Coagulation Combined with Electro-Fe0/H2O2 Reaction for Effective Treatment of Landfill Leachate Effluent of Membrane Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leachate and Material
2.2. Chemicals
2.3. Experimental Procedure
2.3.1. Coagulation Process
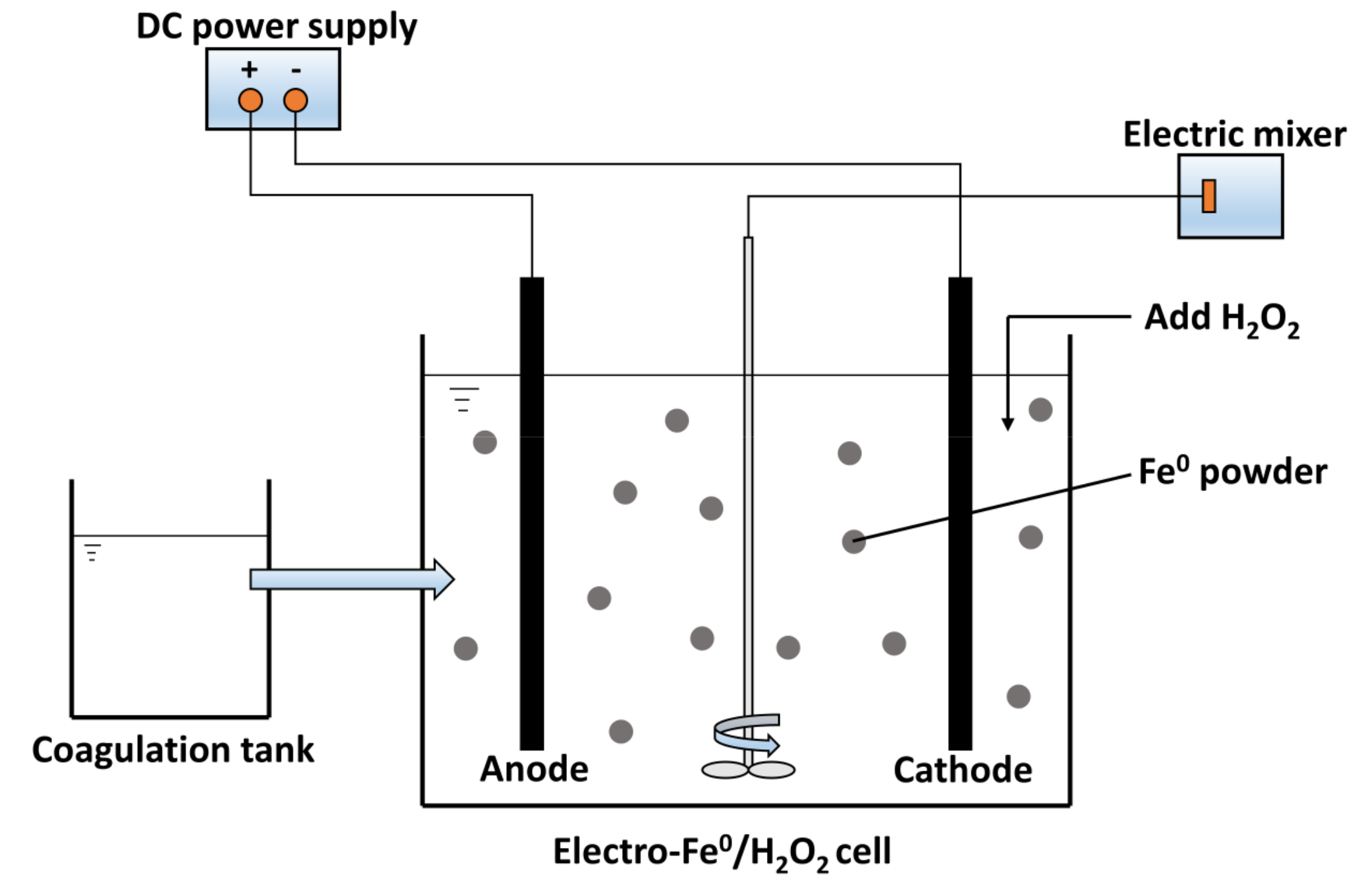
2.3.2. Electro-Fe0/H2O2 Reaction
2.4. Analytical Methods
3. Results and Discussion
3.1. Coagulation Process
3.2. The Electro-Fe0/H2O2 Reaction
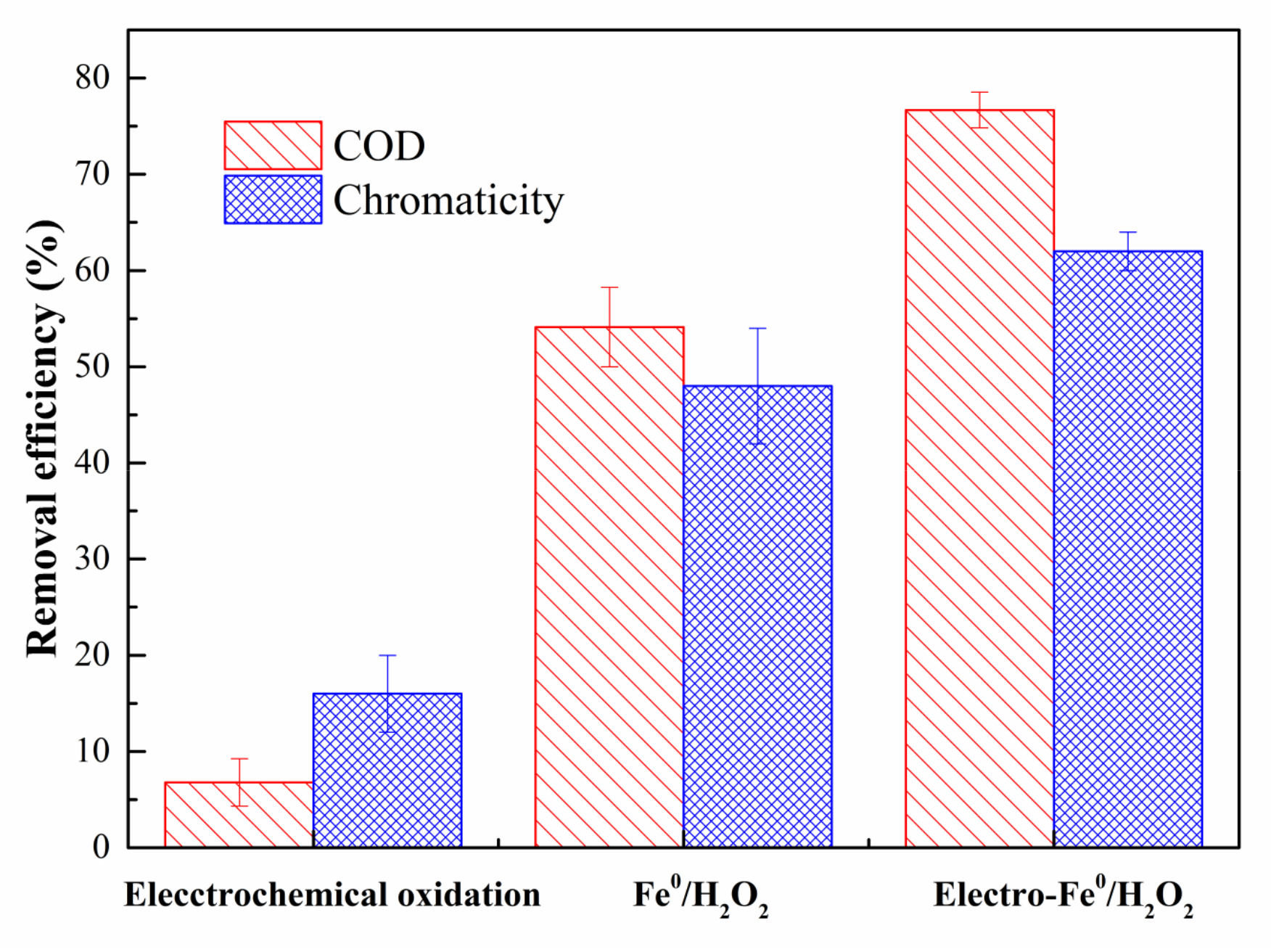
3.2.1. Comparison Experiments
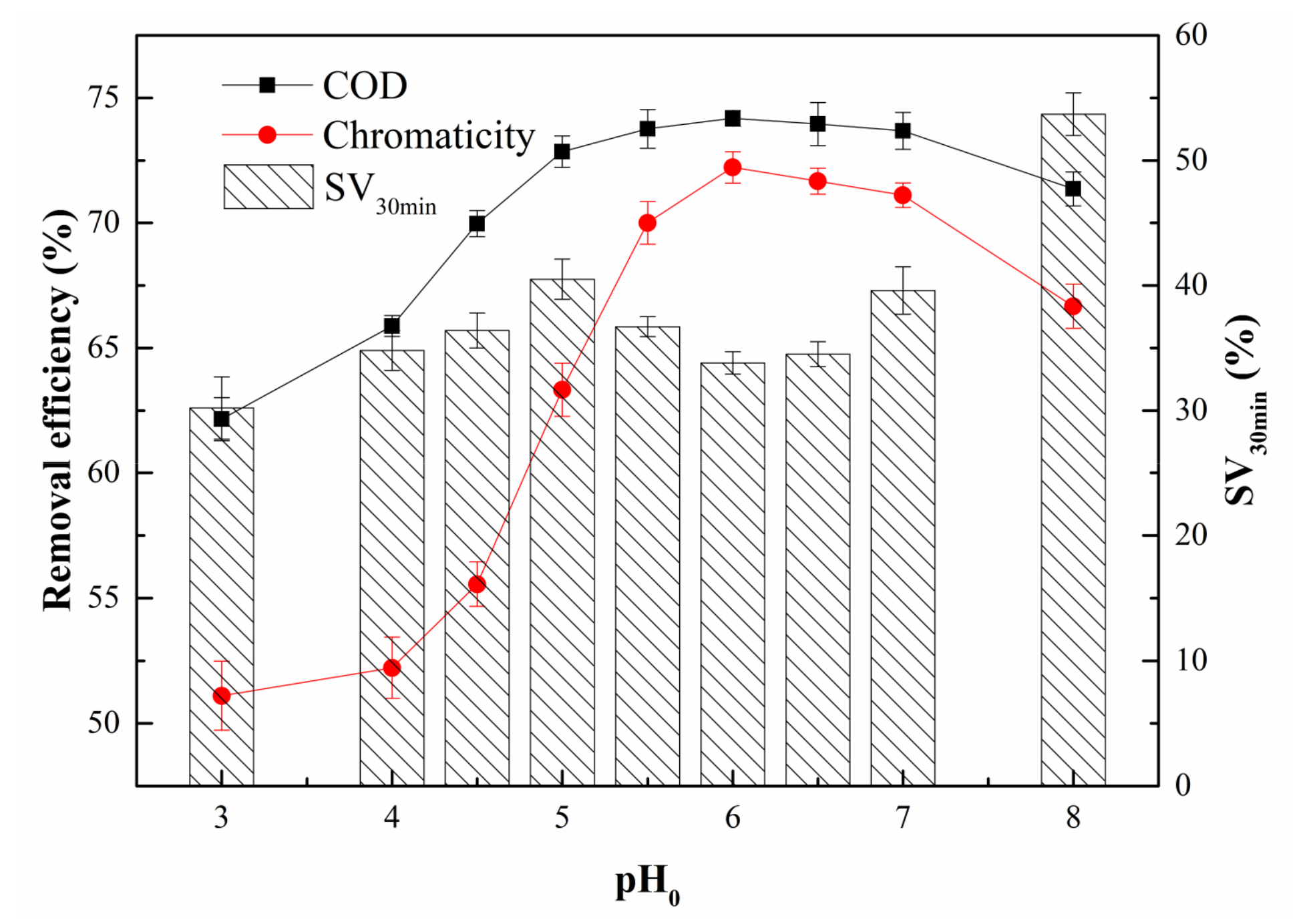
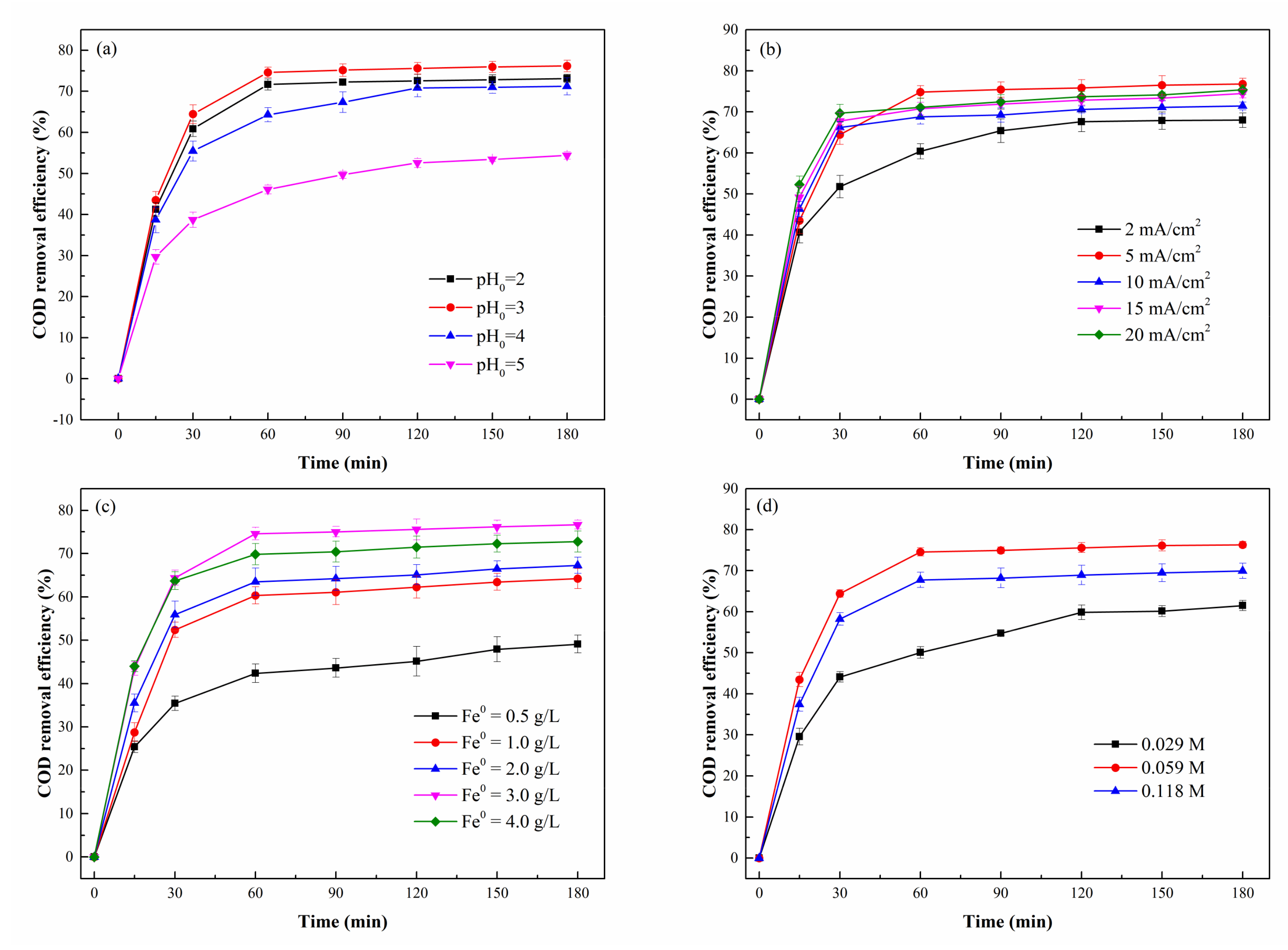
3.2.2. Effect of pH0
3.2.3. Effect of Current Density
3.2.4. Effect of Fe0 Powder and H2O2 Dosage
3.3. Organic Matter Conversion and Biodegradability Evaluation
3.3.1. UV-vis Spectra Analysis
3.3.2. FRI Analysis of 3D-EEM Spectra
3.4. Operating Cost
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Zhou, M.; Qin, X. A feasible electro-Fenton treatment of landfill leachate diluted by electro-Fenton effluent: Evaluation of operational parameters, effect of dilution ratio and assessment of treatment cost. J. Water Process Eng. 2022, 47, 102754. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- Moradian, F.; Ramavandi, B.; Jaafarzadeh, N.; Kouhgardi, E. Effective treatment of high-salinity landfill leachate using ultraviolet/ultrasonication/ peroxymonosulfate system. Waste Manag. 2020, 118, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Pacheco, M.J.; Ciríaco, L.; Lopes, A. Review on the electrochemical processes for the treatment of sanitary landfill leachates: Present and future. Appl. Catal. B Environ. 