Effects of Probiotics on the Water Quality, Growth Performance, Immunity, Digestion, and Intestinal Flora of Giant Freshwater Prawn (Macrobrachium rosenbergii) in the Biofloc Culture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotics
2.2. Experimental Design and Diet
2.3. Water Quality Monitoring and Measurement
2.4. Growth Performance
2.5. Sample Collection and Analysis
2.6. Microbiota Analysis
2.7. Statistical Analysis
3. Results
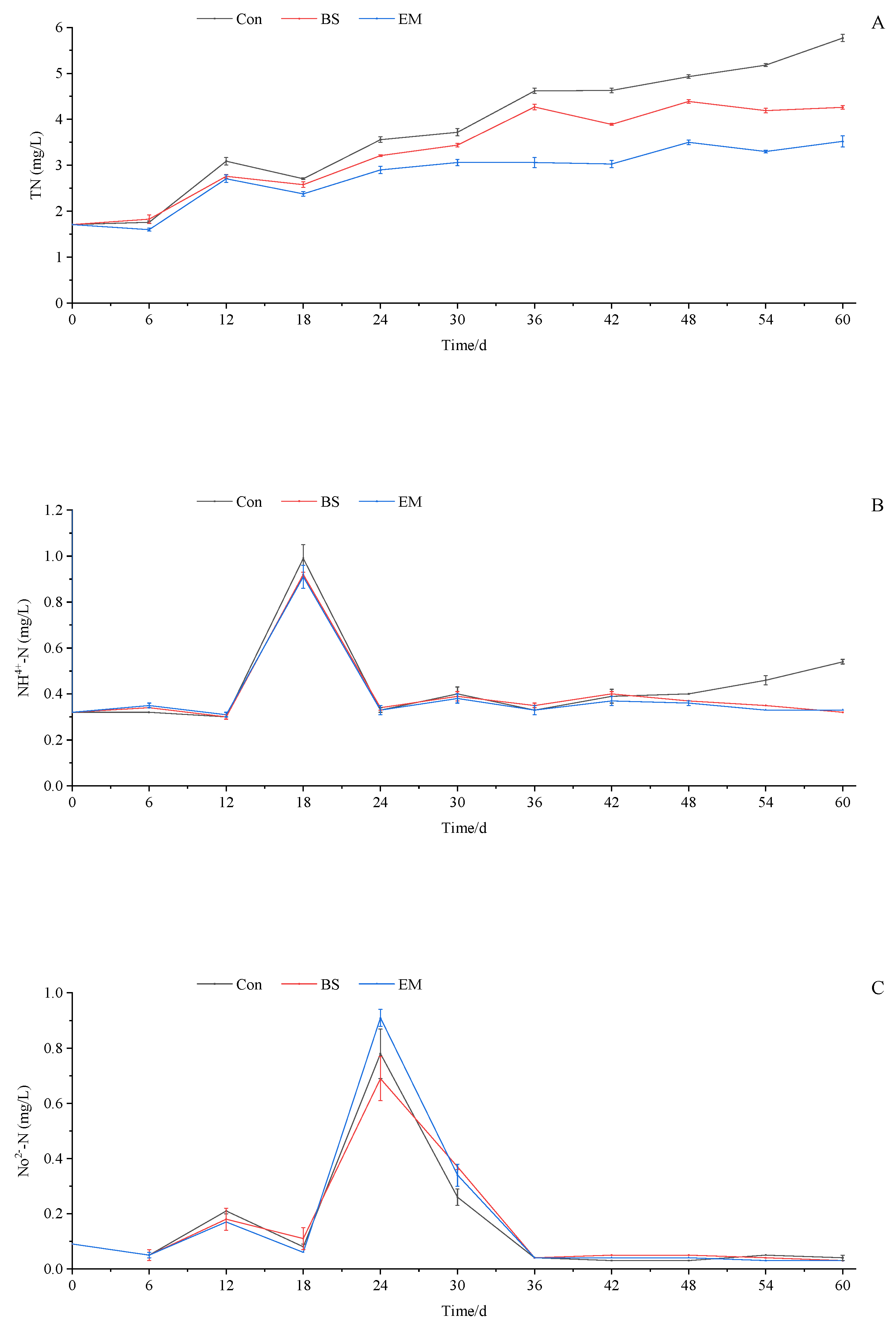
3.1. Water Quality Parameter
3.2. Growth Performance
3.3. Plasma Immune Indices
3.4. Intestinal Digestive Enzyme Activity
3.5. Analysis of Intestinal Microbial Diversity
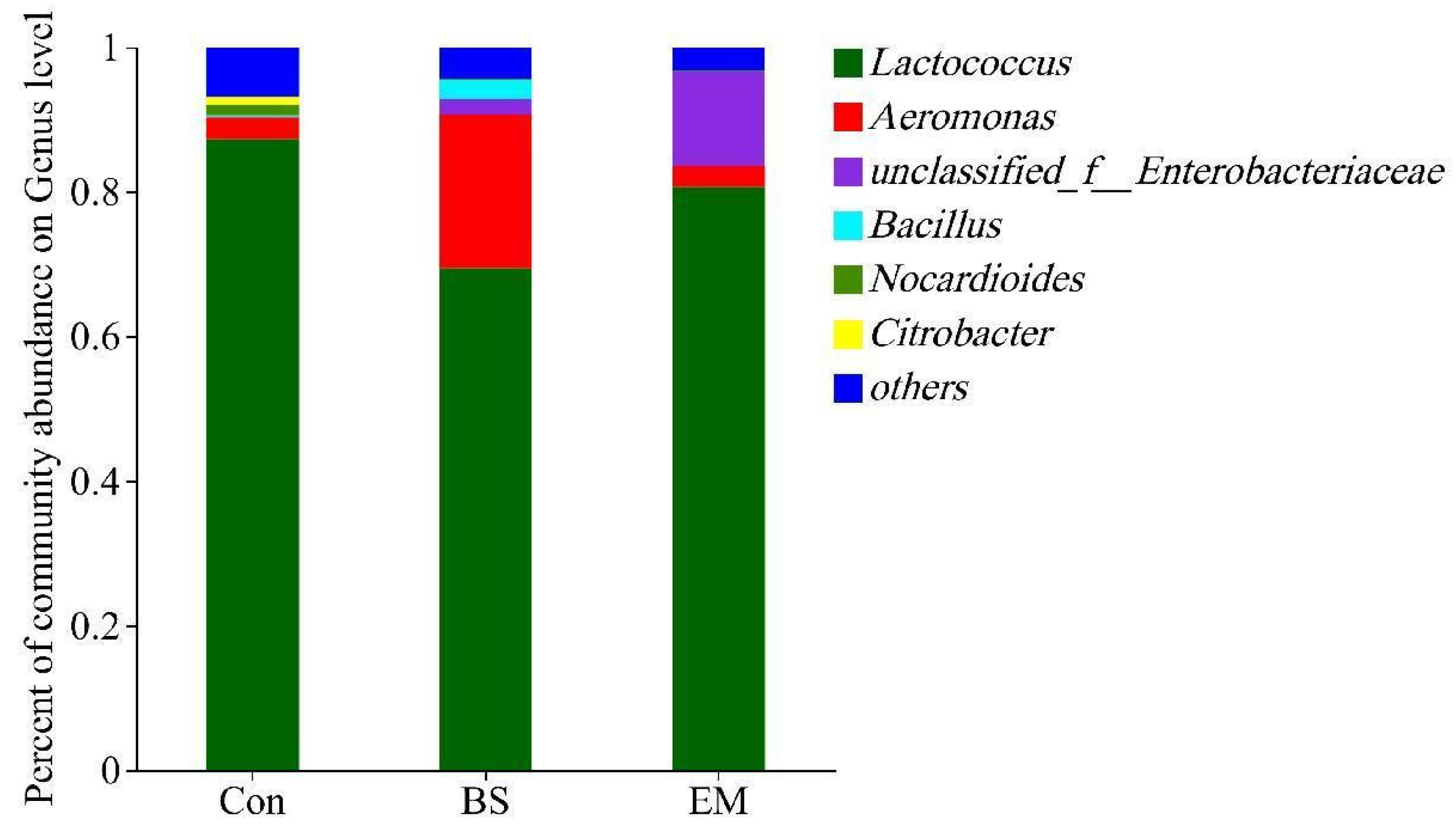
3.6. Analysis of Predominant Intestinal Flora
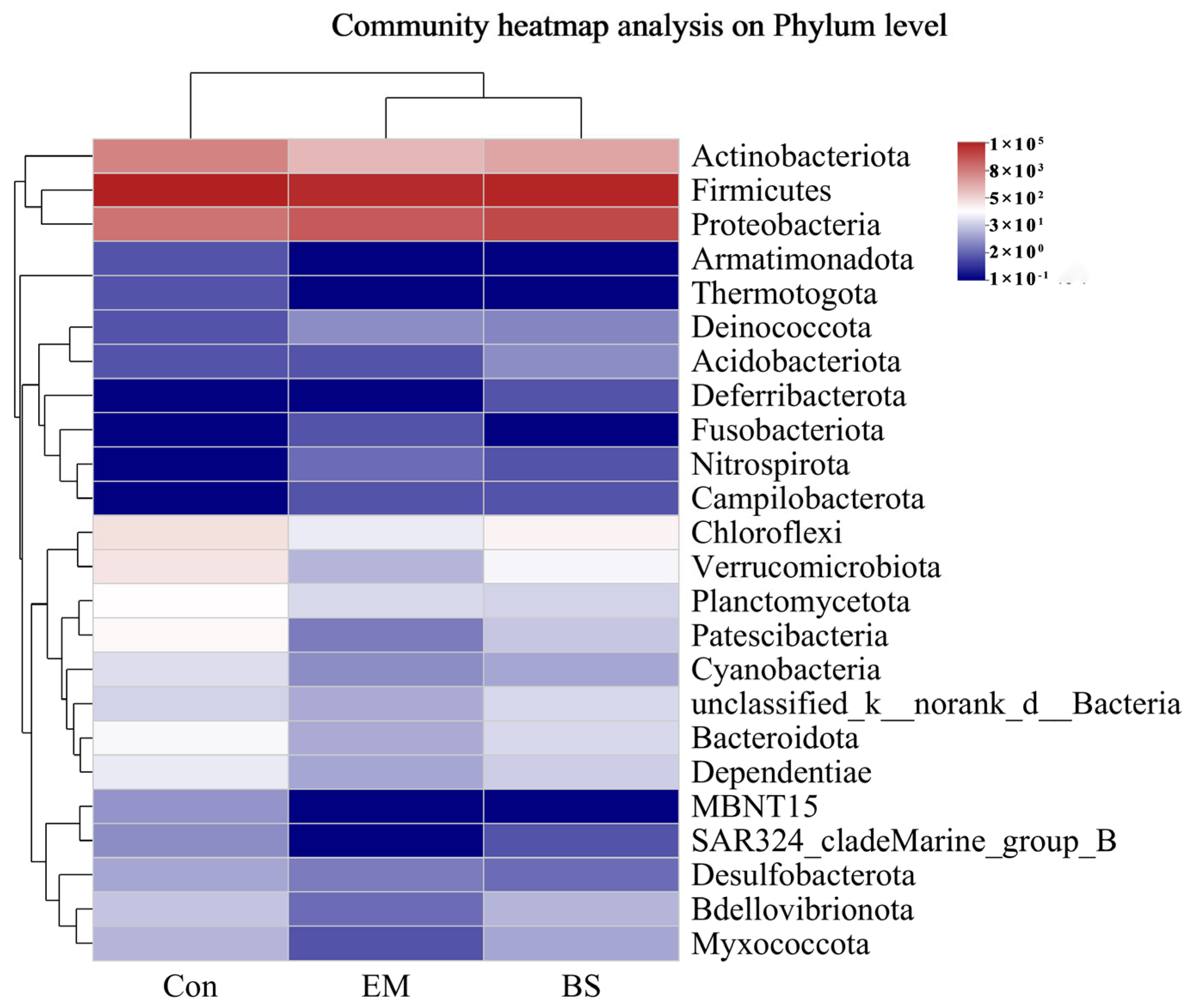
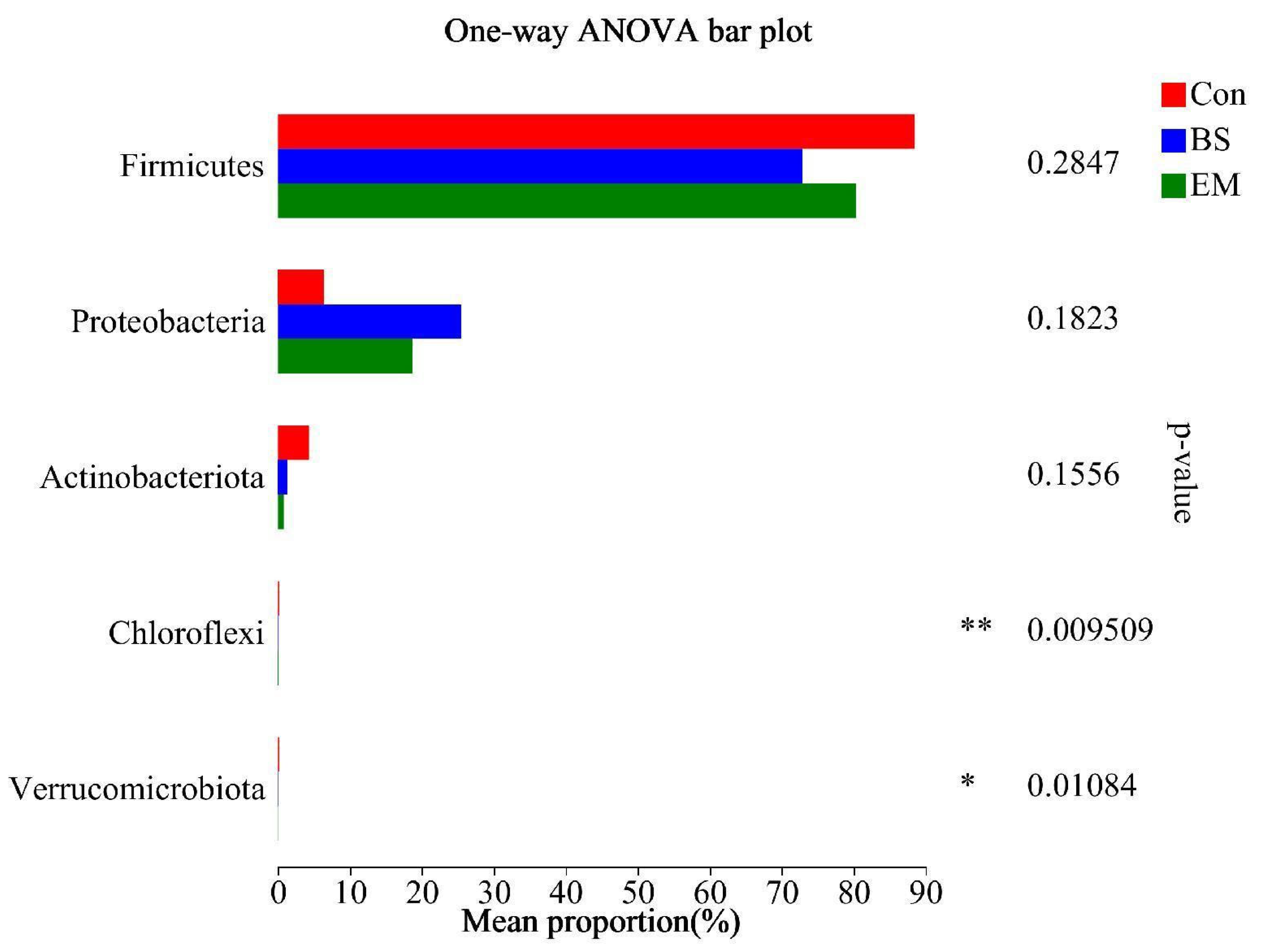
3.7. Microbial Community Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Silva, K.R.; Wasielesky, W., Jr.; Abreu, P.C. Nitrogen and phosphorus dynamics in the biofloc production of the pacific white shrimp, Litopenaeus vannamei. J. World Aquacult. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, B.; Liu, M.; Jiang, K.; Wang, L. Effects of the non-chlorine oxidizer potassium monopersulfate on the water quality, growth performance and microbial community of Pacific white shrimp (Penaeus vannamei) culture systems with limited water exchange. Aquac. Res. 2022, 53, 3555–3567. [Google Scholar] [CrossRef]
- Twarowska, J.G.; Westerman, P.W.; Losordo, T.M. Water treatment and waste characterization evaluation of an intensive recirculating fish production system. Aquacult. Eng. 1997, 16, 133–147. [Google Scholar] [CrossRef]
- Burford, M.A.; Thompson, P.J.; McIntosh, R.P.; Bauman, R.H.; Pearson, D.C. The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system. Aquaculture 2004, 232, 525–537. [Google Scholar] [CrossRef] [Green Version]
- De Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bio-flocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Kochba, M. Evaluation of nitrogen uptake and excretion by tilapia in bio floc tanks, using 15N tracing. Aquaculture 2009, 287, 163–168. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, Q.; Luo, L.; Wang, C.; Li, J.; Wang, L. Effect of feed C/N ratio promoted bioflocs on water quality and production performance of bottom and filter feeder carp in minimum-water exchanged pond polyculture system. Aquaculture 2014, 434, 442–448. [Google Scholar] [CrossRef]
- Emerenciano, M.; Ballester, E.L.; Cavalli, R.O.; Wasielesky, W. Effect of biofloc technology (BFT) on the early postlarval stage of pink shrimp Farfantepenaeus paulensis: Growth performance, floc composition and salinity stress tolerance. Aquacult. Int. 2011, 19, 891–901. [Google Scholar] [CrossRef]
