Review and Opinions on the Research, Development and Application of Microalgae Culture Technologies for Resource Recovery from Wastewater
Abstract
1. Introduction
2. Mechanism, Culture, and Configuration in the Microalgal Wastewater Treatment Technologies
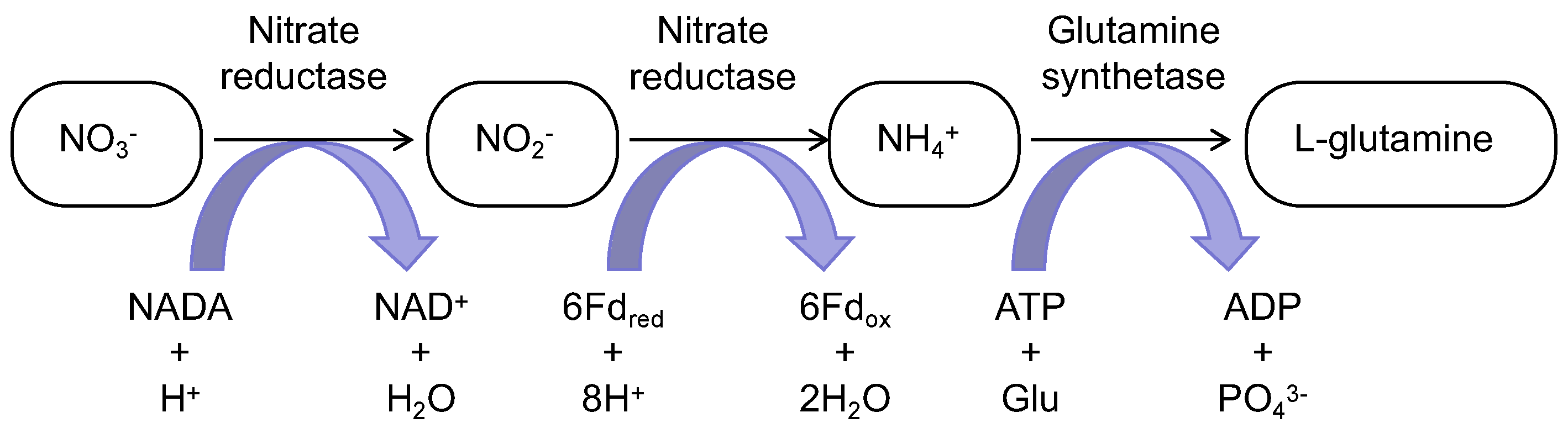
2.1. Enclosed Mechanisms of Microalgae for Nitrogen, Phosphorus, and Carbon Recovery
2.2. Selection of Suitable Microalgae Cultures
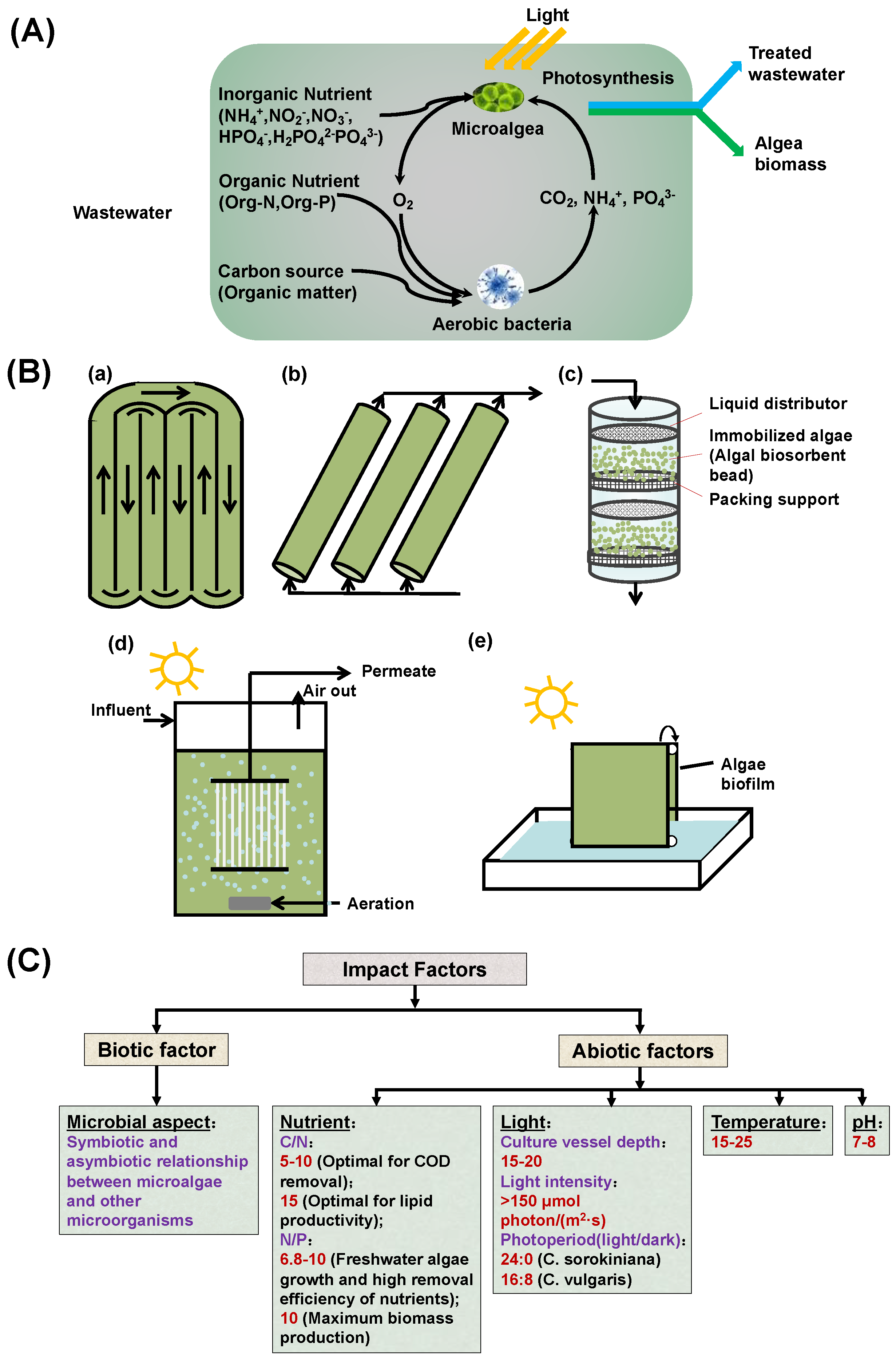
2.3. Configurations of the Microalgal Wastewater Treatment System
3. Factors Influencing the Performance of Microalgal Wastewater System
3.1. Biotic Consortia and Infections
3.2. Nutrient Balance
3.3. Operational Parameters
3.3.1. Light Intensity and Photoperiod
3.3.2. Temperature
3.3.3. pH
4. Pathways to Enhance Microalgae Biomass Harvesting
5. Application of Microalgal Processes for Various Wastewater Treatment
6. Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smol, M.; Adam, C.; Preisner, M. Circular economy model framework in the European water and wastewater sector. J. Mater. Cycles Waste Manag. 2020, 22, 682–697. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Deng, S.; An, D.; Hoang, N.B. Impact factors and novel strategies for improving biohydrogen production in microbial electrolysis cells. Bioresour. Technol. 2022, 346, 126588. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, Y.M. Carbon-Neutrality in Wastewater Treatment Plants: Advanced Technologies for Efficient Operation and Energy/Resource Recovery. Energies 2021, 14, 8514. [Google Scholar] [CrossRef]
- Deng, S.; Peng, S.; Ngo, H.H.; Oh, S.J.-A.; Hu, Z.; Yao, H.; Li, D. Characterization of nitrous oxide and nitrite accumulation during iron (Fe(0))- and ferrous iron (Fe(II))-driven autotrophic denitrification: Mechanisms, environmental impact factors and molecular microbial characterization. Chem. Eng. J. 2022, 438, 135627. [Google Scholar] [CrossRef]
- Deng, S.; Wang, Q.; Cai, Q.; Ong, S.L.; Hu, J. Efficient bio-refractory industrial wastewater treatment with mitigated membrane fouling in a membrane bioreactor strengthened by the micro-scale ZVI@GAC galvanic-cells-initiated radical generation and coagulation processes. Water Res. 2021, 209, 117943. [Google Scholar] [CrossRef]