2015, 176–177, 183–200. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, C.; Chen, N.; Hu, W.; Kuang, P.; Liu, H.; Hu, Z.; Li, R. Research on the treatment of biologically treated landfill leachate by joint electrochemical system. Waste Manag. 2018, 82, 177–187. [Google Scholar] [CrossRef]
- Fang, Y.; Yin, W.; Jiang, Y.; Ge, H.; Li, P.; Wu, J. Depth treatment of coal-chemical engineering wastewater by a cost-effective sequential heterogeneous Fenton and biodegradation process. Environ. Sci. Pollut. Res. Int. 2018, 25, 13118–13126. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Tian, Z.; Xu, R.; Wu, Y.; Song, Y. Pollutant removal from landfill leachate via two-stage anoxic/oxic combined membrane bioreactor: Insight in organic characteristics and predictive function analysis of nitrogen-removal bacteria. Bioresour. Technol. 2020, 317, 124037. [Google Scholar] [CrossRef]
- Ahmed, F.N.; Lan, C.Q. Treatment of landfill leachate using membrane bioreactors: A review. Desalination 2012, 287, 41–54. [Google Scholar] [CrossRef]
- Amor, C.; Torres-Socías, E.D.; Peres, J.A.; Maldonado, M.I.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Chan, G.Y. Degradation of recalcitrant compounds from stabilized landfill leachate using a combination of ozone-GAC adsorption treatment. J. Hazard. Mater. 2006, 137, 443–455. [Google Scholar] [CrossRef]
- Xu, Q.; Siracusa, G.; Di Gregorio, S.; Yuan, Q. COD removal from biologically stabilized landfill leachate using Advanced Oxidation Processes (AOPs). Process Saf. Environ. Prot. 2018, 120, 278–285. [Google Scholar] [CrossRef]