- El-sayed, A. Use of biofloc technology in shrimp aquaculture: A comprehensive review, with emphasis on the last decade. Rev. Aquacult. 2020, 13, 676–705. [Google Scholar] [CrossRef]
- Pacheco-Vega, J.M.; Cadena-Roa, M.A.; Leyva-Flores, J.A.; Zavala-Leal, O.I.; Pérez-Bravo, E.; Ruiz-Velazco, J.M.J. Effect of isolated bacteria and microalgae on the biofloc characteristics in the Pacific white shrimp culture. Aquacult. Rep. 2018, 11, 24–30. [Google Scholar] [CrossRef]
- Diana, A.R.; Alejandra, P.D.; Gabriela, R.F.; Susana, E.; Gabriela, G. A vibriosis outbreak in the Pacific white shrimp, Litopenaeus vannamei reared in biofloc and clear seawater. J. Invertebr. Pathol. 2019, 167, 107246. [Google Scholar]
- Wang, M.; Lv, C.; Chen, Y.; Bi, X.; Yang, D.; Zhao, J. Effects of the potential probiotic Bacillus subtilis D1-2 on growth, digestion, immunity and intestinal flora in juvenile sea cucumber, Apostichopus japonicus. Fish Shellfish Immun. 2022, 124, 12–20. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, S.; Wang, A. The effect of different carbon sources on the nutritional composition, microbial community and structure of bioflocs. Aquaculture 2016, 465, 88–93. [Google Scholar] [CrossRef]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Vinoth Kannan, S.R.; Das, R.R.; Satish Kumar, R.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C:N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish Immun. 2018, 81, 329–337. [Google Scholar] [CrossRef]
- Flores-Higuera, F.A.; Luis-Villaseñor, I.E.; Rochin-Arenas, J.A.; Gómez-Gil, B.; Mazón-Suástegui, J.M.; Voltolina, D.; Medina-Hernández, D. Effect of pH on the bacterial community present in larvae and spat of Crassostrea gigas. Lat. Am. J. Aquat. Res. 2019, 47, 513–523. [Google Scholar] [CrossRef]
- Ghori, I.; Tubassam, M.; Ahmad, T.; Zuberi, A.; Imran, M. Gut microbiome modulation mediated by probiotics: Positive impact on growth and health status of Labeo rohita. Front. Physiol. 2022, 13, 949559. [Google Scholar] [CrossRef]
- Flores-Valenzuela, E.; Miranda-Baeza, A.; Rivas-Vega, M.E.; Miranda-Arizmendi, V.; Beltrán-Ramírez, O.; Emerenciano, M.G. Water quality and productive response of Litopenaeus vannamei reared in biofloc with addition of commercial strains of nitrifying bacteria and Lactobacillus rhamnosus. Aquaculture 2021, 542, 736869. [Google Scholar] [CrossRef]
- Luo, N.; Wang, L.; Wang, Z.; Xiao, B.; Wang, N.; Yu, X.; Wu, D.; Song, Z. Effects of dietary supplementation of duo-strain probiotics with post-spraying technology on growth performance, digestive enzyme, antioxidant capacity and intestinal microbiota of grass carp (Ctenopharyngodon idella). Aquacult. Rep. 2022, 26, 101301. [Google Scholar] [CrossRef]