- Robles, A.; Aguado, D.; Barat, R.; Borras, L.; Bouzas, A.; Gimenez, J.B.; Marti, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef]
- Wang, C.; Tan, Y.; Zhu, L.; Zhou, C.; Yan, X.; Xu, Q.; Ruan, R.; Cheng, P. The intrinsic characteristics of microalgae biofilm and their potential applications in pollutants removal—A review. Algal Res. 2022, 68, 102849. [Google Scholar] [CrossRef]
- Deng, S.; Li, D.; Yang, X.; Xing, W.; Li, J.; Zhang, Q. Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Bioresour. Technol. 2016, 219, 677–686. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Hongyang, S.; Yalei, Z.; Chunmin, Z.; Xuefei, Z.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour. Technol. 2011, 102, 9884–9890. [Google Scholar] [CrossRef]
- Sukla, L.B.; Subudhi, E.; Pradhan, D. The Role of Microalgae in Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Judd, S.; van den Broeke, L.J.P.; Shurair, M.; Kuti, Y.; Znad, H. Algal remediation of CO₂ and nutrient discharges: A review. Water Res. 2015, 87, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Guldhe, A.; Kumari, S.; Ramanna, L.; Ramsundar, P.; Singh, P.; Rawat, I.; Bux, F. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J. Environ. Manag. 2017, 203 Pt 1, 299–315. [Google Scholar] [CrossRef]
- Whitton, R.; Ometto, F.; Pidou, M.; Jarvis, P.; Villa, R.; Jefferson, B. Microalgae for municipal wastewater nutrient remediation: Mechanisms, reactors and outlook for tertiary treatment. Environ. Technol. Rev. 2015, 4, 133–148. [Google Scholar] [CrossRef]
- Raheem, A.; Azlina, W.W.; Yap, Y.T.; Danquah, M.K.; Harun, R. Thermochemical conversion of microalgal biomass for biofuel production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Chalivendra, S. Bioremediation of Wastewater Using Microalgae. Ph.D. Thesis, National University of Singapore, Singapore, 2014. Available online: https://go.exlibris.link/7fgk20Hj (accessed on 15 March 2023).