- Wang, G.; Fan, Z.; Wu, D.; Qin, L.; Zhang, G.; Gao, C.; Meng, Q. Anoxic/aerobic granular active carbon assisted MBR integrated with nanofiltration and reverse osmosis for advanced treatment of municipal landfill leachate. Desalination 2014, 349, 136–144. [Google Scholar] [CrossRef]
- Bogacki, J.; Marcinowski, P.; El-Khozondar, B. Treatment of Landfill Leachates with Combined Acidification/Coagulation and The Fe0/H2O2 Process. Water 2019, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Yusoff, M.S.; Aziz, H.A.; Zamri, M.; Suja, F.; Abdullah, A.Z.; Basri, N.E.A. Floc behavior and removal mechanisms of cross-linked Durio zibethinus seed starch as a natural flocculant for landfill leachate coagulation-flocculation treatment. Waste Manag. 2018, 74, 362–372. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Wen, P.; Li, Q. Degradation of refractory organic contaminants in membrane concentrates from landfill leachate by a combined coagulation-ozonation process. Chemosphere 2019, 217, 411–422. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by the Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- da Costa, F.M.; Daflon, S.D.A.; Bila, D.M.; da Fonseca, F.V.; Campos, J.C. Evaluation of the biodegradability and toxicity of landfill leachates after pretreatment using advanced oxidative processes. Waste Manag. 2018, 76, 606–613. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Gu, X.; Wang, K. Pilot study on the advanced treatment of landfill leachate using a combined coagulation, fenton oxidation and biological aerated filter process. Waste Manag. 2009, 29, 1354–1358. [Google Scholar] [CrossRef]