- Zhu, L.; Kong, Y.; Chang, X.; Feng, J.; Wang, X.; Hou, L.; Zhao, X.; Pei, C.; Kong, X. Effects of two fish-derived probiotics on growth performance, innate immune response, intestinal health, and disease resistance of Procambarus clarkii. Aquaculture 2023, 562, 738765. [Google Scholar] [CrossRef]
- Hossain, M.K.; Hossain, M.M.; Mim, Z.T.; Khatun, H.; Hossain, M.T.; Shahjahan, M. Multi-species probiotics improve growth, intestinal microbiota and morphology of Indian major carp mrigal Cirrhinus cirrhosus. Saudi. J. Biol. Sci. 2022, 29, 103399. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
- Masithah, E.D.; Kurniawati, F.A.; Helmi, A.M. The Influence of the Different Commercial Probiotic on the Biofloc Nutrition; Atlantis Press: Dordrecht, The Netherlands, 2017; pp. 195–198. [Google Scholar]
- de Andrade, R.J.V.; Dos Santos, E.P.; de Almeida Costa, G.K.; Da Silva Campos, C.V.F.; Da Silva, S.M.B.C.; Gálvez, A.O.; Brito, L.O. Effect of different frequencies of the addition of Brachionus plicatilis on the performance of Litopenaeus vannamei in a nursery biofloc system with rice bran (anaerobic and aerobic) as an organic carbon source. Aquaculture 2021, 540, 736669. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Y.; Zhang, Y.; Cao, Y.; Chen, S.; Tian, Z.; Li, Q.; Sun, X.; Chen, A. Effects of adding EM bacteria and mechanical aeration on water quality, growth and antioxidant status of Meretrix meretrix and Exopalaemon carinicauda farmed in the clam–shrimp polyculture system. Aquac. Res. 2022, 53, 1823–1832. [Google Scholar] [CrossRef]
- He, X.; Abakari, G.; Tan, H.; Wenchang, L.; Luo, G. Effects of different probiotics (Bacillus subtilis) addition strategies on a culture of Litopenaeus vannamei in biofloc technology (BFT) aquaculture system. Aquaculture 2023, 566, 739216. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, L.; Pan, X.; Huang, Y.; Du, X.; Qin, J.; Zhou, K.; Wei, Z.; Chen, Z.; Ma, H. Dietary supplementation of Bacillus subtilis and Enterococcus faecalis can effectively improve the growth performance, immunity, and resistance of tilapia against Streptococcus agalactiae. Aquacult. Nutr. 2021, 27, 1160–1172. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Role of Bacillus subtilis VSG4-derived biosurfactant in mediating immune responses in Labeo rohita. Fish Shellfish Immun. 2016, 54, 220–229. [Google Scholar] [CrossRef]
- Eissa, E.H.; Ahmed, N.H.; El-Badawi, A.A.; Munir, M.B.; Abd Al-Kareem, O.M.; Eissa, M.E.H.; Hussien, E.H.M.; Sakr, S.E. Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac. Res. 2022, 53, 6606–6615. [Google Scholar] [CrossRef]
- Wu, D.X.; Zhao, S.M.; Peng, N.; Xu, C.P.; Wang, J.; Liang, Y.X. Effects of a probiotic (Bacillus subtilis FY99-01) on the bacterial community structure and composition of shrimp (Litopenaeus vannamei, Boone) culture water assessed by denaturing gradient gel electrophoresis and high-throughput sequencing. Aquac. Res. 2016, 47, 857–869. [Google Scholar] [CrossRef]
