate genesis in these waters: the greater part is a biogenic (soil) component and the minor part comes from the dissolution of carbonate material of water-bearing strata. Since the amount of soil carbonic acid is insignificant and the deep carbonic acid fluid does not enter this horizon, the interaction reactions in the water-water-bearing strata system (terrigenous rocks of the horizon) are very weak at temperatures up to 45 °C and the water is formed fresh.
The values of δ
13C
DIC in waters drawn by wells 71, 46, and 49 from the Cenomanian-Maastrichtian aquifers (K
2) aquifer are positive and quite close to 1.68, 2.11 and 2.10‰, respectively. Such similar values indicate a single source of carbonates in these waters, the carbonate material of water-bearing strata. H
2S-bearing and fresh waters of this horizon are characterized by a lighter carbon isotopic composition (
Table 4), which is caused by the presence of significant amounts of soil carbonic acid in them. The intermediate δ
13C
DIC value in waters of wells 1-e and 2-e confirms their formation as a result of mixing of local fresh waters and CO
2-rich mineral waters moving from the north.
The Elburg horizon waters (₽
1d–sl), except for waters in wells 34-bis, 33-bis, 36-bis, and 56, are characterized by about zero or weakly negative δ
13C
DIC values (
Table 4). This lightening of the isotopic value of dissolved carbon is the result of δ
13C
DIC fractionation during mixing of deeper CO
2-rich mineral waters with fresh waters from local recharge zones. Strongly negative δ
13C
DIC values in wells 34-bis, 33-bis, and 36-bis (−10.93; −13.73 and −9.34‰, respectively) are caused by changes in δ
13C
DIC values when using gaslift during well operation, which ensures water saturation with carbonic acid. The depleted δ
13C
DIC values in well 56 are apparently caused by biogenic carbonic acid entering the water (from soil or formed during the oxidation of dispersed organic matter of the Elburg marl), since bicarbonate ion inherits the isotopic composition of the initial carbon dioxide.
In general, it is evident that highly saline groundwater, except for forced gaslift waters, contains more isotope-heavy carbonic acid than waters with low TDS (
Figure 11). The obtained δ
18O
DIC values are negative ranging from −14.52 to −6.58‰ (
Table 4).
Mineral waters of the Essentuki field are characterized by high gas saturation (0.5–3.5 dm3/dm3) varying from well to well. In the deepest Titon-Valanginian aquifer, which lies directly on crystalline rocks of the basement, abnormally high content of carbon dioxide was observed, while the intermediate Aptian-Lower Albian aquifer in this field is characterized by the lowest gas content (it is often almost completely absent).
Uneven gas saturation of groundwater in the Essentuki field, which is typical for the Titon-Valanginian horizon, is limited by the openness (or closure) of tectonic faults in the Proterozoic-Paleozoic basement. A zone of increased fracture permeability confined to the deep latitudinal North-Buguntinsky fault was reported in the area of well 75-bis [
14].
4.6. Gas Composition of Groundwater
Generally, CO
2 is predominant in the free gas phase of the Essentuki field waters (its content often reaches 98 volume%), followed by N
2 (up to 66 vol.%), and NH
4 (up to 29 vol.%). Oxygen (13 vol.%), argon (1.86 vol.%), and helium (0.98 vol.%) are present in insignificant amounts (
Table 5,
Figure 12).
N2/Ar ratio values vary from 1 to 190. Its lowest values (1–15) are common for samples taken from wells draining water from the Titon-Valanginian horizon (J3tt–K1v). In the gases samples (wells 9 and 55) taken from the Aptian-Lower Albian (K1a-al1) aquifer, N2/Ar values range from 60 to 100, which is higher than the values of this ratio in the air. In free gas samples taken from CO2-rich mineral waters of the Cenomanian-Maastrichtian aquifer, N2/Ar ratio values range 1–40, while in H2S-bearing waters from this aquifer this ratio reaches higher values of 72–96. Fresh water from well 70 is characterized by a very high N2/Ar value (280), much higher than N2/Ar in the air, probably indicating a non-atmospheric nitrogen admixture. In gas samples from the Elburg horizon, N2/Ar values vary from well to well within the 60–270 range.