- Jurczyk, L.; Koc-Jurczyk, J. Quantitative dynamics of ammonia-oxidizers during biological stabilization of municipal landfill leachate pretreated by Fenton’s reagent at neutral pH. Waste Manag. 2017, 63, 310–326. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Zhou, J. Removal of COD from landfill leachate by electro-Fenton method. J. Hazard. Mater. 2006, 135, 106–111. [Google Scholar] [CrossRef]
- Kallel, M.; Belaid, C.; Mechichi, T.; Ksibi, M.; Elleuch, B. Removal of organic load and phenolic compounds from olive mill wastewater by Fenton oxidation with zero-valent iron. Chem. Eng. J. 2009, 150, 391–395. [Google Scholar] [CrossRef]
- Martins, R.C.; Lopes, D.V.; Quina, M.J.; Quinta-Ferreira, R.M. Treatment improvement of urban landfill leachates by Fenton-like process using ZVI. Chem. Eng. J. 2012, 192, 219–225. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Tan, W.; Wu, X.; Lin, H.; Zhang, H. Removal of COD from landfill leachate by advanced Fenton process combined with electrolysis. Sep. Purif. Technol. 2019, 208, 3–11. [Google Scholar] [CrossRef]
- Qiang, Z.; Chang, J.-H.; Huang, C.-P. Electrochemical regeneration of Fe2+ in Fenton oxidation processes. Water Res. 2003, 37, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Jiang, M.; Zhao, G.; Wei, L.; Wang, S.; Zhao, Q. Treatment of leachate concentrate by electrocoagulation coupled with electro-Fenton-like process: Efficacy and mechanism. Sep. Purif. Technol. 2021, 255, 117668. [Google Scholar] [CrossRef]