- de Paiva-Maia, E.; Alves-Modesto, G.; Otavio-Brito, L.; Vasconcelos-Gesteira, T.C.; Olivera, A. Effect of a commercial probiotic on bacterial and phytoplankton concentration in intensive shrimp farming (Litopenaeus vannamei) recirculation systems. Lat. Am. J. Aquat. Res. 2013, 41, 126–137. [Google Scholar] [CrossRef]
- Tao, X.; He, J.; Lu, J.; Chen, Z.; Jin, M.; Jiao, L.; Masagounder, K.; Liu, W.; Zhou, Q. Effects of Bacillus subtilis DSM 32315 (Gutcare®) on the growth performance, antioxidant status, immune ability and intestinal function for juvenile Litopenaeus vannamei fed with high/low-fishmeal diets. Aquacult. Rep. 2022, 26, 101282. [Google Scholar] [CrossRef]
- Dos Santos, D.K.M.; Kojima, J.T.; Santana, T.M.; de Castro, D.P.; Serra, P.T.; Dantas, N.S.M.; Da Fonseca, F.A.L.; Mariúba, L.A.M.; Gonçalves, L.U. Farming tambaqui (Colossoma macropomum) in static clear water versus a biofloc system with or without Bacillus subtilis supplementation. Aquacult. Int. 2021, 29, 207–218. [Google Scholar] [CrossRef]
- Cha, J.; Rahimnejad, S.; Yang, S.; Kim, K.; Lee, K. Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 2013, 402, 50–57. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Wang, Y.; Liang, Q.; Deng, B.; Li, W.; Fu, L.; Yu, D.; Shen, W.; Wang, Z. Effect of dietary supplementation of probiotic on performance and intestinal microflora of C hinese soft-shelled turtle (Trionyx sinensis). Aquacult. Nutr. 2014, 20, 667–674. [Google Scholar] [CrossRef]
- Rengpipat, S.; Rukpratanporn, S.; Piyatiratitivorakul, S.; Menasaveta, P. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 2000, 191, 271–288. [Google Scholar] [CrossRef]
- Gill, H.S. Probiotics to enhance anti-infective defences in the gastrointestinal tract. Best Pract. Res. Cl Ga 2003, 17, 755–773. [Google Scholar] [CrossRef]
- Khouadja, S.; Haddaji, N.; Hanchi, M.; Bakhrouf, A. Selection of lactic acid bacteria as candidate probiotics for Vibrio parahaemolyticus depuration in pacific oysters (Crassostrea gigas). Aquac. Res. 2017, 48, 1885–1894. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Gallardo-Collí, A.; Pérez-Rostro, C.I.; Hernández-Vergara, M.P.; Pérez-Legaspi, I.A. Microeukaryote community and the nutritional composition of the biofloc during Nile tilapia culture in water-reusing biofloc systems. Aquacult. Int. 2019, 27, 381–398. [Google Scholar] [CrossRef]
- Xu, W.; Wen, G.; Su, H.; Xu, Y.; Hu, X.; Cao, Y. Effect of input C/N ratio on bacterial community of water biofloc and shrimp gut in a commercial zero-exchange system with intensive production of Penaeus vannamei. Microorganisms 2022, 10, 1060. [Google Scholar] [CrossRef]