- Maestrini, S.Y.; Robert, J.-M.; Leftley, J.W.; Collos, Y. Ammonium thresholds for simultaneous uptake of ammonium and nitrate by oyster-pond algae. J. Exp. Mar. Biol. Ecol. 1986, 102, 75–98. [Google Scholar] [CrossRef]
- Collos, Y.; Berges, J. Nitrogen metabolism in phytoplankton. Mar. Ecol 2011, 1. [Google Scholar]
- Martinez, M.; Jimenez, J.; El Yousfi, F. Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour. Technol. 1999, 67, 233–240. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Aparicio, S.; Ruano, M.; Borrás, L.; Barat, R.; Ferrer, J. Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in algal-prokaryotic wastewater treatment: A review of nitrogen transformations, reactor configurations and molecular tools. J. Environ. Manag. 2018, 217, 845–857. (In English) [Google Scholar] [CrossRef] [PubMed]
- You, X.; Yang, L.; Zhou, X.; Zhang, Y. Sustainability and carbon neutrality trends for microalgae-based wastewater treatment: A review. Environ. Res. 2022, 209, 112860. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D.; Elboughdiri, N.; Ghareba, S.; Salih, A. Coagulation Process for Removing Algae and Algal Organic Matter—An Overview. Open Access Libr. J. 2020, 7, 1–21. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Bangwal, D.; Kaul, S.; Garg, M. Algae based biorefinery—How to make sense? Renew. Sustain. Energy Rev. 2015, 47, 295–307. [Google Scholar] [CrossRef]
- Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Microalgal rainbow colours for nutraceutical and pharmaceutical applications. In Plant Biology and Biotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 777–791. [Google Scholar]
- Grobbelaar, J.U. Microalgal biomass production: Challenges and realities. Photosynth. Res. 2010, 106, 135–144. [Google Scholar] [CrossRef]
- Arbib, Z.; de Godos, I.; Ruiz, J.; Perales, J.A. Optimization of pilot high rate algal ponds for simultaneous nutrient removal and lipids production. Sci. Total Environ. 2017, 589, 66–72. [Google Scholar] [CrossRef]
- Xu, X.; Gu, X.; Wang, Z.; Shatner, W.; Wang, Z. Progress, challenges and solutions of research on photosynthetic carbon sequestration efficiency of microalgae. Renew. Sustain. Energy Rev. 2019, 110, 65–82. [Google Scholar] [CrossRef]
- Acién, F.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the production of microalgae. In Microalgae-Based Biofuels Bioproducts; Woodhead Publishing: Sawston, UK, 2017; pp. 1–44. [Google Scholar]
- Mallick, N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: A review. Biometals 2002, 15, 377–390. [Google Scholar] [CrossRef]
- Ruiz-Marin, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef]
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Liu, Y.; Liu, Y.; Nguyen, D.D.; Chang, S.W. A critical review on designs and applications of microalgae-based photobioreactors for pollutants treatment. Sci. Total Environ. 2019, 651, 1549–1568. [Google Scholar] [CrossRef]
- Bhave, R.; Kuritz, T.; Powell, L.; Adcock, D. Membrane-based energy efficient dewatering of microalgae in biofuels production and recovery of value added co-products. Environ. Sci. Technol. 2012, 46, 5599–5606. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.; Discart, V.; Vandamme, D.; Foubert, I.; Muylaert, K.; Vankelecom, I.F. Coupled cultivation and pre-harvesting of microalgae in a membrane photobioreactor (MPBR). Bioresour. Technol. 2014, 155, 410–417. [Google Scholar] [CrossRef]
- Senatore, V.; Buonerba, A.; Zarra, T.; Oliva, G.; Belgiorno, V.; Boguniewicz-Zablocka, J.; Naddeo, V. Innovative membrane photobioreactor for sustainable CO2 capture and utilization. Chemosphere 2021, 273, 129682. [Google Scholar] [CrossRef]
- Mantzorou, A.; Ververidis, F. Microalgal biofilms: A further step over current microalgal cultivation techniques. Sci. Total Environ. 2019, 651, 3187–3201. [Google Scholar] [CrossRef]
- Lee, S.-H.; Oh, H.-M.; Jo, B.-H.; Lee, S.-A.; Shin, S.-Y.; Kim, H.-S.; Lee, S.-H.; Ahn, C.-Y. Higher biomass productivity of microalgae in an attached growth system, using wastewater. J. Microbiol. Biotechnol. 2014, 24, 1566–1573. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Microalgae–bacteria biofilms: A sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2018, 102, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Qiu, S.; Li, M.; Yuan, W.; Ge, S. Granular indigenous microalgal-bacterial consortium for wastewater treatment: Establishment strategy, functional microorganism, nutrient removal, and influencing factor. Bioresour. Technol. 2022, 353, 127130. (In English) [Google Scholar] [CrossRef]