- Liang, L.; Cheng, L.; Zhang, Y.; Wang, Q.; Wu, Q.; Xue, Y.; Meng, X. Efficiency and mechanisms of rhodamine B degradation in Fenton-like systems based on zero-valent iron. RSC Adv. 2020, 10, 28509–28515. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, A.; Gu, Z.; Li, Q. Enhanced degradation of refractory organics in concentrated landfill leachate by Fe0/H2O2 coupled with microwave irradiation. Chem. Eng. J. 2018, 354, 680–691. [Google Scholar] [CrossRef]
- Deng, Y.; Englehardt, J.D. Kinetics and oxidative mechanism for H2O2-enhanced iron-mediated aeration (IMA) treatment of recalcitrant organic compounds in mature landfill leachate. J. Hazard. Mater. 2009, 169, 370–375. [Google Scholar] [CrossRef]
- Bu, Z.; Li, X.; Xue, Y.; Ye, J.; Zhang, J.; Pan, Y. Hydroxylamine enhanced treatment of highly salty wastewater in Fe0/H2O2 system: Efficiency and mechanism study. Sep. Purif. Technol. 2021, 271, 118847. [Google Scholar] [CrossRef]
- Bremner, D.H.; Burgess, A.E.; Houllemare, D.; Namkung, K.-C. Phenol degradation using hydroxyl radicals generated from zero-valent iron and hydrogen peroxide. Appl. Catal. B Environ. 2006, 63, 15–19. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef]
- Kayan, B.; Gozmen, B.; Demirel, M.; Gizir, A.M. Degradation of acid red 97 dye in aqueous medium using wet oxidation and electro-Fenton techniques. J. Hazard. Mater. 2010, 177, 95–102. [Google Scholar] [CrossRef]
- Kocanova, V.; Dusek, L. Electrochemical dissolution of steel as a typical catalyst for electro-Fenton oxidation. Mon. Chem. 2016, 147, 935–941. [Google Scholar] [CrossRef] [Green Version]
- Apha, A. WEF (2005) Standard Methods for the Examination of Water and Wastewater; National Government Publication: Washington, DC, USA, 2007; Available online: https://www.worldcat.org (accessed on 28 June 2022).