- Huerta-Rábago, J.A.; Martínez-Porchas, M.; Miranda-Baeza, A.; Nieves-Soto, M.; Rivas-Vega, M.E.; Martínez-Córdova, L.R. Addition of commercial probiotic in a biofloc shrimp farm of Litopenaeus vannamei during the nursery phase: Effect on bacterial diversity using massive sequencing 16S rRNA. Aquaculture 2019, 502, 391–399. [Google Scholar] [CrossRef]
- Dong, S.; Li, Y.; Huang, F.; Lin, L.; Li, Z.; Li, J.; Zhang, Y.; Zheng, Y. Enhancing effect of Platymonas addition on water quality, microbial community diversity and shrimp performance in biofloc-based tanks for Penaeus vannamei nursery. Aquaculture 2022, 554, 738057. [Google Scholar] [CrossRef]
- Tabassum, T.; Sofi Uddin Mahamud, A.G.M.; Acharjee, T.K.; Hassan, R.; Akter Snigdha, T.; Islam, T.; Alam, R.; Khoiam, M.U.; Akter, F.; Azad, M.R.; et al. Probiotic supplementations improve growth, water quality, hematology, gut microbiota and intestinal morphology of Nile tilapia. Aquacult. Rep. 2021, 21, 100972. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.; Zheng, G.; Lin, Q.; Zhuo, X.; Zhang, G. Effects of Addition of Sucrose and Probiotics on Whiteleg Shrimp Litopenaeus vannamei Postlarvae Performance, Water Quality, and Microbial Community. N. Am. J. Aquacult. 2020, 82, 43–53. [Google Scholar] [CrossRef]
- Chen, X.; Luo, G.; Tan, J.; Tan, H.; Yao, M. Effects of carbohydrate supply strategies and biofloc concentrations on the growth performance of African catfish (Clarias gariepinus) cultured in biofloc systems. Aquaculture 2020, 517, 734808. [Google Scholar] [CrossRef]
- Irani, M.; Islami, H.R.; Bahabadi, M.N.; Shekarabi, S.P.H. Production of Pacific white shrimp under different stocking density in a zero-water exchange biofloc system: Effects on water quality, zootechnical performance, and body composition. Aquacult. Eng. 2023, 100, 102313. [Google Scholar] [CrossRef]
- Kathyayani, S.A.; Poornima, M.; Sukumaran, S.; Nagavel, A.; Muralidhar, M. Effect of ammonia stress on immune variables of Pacific white shrimp Penaeus vannamei under varying levels of pH and susceptibility to white spot syndrome virus. Ecotox Environ. Safe 2019, 184, 109626. [Google Scholar] [CrossRef]
- Roumieh, R.; Barakat, A.; Abdelmeguid, N.E.; Ghanawi, J.; Patrick Saoud, I. Acute and chronic effects of aqueous ammonia on marbled spinefoot rabbitfish, Siganus rivulatus (Forsskål 1775). Aquac. Res. 2013, 44, 1777–1790. [Google Scholar] [CrossRef]
- Abasolo-Pacheco, F.; Campa-Córdova, Á.I.; Mazón-Suástegui, J.M.; Tovar-Ramírez, D.; Araya, R.; Saucedo, P.E. Enhancing growth and resistance to Vibrio alginolyticus disease in catarina scallop (Argopecten ventricosus) with Bacillus and Lactobacillus probiotic strains during early development. Aquac. Res. 2017, 48, 4597–4607. [Google Scholar] [CrossRef]