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R.P. Review of Challenges for Algae-Based Wastewater Treatment: Strain Selection, Wastewater Characteristics, Abiotic, and Biotic Factors. J. Hazard. Toxic Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Borowitzka, M. Limits to Growth in Wastewater Treatment with Algae; Wong, Y.S., Tam, N.F.Y., Eds.; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Moreno-Andrés, J.; Rivas-Zaballos, I.; Acevedo-Merino, A.; Nebot, E. On the Efficacy of H2O2 or S2O82− at Promoting the Inactivation of a Consortium of Cyanobacteria and Bacteria in Algae-Laden Water. Microorganisms 2022, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Grobbelaar, J.U. Potential of algal production. Water 1982, 8, 79–85. [Google Scholar]
- Lee, S.-A.; Kim, M.; Kim, H.-S.; Ahn, C.-Y. Extra benefit of microalgae in raw piggery wastewater treatment: Pathogen reduction. Microbiome 2022, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Michelon, W.; da Silva, M.L.B.; Matthiensen, A.; Silva, E.; Pilau, E.J.; de Oliveira Nunes, E.; Soares, H.M. Microalgae produced during phycoremediation of swine wastewater contains effective bacteriostatic compounds against antibiotic-resistant bacteria. Chemosphere 2021, 283, 131268. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El-Dalatony, M.M.; Thakur, N.; Zheng, Y.; Salama, E.-S. Role of microalgae and cyanobacteria in wastewater treatment: Genetic engineering and omics approaches. Int. J. Environ. Sci. Technol. 2022, 19, 2173–2194. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, M.; Lu, Q.; Wu, X.; Ma, Y.; Cheng, Y.; Addy, M.; Liu, Y.; Ruan, R. Balancing carbon/nitrogen ratio to improve nutrients removal and algal biomass production in piggery and brewery wastewaters. Bioresour. Technol. 2018, 249, 479–486. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Liu, J.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zeng, Q.; Li, H.; Zhong, Y.; Tong, L.; Ruan, R.; Liu, H. Contribution of glycerol addition and algal–bacterial cooperation to nutrients recovery: A study on the mechanisms of microalgae-based wastewater remediation. J. Chem. Technol. Biotechnol. 2020, 95, 1717–1728. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Bui, X.-T.; Ngo, H.H.; Nguyen, K.-Q.; Nguyen, H.-H.; Némery, J.; Fujioka, T.; Duong, C.H.; Dang, B.-T.; Varjani, S. Nutrient recovery and microalgae biomass production from urine by membrane photobioreactor at low biomass retention times. Sci. Total Environ. 2021, 785, 147423. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Cabanelas, I.T.D.; Ruiz, J.; Arbib, Z.; Chinalia, F.A.; Garrido-Pérez, C.; Rogalla, F.; Nascimento, I.A.; Perales, J.A. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour. Technol. 2013, 131, 429–436. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Hong, Y.; Liu, X.; Wang, Q.; Zhai, Q.; Zhang, H. Attached cultivation of microalgae on rational carriers for swine wastewater treatment and biomass harvesting. Bioresour. Technol. 2022, 351, 127014. [Google Scholar] [CrossRef]
- Shao, H.; Sun, Y.; Jiang, X.; Hu, J.; Guo, C.; Lu, C.; Guo, F.; Sun, C.; Wang, Y.; Dai, C. Towards biomass production and wastewater treatment by enhancing the microalgae-based nutrients recovery from liquid digestate in an innovative photobioreactor integrated with dialysis bag. J. Environ. Manag. 2022, 317, 115337. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M.J.W.; Valorization, B. Enhanced microalgae growth for biodiesel production and nutrients removal in raw swine wastewater by carbon sources supplementation. Waste Biomass Valorization 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.-K.; Kim, H.-C.; Sapireddy, V.R.; Yun, H.-S.; Abou-Shanab, R.A.; Choi, J.; Lee, W.; Timmes, T.C.; Jeon, B.-H. Simultaneous nutrient removal and lipid production from pretreated piggery wastewater by Chlorella vulgaris YSW-04. Appl. Microbiol. Biotechnol. 2013, 97, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Miao, Z.H.; Wyatt, S.K. Influence of nutrient loads, feeding frequency and inoculum source on growth of Chlorella vulgaris in digested piggery effluent culture medium. Bioresour. Technol. 2010, 101, 6012–6018. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Sayedin, F.; Kermanshahi-pour, A.; He, Q.S.; Tibbetts, S.M.; Lalonde, C.G.; Brar, S.K. Microalgae cultivation in thin stillage anaerobic digestate for nutrient recovery and bioproduct production. Algal Res. 2020, 47, 101867. [Google Scholar] [CrossRef]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus sp. Z-4. Biotechnol. Biofuels 2017, 10, 111. [Google Scholar] [CrossRef]