- Wang, F.; Luo, Y.; Ran, G.; Li, Q. Sequential coagulation and Fe(0)-O3/H2O2 process for removing recalcitrant organics from semi-aerobic aged refuse biofilter leachate: Treatment efficiency and degradation mechanism. Sci. Total. Environ. 2020, 699, 134371. [Google Scholar] [CrossRef]
- Mandal, P.; Dubey, B.K.; Gupta, A.K. Review on landfill leachate treatment by electrochemical oxidation: Drawbacks, challenges and future scope. Waste Manag. 2017, 69, 250–273. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in electro-Fenton process for water and wastewater treatment: An overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Yun, W.K.; Hwang, K.Y. Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res. 2000, 34, 2786–2790. [Google Scholar]
- Wang, Y.; Li, X.; Zhen, L.; Zhang, H.; Zhang, Y.; Wang, C. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate. J. Hazard. Mater. 2012, 229–230, 115–121. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S.; Bro, R. Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 2003, 82, 239–254. [Google Scholar] [CrossRef]
- He, X.S.; Xi, B.D.; Wei, Z.M.; Jiang, Y.H.; Yang, Y.; An, D.; Cao, J.L.; Liu, H.L. Fluorescence excitation-emission matrix spectroscopy with regional integration analysis for characterizing composition and transformation of dissolved organic matter in landfill leachates. J. Hazard. Mater. 2011, 190, 293–299. [Google Scholar] [CrossRef]