To identify the genesis of nitrogen we studied δ
15N in the N
2 free gas from several wells, and additional data on δ
15N were taken from the manuscript [
18]. δ
15N
gas values in mineral waters of the Essentuki field vary quite widely from −2.31 to 2.50‰ (
Table 6) indicating a different source of this gas. Typically atmogenic N
2 (δ
15N close to 0 ± 0.5‰) is characteristic of most mineral waters from wells draining water from the Paleogene (wells 57-re-bis, 41-bis, 418-bis, 56, and 39-bis), and Cenomanian-Maastrichtian aquifers (except for well 71). Gas phase of samples taken from wells 34-bis, 17-bis, and 36-bis shows weakly negative δ
15N values from −1.54 to −2.31‰. N
2 from the deepest well 1-kmv-bis (J
3tt-K
1v aquifer), is also characterized by isotope-light δ
15N values of −2.0‰. These values indicate a possible admixture of mantle nitrogen (δ
15N
mantle ≈ −5‰) in N
2gas of mineral waters according to [
19]. Isotopic heavy nitrogen δ
15N (from +1.2 to +2.5) was observed in the associated gas from wells 55, 71, and 24-bis-1 (
Table 6). These values indicate an admixture of crustal nitrogen (δ
15N
crust ≈ +6‰) according to [
19]. Therefore, the isotopic characteristics of nitrogen in the gas phase of the Essentuki field indicate its polygenetic genesis. N
2 is predominantly of atmospheric origin, but in some local environments an additional source of gas is possible either from deep parts of the Earth’s crust (N
mantle) or as a result of the degradation of water-bearing strata (N
crust).
4.7. Isotopic Composition of Carbon in the Gas Phase
δ
13C
CO2 and δ
13C
CH4 revealed the genesis of carbon-bearing gases in the mineral waters of the Essentuki field (
Figure 13,
Table 6). δ
13C
CO2 values vary in a wide range from −23.34 to −6.03‰, and the maximum δ
13C
CO2 (−1.96 ÷ −2.46‰) are typical for gases from the deepest Titon-Valanginian aquifer. Methane is practically absent in free gases from this horizon. These figures are very close to δ
13C
CO2 (δ
13C
CO2carb. varies from −2 to +2‰) which was formed during the thermal decomposition of carbonate complexes. Two sources of CO
2 are most probable here: a significant part is supplied during the metamorphogenic transformation of the carbonate bed, and a minor admixture of mantle gas from the basement through open fractures cannot also be ruled out.
Figure 13 clearly shows a group of wells (36-bis, 33-bis, and 34-bis) delivering mineral water from the Elburg horizon strongly depleted with δ
13C
CO2, which are typical for biogenic gases [
20]. As discussed earlier, these wells are operating in a forced gaslift mode and the values obtained show δ
13C
CO2 fractionation during this process. Other wells draining water from the ₽
1d–sl stratum on the contrary enriched with δ
13C
CO2 −9.59 to −6.87‰ (
Table 6). Very similar δ
13C
CO2 (−9.52 ÷ −6.03‰) are typical for mineral waters from the Cenomanian-Maastrichtian (K
2) aquifer. These similar δ
13C
CO2 data confirm the hypothesis of a single source of CO
2 gas in mineral waters in both aquifers, which is probably the mantle CO
2 with the values of −8 ÷ −3‰ [
21]. The low δ
13C
CO2 data recorded in a number of wells (24-bis-1, 1-e, and 2-e) possibly indicate the input of the biogenic CO
2.
The content of δ
13C
CO2 in waters from the Aptian-Lower Albian (K
1a–al
1) aquifer are quite low (−17.35 ÷ −16.37‰) and close to the upper threshold of biogenic (soil) CO
2 formed by the oxidation of organic matter. δ
13C
CO2soil varies from −30 to −18‰ [
22] suggesting the soil genesis of this gas.