- Dou, L.; Chen, W.; Pan, L.; Huang, F. Characterization of Vibrio sp. strain AB15 and Pseudomonas fluorescens strain NB14 from the biofloc of shrimp culture ponds capable of high ammonia and nitrite removal efficiency. J. World Aquacult. Soc. 2021, 52, 843–858. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Z.; Wang, J.; Liu, Y.; Chen, S.; Li, J. Water quality, microbial community and shrimp growth performance of Litopenaeus vannamei culture systems based on biofloc or biofilters. Aquac. Res. 2021, 52, 6656–6666. [Google Scholar] [CrossRef]
- Miao, S.; Hu, J.; Wan, W.; Han, B.; Zhou, Y.; Xin, Z.; Sun, L. Biofloc technology with addition of different carbon sources altered the antibacterial and antioxidant response in Macrobrachium rosenbergii to acute stress. Aquaculture 2020, 525, 735280. [Google Scholar] [CrossRef]
- de Souza, D.M.; Suita, S.M.; Leite, F.P.L.; Romano, L.A.; Wasielesky, W.; Ballester, E.L.C. The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system. Aquac. Res. 2012, 43, 1828–1837. [Google Scholar] [CrossRef]
- Krummenauer, D.; Poersch, L.; Romano, L.A.; Lara, G.R.; Encarnação, P.; Wasielesky, W., Jr. The effect of probiotics in a Litopenaeus vannamei biofloc culture system infected with Vibrio parahaemolyticus. J. Appl. Aquacult. 2014, 26, 370–379. [Google Scholar] [CrossRef]
- Xu, W.; Pan, L. Enhancement of immune response and antioxidant status of Litopenaeus vannamei juvenile in biofloc-based culture tanks manipulating high C/N ratio of feed input. Aquaculture 2013, 412, 117–124. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. Transl. Integr. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Bibiano Melo, J.F.; Lundstedt, L.M.; Metón, I.; Baanante, I.V.; Moraes, G. Effects of dietary levels of protein on nitrogenous metabolism of Rhamdia quelen (Teleostei: Pimelodidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 145, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Tilwani, Y.M.; Sivagnanavelmurugan, M.; Lakra, A.K.; Jha, N.; Arul, V. Enhancement of growth, innate immunity, and disease resistance by probiotic Enterococcus faecium MC-5 against Aeromonas hydrophila in Indian major carp Cirrhinus mrigala. Vet. Immunol. Immunop. 2022, 253, 110503. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Wang, J.; Longshaw, M.; Song, K.; Wang, L.; Zhang, C. Substituting fish meal with a bacteria protein (Methylococcus capsulatus, Bath) grown on natural gas: Effects on growth non-specific immunity and gut health of spotted seabass (Lateolabrax maculatus). Anim. Feed Sci. Tech. 2023, 296, 115556. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Saravana, B.P.; Seenivasan, C.; Muralisankar, T. Effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbergii postlarvae. Int. J. Fish. Aquac. 2015, 7, 62–70. [Google Scholar]