- Phang, S.; Miah, M.; Yeoh, B.; Hashim, M. Spirulina cultivation in digested sago starch factory wastewater. J. Appl. Phycol. 2000, 12, 395–400. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, G.; Dai, X.; Yuan, G.; Cao, W.; Zhang, Y.; Li, B. Comparing three methods for photosynthetic bacteria separation and recycling during wastewater treatment. Desalination Water Treat. 2016, 57, 12467–12477. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour. Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef]
- Gao, F.; Yang, Z.-H.; Li, C.; Zeng, G.-M.; Ma, D.-H.; Zhou, L. A novel algal biofilm membrane photobioreactor for attached microalgae growth and nutrients removal from secondary effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef]
- Wong, Y.K.; Yung, K.K.L.; Tsang, Y.F.; Xia, Y.; Wang, L.; Ho, K.C. Scenedesmus quadricauda for nutrient removal and lipid production in wastewater. Water Environ. Res. 2015, 87, 2037–2044. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, M.; Park, C.; Sahu, A.K.; Ergas, S.J. Microalgae Growth Using High-Strength Wastewater Followed by Anaerobic Co-Digestion. Water Environ. Res. 2012, 84, 396–404. [Google Scholar] [CrossRef]
- Fettah, N.; Derakhshandeh, M.; Tezcan Un, U.; Mahmoudi, L. Effect of light on growth of green microalgae Scenedesmus quadricauda: Influence of light intensity, light wavelength and photoperiods. Int. J. Energy Environ. Eng. 2022, 13, 703–712. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal growth coupled with wastewater treatment in open and closed systems for advanced biofuel generation. Biomass Convers. Biorefinery 2022, 12, 1939–1958. [Google Scholar] [CrossRef]
- Aleya, L.; Dauta, A.; Reynolds, C.S. Endogenous regulation of the growth-rate responses of a spring-dwelling strain of the freshwater alga, Chlorella minutissima, to light and temperature. Eur. J. Protistol. 2011, 47, 239–244. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Serralta, J.; Seco, A.; Ferrer, J. Effect of temperature on ammonium removal in Scenedesmus sp. Bioresour. Technol. 2015, 191, 346–349. [Google Scholar] [CrossRef]
- Filippino, K.C.; Mulholland, M.R.; Bott, C.B. Phycoremediation strategies for rapid tertiary nutrient removal in a waste stream. Algal Res. 2015, 11, 125–133. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Li, F.; Ho, S.-H. Data-driven analysis on immobilized microalgae system: New upgrading trends for microalgal wastewater treatment. Sci. Total Environ. 2022, 852, 158514. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.I.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Barsanti, L.; Gualtieri, P. Is exploitation of microalgae economically and energetically sustainable? Algal Res. 2018, 31, 107–115. [Google Scholar] [CrossRef]
- Han, F.; Huang, J.; Li, Y.; Wang, W.; Wan, M.; Shen, G.; Wang, J. Enhanced lipid productivity of Chlorella pyrenoidosa through the culture strategy of semi-continuous cultivation with nitrogen limitation and pH control by CO2. Bioresour. Technol. 2013, 136, 418–424. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Song, X.; Yu, M.; Tong, Y.; Zhang, W. Determining the effects of polyaluminum chloride alkalinities and dosage treatments on various microalgal growth phases for the treatment of microalgae-laden water. Sep. Purif. Technol. 2019, 209, 202–210. [Google Scholar] [CrossRef]
- de Moraes, A.P.J.; Teixeira, C.M.L.L.; Faria-Machado, A.F.; Lage, C.L.S. Effects of bioflocculants on lipid extraction, fatty acid composition and reuse of the culture media for biodiesel production using Chlorella vulgaris. Sep. Sci. Technol. 2021, 56, 2609–2618. (In English) [Google Scholar] [CrossRef]
- Teixeira, C.M.L.L.; Kirsten, F.V.; Teixeira, P.C.N. Evaluation of Moringa oleifera seed flour as a flocculating agent for potential biodiesel producer microalgae. J. Appl. Phycol. 2012, 24, 557–563. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Saliu, T.D.; Ololade, I.A.; Anthony, E.T.; Bello, G.A. A new indigenous green option for turbidity removal from aqueous system. Sep. Purif. Technol. 2017, 186, 166–174. [Google Scholar] [CrossRef]