Therefore, it is possible to argue that CO2 gas in mineral waters of the Essentuki field does not have a single source, but it is a mixture of gases of mantle, biogenic, and metamorphogenic origin.
δ
13C
CH4 values are very similar in all studied samples (except for well 55). They vary insignificantly from −66.41 to −59.20‰ indicating this methane is of biogenic genesis (
Figure 13). The only exception is the sample taken from well 55 (K
1a–al
1 aquifer) characterized by heavier δ
13C
CH4 values (−23.66‰), which are typical for abiotic methane (
Figure 13).
4.8. Isotopic Determinations of Sulphate Forms of Sulfur
In order to identify the sulfate-ion genesis we carried out the δ
34S
VCDT determination in several groundwaters. In general, the groundwaters within the Essentuki field are characterized by insignificant concentrations of sulfate ion, which increase further down the section (
Table 2). The highest concentrations of SO
42− were determined in waters from the Titon-Valanginian (J
3tt–K
1v) aquifer (up to 831.1 mg/L), whereas samples from of the Elburg (₽
1d–sl) and Cenomanian-Maastrichtian (K
2) aquifers content the negligible amount of the sulfate ion (less than 10 mg/L), excluding waters from four wells: 24-bis-1, 70, 1-e and 2-e (
Table 2). Waters taken from K
1a–al
1 aquifer contain 142.1 mg/L of sulfate ion on average.
δ
34S
VCDT data demonstrated that mineral waters of the Elburg horizon (well 24-bis-1) are enriched with δ
34S
VCDT +17.2‰ (
Table 7,
Figure 14), which corresponds to the isotopic composition of sulfates from marls of the Elburg horizon where δ
34S
VCDT ≈+12.4‰, according to [
23]. A comparable isotopic value of δ
34S
VCDT is observed in waters of the Titon-Valanginian (J
3tt–K
1v) horizon, where δ
34S
VCDT also has positive values +17.3 ÷ 17.8‰. Similar values (+12.2 ÷ 20.4‰) were reported in gypsum and anhydrite forming this complex [
24].
Mineral waters from the Cenomanian-Maastrichtian (K
2) aquifer are also characterized by positive but lower values of δ
34S
VCDT = +2.6 ÷ 4.7‰ (
Table 7,
Figure 14) compared to waters of overlying and underlying aquifers. δ
34S
VCDT values of carbon-free waters (well 70) and H
2S-bearing waters (wells 1-e and 2-e) are close, which indicates a single source of sulfur in these waters.
Negative values δ
34S
VCDT are typical both for waters of the Aptian-Lower Albian aquifer (from −16.8 to −23.6‰) and for pyrite from the Lower Cretaceous sandstone (δ
34S
VCDT = −11.0‰) [
23]. It is likely that sulfate in waters of this aquifer complex is formed by the dissolution of sulfides in sandstone and mudstone.
This means that SO42− appears in mineral waters of different aquifers of the Essentuki field during the dissolution of water-bearing strata and there is no evidence of possible groundwater exchange between the aquifers. In general, the dissolution of gypsum and anhydrite of water-bearing sediments causes the appearance of SO42− in waters of the Elburg and Titon-Valanginian aquifers. Sulfate ion come to the Aptian-Lower Albian horizon waters during the oxidation of pyrite spreading within host-rocks. Besides, additional sulfate come to CO2 rich mineral water K2 aquifer from the aquifer recharge area.
The main sources of H
2S
dis. in waters from wells 1-e and 2-e are the products of sulfate-reducing bacteria which produce H
2S by consuming organic matter from sedimentary rocks in the area. According to previous studies [
23], almost all groundwater in main productive aquifers of the studied region contains sulfate-reducing bacteria which actively grow on various nutrient media (water-dissolved organic matter, hydrogen, methane, etc.). As a result of bacterial anaerobic sulfate reduction of source waters with significant sulfate ion content, sulfate concentration is notably reduced and dissolved hydrogen sulfide is formed. The data we obtained are in good agreement with the available literature on the formation of sulfurous carbonated waters in the Essentuki field [
23,
24,
25].