- Wen, C.; Paul, W.; Leenheer, J.A.; Karl, B. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar]
- He, X.S.; Xi, B.D.; Li, X.; Pan, H.W.; An, D.; Bai, S.G.; Li, D.; Cui, D.Y. Fluorescence excitation-emission matrix spectra coupled with parallel factor and regional integration analysis to characterize organic matter humification. Chemosphere 2013, 93, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.; Bila, D.M.; Quintaes, B.R.; Campos, J.C. Cost estimation of landfill leachate treatment by reverse osmosis in a Brazilian landfill. Waste Manag. Res. 2020, 38, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.; de Souza Couto, J.M.; Gouvea, R.M.; de Almeida Oroski, F.; Bila, D.M.; Quintaes, B.R.; Campos, J.C. Nanofiltration applied to the landfill leachate treatment and preliminary cost estimation. Waste Manag. Res. 2020, 38, 1119–1128. [Google Scholar] [CrossRef]


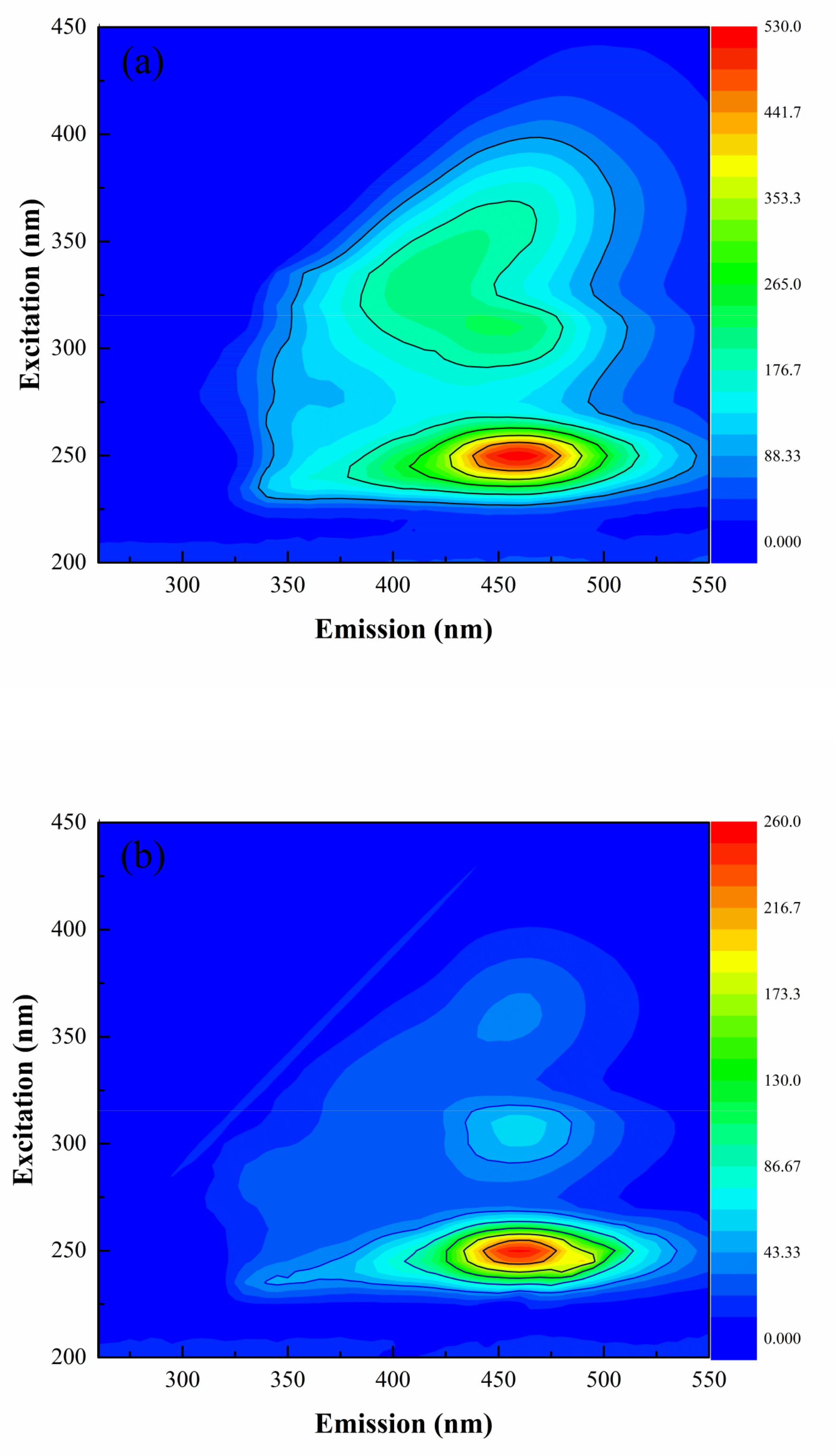
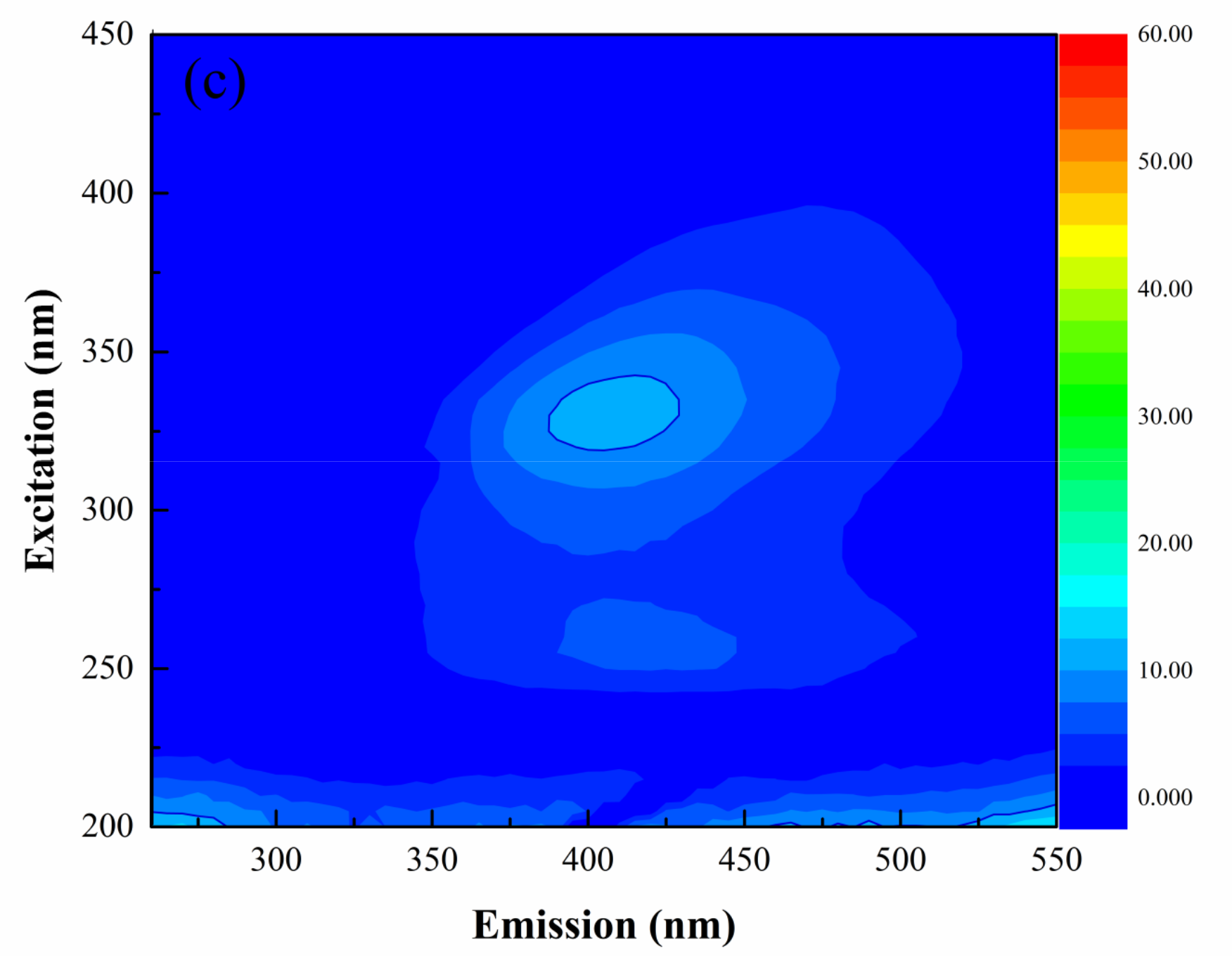
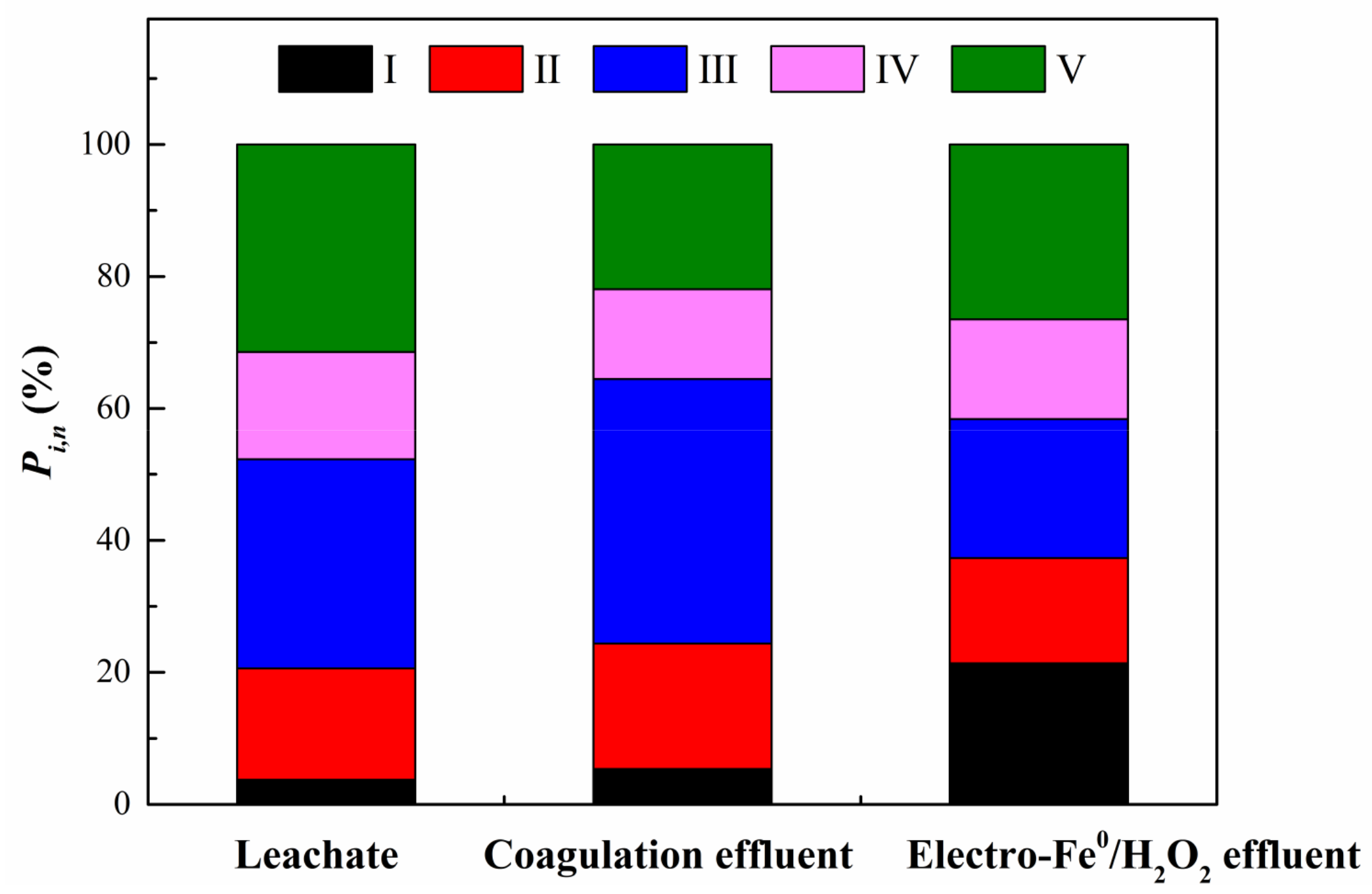
| Parameters | Value |
|---|---|
| COD (mg/L) | 3378.5 |
| BOD5 (mg/L) | 166.3 |
| BOD5/COD | 0.049 |
| pH | 7.2 |