- Diaz, A.; Rincon, N.; Escorihuela, A.; Fernandez, N.; Chacin, E.; Forster, C.F. A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochem. 1999, 35, 391–395. [Google Scholar] [CrossRef]
- Mohd Yunos, F.H.; Nasir, N.M.; Wan Jusoh, H.H.; Khatoon, H.; Lam, S.S.; Jusoh, A. Harvesting of microalgae (Chlorella sp.) from aquaculture bioflocs using an environmental-friendly chitosan-based bio-coagulant. Int. Biodeterior. Biodegrad. 2017, 124, 243–249. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L.; Yang, Y.; You, G.; Jia, D.; Gao, M. Removing specific extracellular organic matter from algal bloom water by Tanfloc flocculation: Performance and mechanisms. Sep. Purif. Technol. 2019, 212, 65–74. [Google Scholar] [CrossRef]
- Pritchard, M.; Craven, T.; Mkandawire, T.; Edmondson, A.S.; O’Neill, J.G. A comparison between Moringa oleifera and chemical coagulants in the purification of drinking water—An alternative sustainable solution for developing countries. Phys. Chem. Earth Parts A/B/C 2010, 35, 798–805. [Google Scholar] [CrossRef]
- Lananan, F.; Mohd Yunos, F.H.; Mohd Nasir, N.; Abu Bakar, N.S.; Lam, S.S.; Jusoh, A. Optimization of biomass harvesting of microalgae, Chlorella sp. utilizing auto-flocculating microalgae, Ankistrodesmus sp. as bio-flocculant. Int. Biodeterior. Biodegrad. 2016, 113, 391–396. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Nguyen, T.T.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef] [PubMed]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour. Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Romero-Villegas, G.I.; Fiamengo, M.; Acién-Fernández, F.G.; Molina-Grima, E. Utilization of centrate for the outdoor production of marine microalgae at the pilot-scale in raceway photobioreactors. J. Environ. Manag. 2018, 228, 506–516. [Google Scholar] [CrossRef]
- Sepehri, A.; Sarrafzadeh, M.-H.; Avateffazeli, M. Interaction between Chlorella vulgaris and nitrifying-enriched activated sludge in the treatment of wastewater with low C/N ratio. J. Clean. Prod. 2020, 247, 119164. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, A.; Meng, F.; Zhang, G. Additives for photosynthetic bacteria wastewater treatment: Latest developments and future prospects. Bioresour. Technol. Rep. 2019, 7, 100229. [Google Scholar] [CrossRef]
- Sheng, A.; Bilad, M.; Osman, N.; Arahman, N. Sequencing batch membrane photobioreactor for real secondary effluent polishing using native microalgae: Process performance and full-scale projection. J. Clean. Prod. 2017, 168, 708–715. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.H.; Zeng, G.M.; Mu, J.; Liu, M.; Cui, W. Removal of nutrients, organic matter, and metal from domestic secondary effluent through microalgae cultivation in a membrane photobioreactor. J. Chem. Technol. Biotechnol. 2016, 91, 2713–2719. [Google Scholar] [CrossRef]
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.-C.; Tang, Q.; Shi, L.-D.; Tao, X.-M.; Tian, G.-M. Organic degrading bacteria and nitrifying bacteria stimulate the nutrient removal and biomass accumulation in microalgae-based system from piggery digestate. Sci. Total Environ. 2020, 707, 134442. [Google Scholar] [CrossRef]
- Higgins, B.T.; Gennity, I.; Fitzgerald, P.S.; Ceballos, S.J.; Fiehn, O.; VanderGheynst, J.S. Algal–bacterial synergy in treatment of winery wastewater. NPJ Clean Water 2018, 1, 6. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Yang, Z.-H.; Zeng, G.-M.; Feng, L.-J.; Liu, J.-z.; Liu, M.; Cai, H.-W. Continuous microalgae cultivation in aquaculture wastewater by a membrane photobioreactor for biomass production and nutrients removal. Ecol. Eng. 2016, 92, 55–61. [Google Scholar] [CrossRef]
| Representative MA Species | Composition (% dry w/w) | Lipid Productivity (mg/(L·d)) | Biomass Productivity | |||
|---|---|---|---|---|---|---|
| Protein | Carbohydrate | Lipid (Oil) | Areal (g/(m·d)) | Volumetric (mg/(L·d)) | ||
| Botryococcus braunii | 22 | 18 | 55–60 | 5.5 | 3 | 20 |
| Chlorella protothecoides | 10–20 | 12–20 | 55 | 1214 | - | 2000–7700 |
| Chlorella pyrenoidas | 54–60 | 24–28 | 11–12 | - | 72.5/130 | 2900–3640 |
| Chlorella vulgaris | 51–58 | 12–17 | 4–24/14–22 | 11.2–40 | 0.57–0.95 | 20–200 |