- Afrilasari, W.; Meryandini, A. Effect of probiotic Bacillus megaterium PTB 1.4 on the population of intestinal microflora, digestive enzyme activity and the growth of catfish (Clarias sp.). HAYATI J. Biosci. 2016, 23, 168–172. [Google Scholar] [CrossRef]
- Chang, X.; Kang, M.; Yun, L.; Shen, Y.; Feng, J.; Yang, G.; Zhang, J.; Meng, X. Sodium gluconate increases Bacillus velezensis R-71003 growth to improve the health of the intestinal tract and growth performance in the common carp (Cyprinus carpio L.). Aquaculture 2023, 563, 738980. [Google Scholar] [CrossRef]
- Moss, S.M.; Leamaster, B.R.; Sweeney, J.N. Relative Abundance and Species Composition of Gram-Negative, Aerobic Bacteria Associated with the Gut of Juvenile White Shrimp Litopenaeus vannamei Reared in Oligotrophic Well Water and Eutrophic Pond Water. J. World Aquacult. Soc. 2010, 31, 255–263. [Google Scholar] [CrossRef]
- Tachibana, L.; Telli, G.S.; Dias, D.D.C.; Goncalves, G.S.; Guimaraes, M.C.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Fernandez Alarcon, M.F.; Tapia Paniagua, S. Bacillus subtilis and Bacillus licheniformis in diets for Nile tilapia (Oreochromis niloticus): Effects on growth performance, gut microbiota modulation and innate immunology. Aquac. Res. 2021, 52, 1630–1642. [Google Scholar] [CrossRef]
- Cardman, Z.; Arnosti, C.; Durbin, A.; Ziervogel, K.; Cox, C.; Steen, A.D.; Teske, A. Verrucomicrobia are candidates for polysaccharide-degrading bacterioplankton in an arctic fjord of Svalbard. Appl. Environ. Microb. 2014, 80, 3749–3756. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, A. Effect of two carbon sources in microbial abundance in a Biofloc culture system with Oreochromis niloticus (Linnaeus, 1758). Int. J. Fish. Aquat. Stud. 2016, 4, 421–427. [Google Scholar]
- Cao, H.; Chen, D.; Guo, L.; Jv, R.; Xin, Y.; Mo, W.; Wang, C.; Li, P.; Wang, H. Effects of Bacillus subtilis on growth performance and intestinal flora of Penaeus vannamei. Aquacult. Rep. 2022, 23, 101070. [Google Scholar] [CrossRef]
- Hao, K.; Wu, Z.; Li, D.; Yu, X.; Wang, G.; Ling, F. Effects of dietary administration of Shewanella xiamenensis A-1, Aeromonas veronii A-7, and Bacillus subtilis, single or combined, on the grass carp (Ctenopharyngodon idella) intestinal microbiota. Probiotics Antimicro 2017, 9, 386–396. [Google Scholar] [CrossRef]

| Ingredients | Composition of Diets (%) |
|---|---|
| White fish meal | 32.00 |
| Gluten flour | 8.00 |
| Soybean meal | 16.00 |
| Corn starch | 24.98 |
| Corn protein | 8.00 |
| Soybean oil | 2.00 |
| Soybean lecithin | 2.00 |
| Fish oil | 0.50 |
| Calcium dihydrogen phosphate | 2.00 |
| Choline chloride | 0.50 |
| Arginine | 0.37 |
| Cholesterol | 0.50 |
| Sodium carboxymethylcellulose | 2.00 |