| Chromaticity (times) | 1800 |
| Index | Leachate | Coagulated Effluent | Electro-Fe0/H2O2 Effluent |
|---|---|---|---|
| E254 | 2.0136 | 1.0910 | 0.0894 |
| E280 | 1.6221 | 0.9026 | 0.0786 |
| E250/E365 | 4.1889 | 6.6398 | 9.1463 |
| E300/E400 | 4.8279 | 11.2706 | 17.6339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, C.; Zhu, B.; Liu, W.; Li, Q. Coagulation Combined with Electro-Fe0/H2O2 Reaction for Effective Treatment of Landfill Leachate Effluent of Membrane Bioreactor. Water 2023, 15, 1158. https://doi.org/10.3390/w15061158
Long C, Zhu B, Liu W, Li Q. Coagulation Combined with Electro-Fe0/H2O2 Reaction for Effective Treatment of Landfill Leachate Effluent of Membrane Bioreactor. Water. 2023; 15(6):1158. https://doi.org/10.3390/w15061158
Chicago/Turabian StyleLong, Cheng, Bin Zhu, Wei Liu, and Qixuan Li. 2023. "Coagulation Combined with Electro-Fe0/H2O2 Reaction for Effective Treatment of Landfill Leachate Effluent of Membrane Bioreactor" Water 15, no. 6: 1158. https://doi.org/10.3390/w15061158
APA StyleLong, C., Zhu, B., Liu, W., & Li, Q. (2023). Coagulation Combined with Electro-Fe0/H2O2 Reaction for Effective Treatment of Landfill Leachate Effluent of Membrane Bioreactor. Water, 15(6), 1158. https://doi.org/10.3390/w15061158