| Dunaliella salina | 57 | 32 | 6 | 116 | 1.6–3.5/20–38 | 220–340 |
| Dunaliella tertiolecta | 55–65 | 10–15 | 20 | 20 | - | 120 |
| Euglena gracilis | 39–61 | 14–18 | 14–20 | - | - | 7700 |
| Phaedactulum Tricornutum | 36.4–53.2 | 11.2–26.1 | 8–32.6 | 44.8 | 2.4–21 | 3–1900 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 | 34.8 | - | 370 |
| Scenedesmus obliquus | 50–65 | 10–17/27 | 7/12–14 | 7.14/11.6–58.6 | - | 4–740 |
| Scenedesmus quadricauda | 4.4–9.5 | 3.7–24.8 | 6.9–10.6 | 35.1 | - | 190 |
| Spirulina platensis | 46–63 | 8–14 | 4 -9 | - | 1.5–18.0/24–51 | 60–430 |
| Spirulina maxima | 60–71 | 13–16 | 6–7 | 8.6 | 25 | 210–250 |
| Wastewater Sources | Description | COD (mg/L) | TN (mg/L) | TP (mg/L) | C/N Ratio | N/P Ratio | Reference |
|---|---|---|---|---|---|---|---|
| Municipal wastewater | Sewage centrate | 846 ± 12 | 48.6 ± 1.8 | 49.8 ± 2.2 | 6.53 | 0.98 | [52] |
| Fresh urine | - | 5015 ± 209 | 347 ± 2 | - | 19.3 | [53] | |
| Raw sewage | 231.0 ± 4.2 | 40.65 ± 0.07 | 5.66 ± 0.08 | 2.13 | 7.18 | [54] | |
| Primary settled sewage | 224.0 ± 4.2 | 38.95 ± 1.91 | 6.86 ± 0.05 | 2.16 | 5.68 | [54] | |
| Sludge centrifuge centrate | 2250 ± 99 | 131.5 ± 2.1 | 201.5 ± 10.6 | 6.42 | 0.65 | [54] | |
| Pretreated urban wastewater | 150.0 | 84.42 ± 2.65 | 6.07 ± 0.26 | 0.67 | 13.91 | [55] | |
| Disposing effluent | 90 | 36.44 ± 1.93 | 2.38 ± 0.1 | 0.93 | 15.31 | [55] | |
| Effluent from primary settler | 160 | 33.9 ± 0.83 | 3.20 ± 0.1 | 1.77 | 10.59 | [55] | |
| Animal wastewater | Swine wastewater | 12,000 | 1700 | 80 | 2.65 | 21.25 | [56] |
| Anaerobic digestate of swine manure | - | 1218 NH4+-N | 25 | - | 48.72 | [57] | |
| Raw swine wastewater | 1421 | 326.60 ± 2.98 | 74 ± 0.42 | 1.63 | 4.41 | [58] | |
| Dairy manure | 38,230 | 3305 TKN | 266 | 4.34 | 12.42 | [59] | |
| Digested dairy manure | 23,760 | 3456 TKN | 249.7 | 2.58 | 13.84 | [59] | |
| Pretreated piggery | 840 ± 15 | 512 ± 9 | 57 ± 1 | 0.62 | 8.98 | [60] | |
| Digested piggery effluent | 12,152 | 3304 TKN | 192 | 1.38 | 17.21 | [61] | |
| Digested pig waste | 2746–4157 | 1405–1519 | 164–620 | 0.85–1.16 | 2.21–7.43 | [62] | |
| Industrial wastewater | Anaerobically-digested thin-stillage | 4540 | 130.9 NH4+-N | 21.5 | 13 | 6.09 | [63] |
| Brewery wastewater | 547–6730 | 9–480 | 5–45 | 0.8–70.1 | 1.4–10.7 | [49] | |
| Molasses wastewater | 514,000 | 458 | 67 | 420.85 | 6.84 | [64] | |
| Starch wastewater | 5130 ± 1280 | 2.24 NH4+-N | 18.3 ± 2.95 | 858.82 | 0.12 | [65] | |
| Digested starch wastewater | 1340 ± 520 | 2.87 NH4+-N | 21.0 ± 4.21 | 175.09 | 0.14 | [65] | |
| Soybean processing wastewater | 8087–13,215 | 189.9–267.1 | 45.6–56.3 | 16.0–18.6 | 4.16–4.74 | [10] | |
| Slaughterhouse wastewater | 734–3560 | 64.8- 327.6 | 5.6–46.8 | 3.2–8.4 | 1.4–21.0 | [66] |
| Microalgae Species | Light Intensity (μmol/m2/s) | Photoperiod (Light/Dark Ratio) | Specific Growth Rate (d−1) | Biomass Productivity | References |
|---|---|---|---|---|---|
| Chlorella. vulgaris | 642 | Natural light 15:9 | - | 14.05 g/m2/day | [70] |
| 261 | Natural light 15:9 | - | 8.09 g/m2/day | ||
| Chlorella. vulgaris | 3500 lx | 24:0 | 0.129 | 3.3 g/L | [68] |
| 5000 lx | 24:0 | 0.136 | 3.6 g/L | ||
| 7000 lx | 24:0 | 0.143 | 3.8 g/L | ||
| 5000 lx | 16:8 | - | 3.2 g/L | ||
| 5000 lx | 12:12 | - | 2.7 g/L | ||
| Chlorella. vulgaris | 80 | 24:0 | 1.51 g/L | [2,56] | |
| 110 | 24:0 | 1.79 g/L | |||
| 140 | 24:0 | 1.87 g/L | |||
| Chlorella. vulgaris | 100 | 24:0 | - | 0.03 g/L/day | [71] |
| Chlorella. vulgaris | 8000 lx | 24:0 | - | 0.072 g/L/day | [72] |
| Scenedesmus. quadricauda | 7000 lx | 12:12 | 0.6 | 0.995 g/L | [73] |
| Spirulina platensis | 3000 lx | 24:0 | 0.16 | 1.70 g/L | [58] |
| Chlorella. sorokiniana | 210 | 13:11 | - | 1.63 g/L | [63] |
| Chlorella sp. | 370–430 | 12:12 | 0.19 | 6.8 g/m2/day | [74] |
| 24:0 | 0.32 | 15.6 g/m2/day | |||
| Scenedesmus sp. | 1300 lx | 12:12 | - | 414.47 mg/L | [56] |
| Cyanobacteria, Chlorella sp. and Scenedesmus sp. | 20 | 24:0 | - | 72% N recovery | [69] |
| 50 | 24:0 | - | 44% N recovery | ||
| 100 | 24:0 | - | 46% N recovery | ||
| Scenedesmus quadricauda | 100 | 24:24 | 0.3 | 1.5 g/L | [75] |
| 500 | 24:24 | 1.057 | 4 g/L | ||
| 1000 | 24:24 | 0.8 | 2 g/L | ||
| 500 | 1:1 | 0.85 | 3.5 g/L |