| Vitamin premix 1 | 0.50 |
| Mineral premix 2 | 0.50 |
| Magnesium sulfate | 0.15 |
| Proximate composition (% dry matter) | |
| Crude protein | 39.17 |
| Crude lipid | 6.84 |
| Ash | 10.00 |
| Groups | IBW(g) | FBW (g) | SR (%) | SGR (%/Day) | WG (%) | FCR |
|---|---|---|---|---|---|---|
| Con | 2.09 ± 0.02 | 9.69 ± 0.26 b | 63.57 ± 2.01 | 2.55 ± 0.06 b | 364.37 ± 15.74 b | 1.45 ± 0.03 a |
| BS | 2.09 ± 0.03 | 11.35 ± 0.21 a | 66.43 ± 1.32 | 2.82 ± 0.03 a | 443.10 ± 8.33 a | 1.35 ± 0.01 b |
| EM | 2.09 ± 0.05 | 10.66 ± 0.29 a | 66.19 ± 3.01 | 2.71 ± 0.03 a | 409.09 ± 9.39 a | 1.39 ± 0.01 b |
| Groups | SOD (U/mL) | MDA (nmol/mL) | ACP (U/100 mL) | LZM (μg/mL) |
|---|---|---|---|---|
| Con | 72.16 ± 2.24 b | 37.29 ± 0.26 b | 6.23 ± 0.08 b | 2.84 ± 0.05 b |
| BS | 95.38 ± 1.70 a | 33.11 ± 0.40 c | 15.24 ± 0.30 a | 3.52 ± 0.09 a |
| EM | 68.33 ± 2.28 b | 49.81 ± 0.28 a | 15.01 ± 0.59 a | 2.70 ± 0.17 b |
| Groups | Amylase (U/mgprot) | Trypsin (U/mgprot) | Lipase (U/mgprot) |
|---|---|---|---|
| Con | 0.81 ± 0.12 | 283.41 ± 32.85 b | 11.23 ± 0.99 |
| BS | 0.84 ± 0.14 | 615.66 ± 48.71 a | 14.00 ± 0.94 |
| EM | 0.85 ± 0.14 | 285.08 ± 11.42 b | 11.88 ± 0.65 |
| Groups | Chao | Ace | Shannon |
|---|---|---|---|
| Con | 656.65 ± 35.45 a | 648.90 ± 35.50 a | 1.91 ± 0.10 |
| BS | 572.64 ± 20.86 ab | 610.32 ± 16.37 ab | 2.13 ± 0.15 |
| EM | 450.74 ± 40.94 b | 502.62 ± 28.74 b | 1.66 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Z.; Xu, Q.; Li, S.; Zheng, D.; Zhang, R.; Zhao, J.; Wang, T. Effects of Probiotics on the Water Quality, Growth Performance, Immunity, Digestion, and Intestinal Flora of Giant Freshwater Prawn (Macrobrachium rosenbergii) in the Biofloc Culture System. Water 2023, 15, 1211. https://doi.org/10.3390/w15061211
Qiu Z, Xu Q, Li S, Zheng D, Zhang R, Zhao J, Wang T. Effects of Probiotics on the Water Quality, Growth Performance, Immunity, Digestion, and Intestinal Flora of Giant Freshwater Prawn (Macrobrachium rosenbergii) in the Biofloc Culture System. Water. 2023; 15(6):1211. https://doi.org/10.3390/w15061211
Chicago/Turabian StyleQiu, Zongsheng, Qiyou Xu, Shenghao Li, Dakua Zheng, Rongfei Zhang, Jianhua Zhao, and Ting Wang. 2023. "Effects of Probiotics on the Water Quality, Growth Performance, Immunity, Digestion, and Intestinal Flora of Giant Freshwater Prawn (Macrobrachium rosenbergii) in the Biofloc Culture System" Water 15, no. 6: 1211. https://doi.org/10.3390/w15061211
APA StyleQiu, Z., Xu, Q., Li, S., Zheng, D., Zhang, R., Zhao, J., & Wang, T. (2023). Effects of Probiotics on the Water Quality, Growth Performance, Immunity, Digestion, and Intestinal Flora of Giant Freshwater Prawn (Macrobrachium rosenbergii) in the Biofloc Culture System. Water, 15(6), 1211. https://doi.org/10.3390/w15061211