| Wastewater Type | Reactor/Operation Type | Working Volume (L) | Microalgae Species | Operating Conditions | Treatment Time (day) | Initial TN (mg/L) | TN Removal (%) | Initial TP (mg/L) | TP Removal (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Municipal wastewater | PBR; batch | 0.3 | Chlorella. vulgaris | 300 rpm; IR: 2000 lux | 14 | 40 NH4+-N | 100 | 10 | 45 | [99] |
| Municipal sewage | Batch | - | Chlorella & Scenedesmus | 30 ± 1 °C; IR: all-day light 4000 lx or dark | 7 | 49.4 NH4+-N | 97 NH4+-N | 9.5 | 100 | [100] |
| Municipal wastewater | Sequencing MPBR | 5 | Euglena sp. | SRT: 60 days; HRT: 2–8 days | 2–8 | 24.7 ± 0.5 | 82.8–96% | 3.5±0.5 | 35.7–70% | [101] |
| Municipal wastewater | MPBR | 4 | Chlorella. vulgaris | 25–30 °C; IR: 120.8 μmole∙m−2∙s−1; pH: 6.5–7.8 | 35 | 14.12 ± 0.95 | 87.7 | 0.78 ± 0.11 | 76.7 | [102] |
| Dairy wastewater | Batch | 3 | Chlorella sp. Chlorella. sorokiniana | 30 °C; aeration at 1 vvm; 150 rpm; IR: 250 μE∙m−2∙s−1; Light/darkratio = 16:8, | 10 | 1750 NO3−-N | 85 | 55 | 100 | [103] |
| Digested dairy manure wastewater | Flask; batch | 0.1 | Chlorella sp. | 25 ± 2 °C; 150 rpm; continuous fluorescent light illumination | 21 | 109–239 | 75.7–82.5 | 15.3–29.5 | 62.5–74.7 | [59] |
| Swine wastewater | Flask; batch | - | Spirulina platensis | IR: all day light 3000 ± 100 lux; pH: 8.45 ± 0.01 | 15 | 326.60 ± 2.98 | 91.24 | 74 ± 0.42 | 87.44 | [58] |
| Swine wastewater | Beaker; batch | 0.15 | Scenedesmus sp. | 25 °C; IR: 1300 lx; 12 h light/12 h dark | 10 | 1700 | 60.75 | 80 | 96.13 | [56] |
| Anaerobic digestate of swine manure | Column PBR | 1.5 | Chlorella. vulgaris | 25 °C; IR: 140 μmole∙m−2∙s−1, | 7 | 1218 NH4+-N | 95.12 NH4+-N | 25 | 76.87 | [57] |
| Piggery wastewater | Flask; batch | 0.8 | Desmodesmus sp. | 25 °C; 150 rpm mixing; IR: 8000 lx | 8 | 393.82 ± 15.98 | 52 | 15.61 ± 0.76 | 100 | [104] |
| Mixed piggery-brewery wastewater | Flask; batch | 0.75 | Chlorella. vulgaris | 25 °C; IR: 200 μmole∙m−2∙s−1; 12 h light/12 h dark | 7 | 9–480 | 32–96 | 5–45 | 28–95 | [49] |
| Winery wastewater | Hybridization tubes; batch | 0.2 | a. Chlorella. Sorokiniana b. Auxenochlorella protothecoides | 28 °C; 150 rpm mixing; pH: 7.5; mixed CO2 125 mL/min | 5 | a. 114 NH4+-N b. 114 NH4+-N | a. 100 100 | a. 44 44 | a. 100 100 | [105] |
| Anaerobically-digested thin-stillage | Glass bottle; batch | 1 | Chlorella. sorokiniana | 23 ± 2 °C; 400 rpm; IR: 210 μmole∙m−2∙s−1; 13 h light/11 h dark; Mixed 2% CO2: 0.01 vvm | 18 | 130.9 NH4+-N | 95.3 NH4+-N | 21.5 | 78.3 | [63] |
| Fresh urine | PBR; | 4 | Chlorella. vulgaris | IR: 3000 lx; 24 h light/24 h dark; CO2/air mixture at flow rate of 2 L/min | 7 | 50.5 | 77.3 | 4.7 | 53.2 | [53] |
| Soybean processing wastewater | Flask; batch and fed-batch | 0.1 | Chlorella. pyrenoidosa | 25 ± 1 °C; intermittent shaking IR: 27 μmole m−2∙s−1; light/dark ratio = 14:10 | 5 | 16.8–17.5 | 88.8 | 16.8–17.5 | 70.3 | [10] |
| Diluted centrate from sewage | High-rate algal pond; semi-continuous | 855 | Nannochloropsis gaditana | 39 °C; pH: 7.3–8.2; air flow rate: 0.3 v∙v−1∙min−1; IR: 20–88 μE∙m−2∙s−1 | 1/6 | 700 NH4+-N | 90 | 11.5 | 82 | [98] |
| Agricultural wastewater | MPBR | 4 | Chlorella. Vulgaris | 25 ± 2 °C; IR: 120.8 μmole∙m−2∙s−1; pH: 6.8–7.2 | 16 | 6.81 ± 0.68 | 86.1% | 0.42 ± 0.05 | 82.7% | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, N.; Deng, S.; Wang, C.; Ngo, H.H.; Wang, X.; Yu, H.; Tang, L.; Han, J. Review and Opinions on the Research, Development and Application of Microalgae Culture Technologies for Resource Recovery from Wastewater. Water 2023, 15, 1192. https://doi.org/10.3390/w15061192
You N, Deng S, Wang C, Ngo HH, Wang X, Yu H, Tang L, Han J. Review and Opinions on the Research, Development and Application of Microalgae Culture Technologies for Resource Recovery from Wastewater. Water. 2023; 15(6):1192. https://doi.org/10.3390/w15061192
Chicago/Turabian StyleYou, Na, Shihai Deng, Chaoqi Wang, Huu Hao Ngo, Xiaowei Wang, Hongbin Yu, Long Tang, and Jie Han. 2023. "Review and Opinions on the Research, Development and Application of Microalgae Culture Technologies for Resource Recovery from Wastewater" Water 15, no. 6: 1192. https://doi.org/10.3390/w15061192
APA StyleYou, N., Deng, S., Wang, C., Ngo, H. H., Wang, X., Yu, H., Tang, L., & Han, J. (2023). Review and Opinions on the Research, Development and Application of Microalgae Culture Technologies for Resource Recovery from Wastewater. Water, 15(6), 1192. https://doi.org/10.3390/w15061192








