Characteristics of Malaysian Crude Oils and Measurement of ASP Flooded Water in Oil Emulsion Stability and Viscosity in Primary Separator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crude Oils
2.2. Malaysian Crude Oils
2.3. Characteristics of Angsi Crude Oil Used for Emulsion
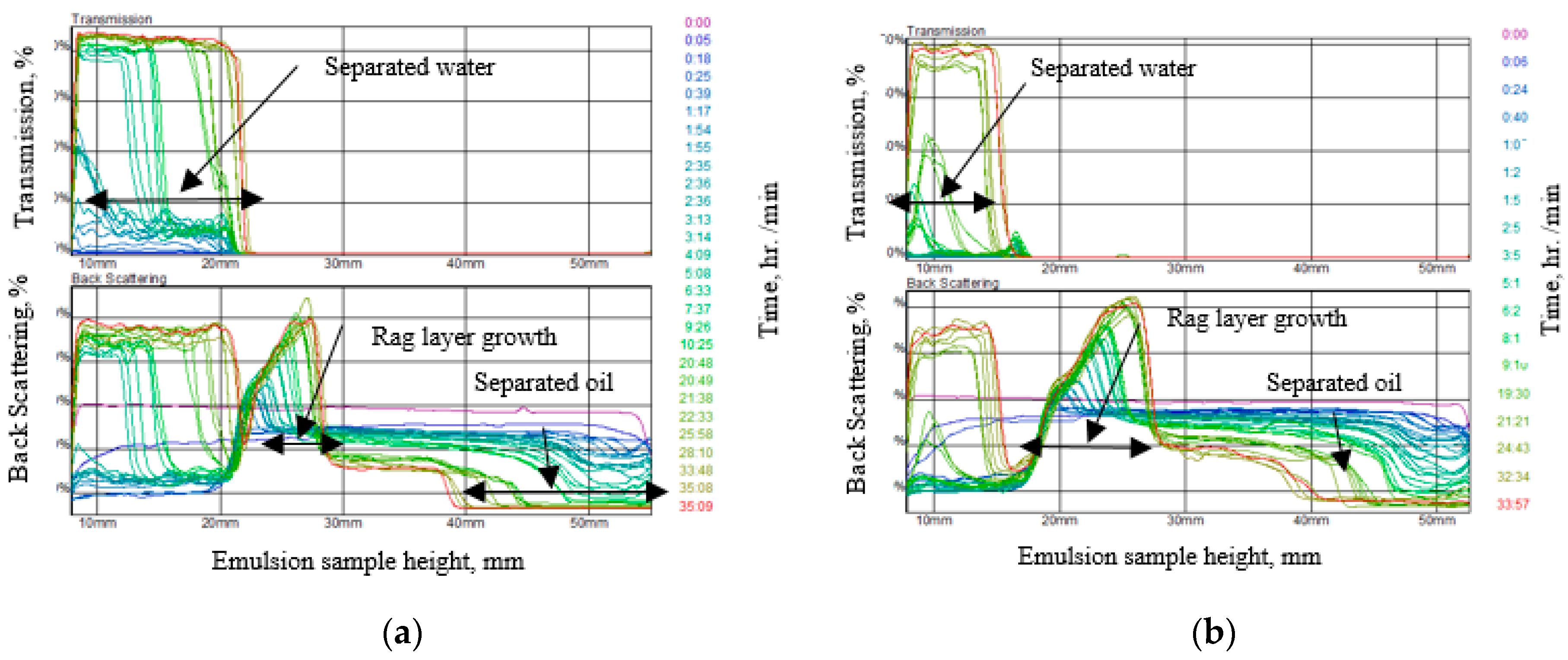
2.4. Emulsion Stability Test
2.5. Rheology Measurement
2.6. Zeta Potential Measurement
2.7. Artificial Neural Network Methodology
2.8. Procedure for Artificial Neural Network Model
2.9. Development of Artificial Neural Networks Model
2.10. Determination of Hidden Layers, Nodes, and Validation
3. Results and Discussion
3.1. Emulsion Stability with Alkali
3.2. Emulsion Stability with Surfactant
3.3. Emulsion Stability with Polymer
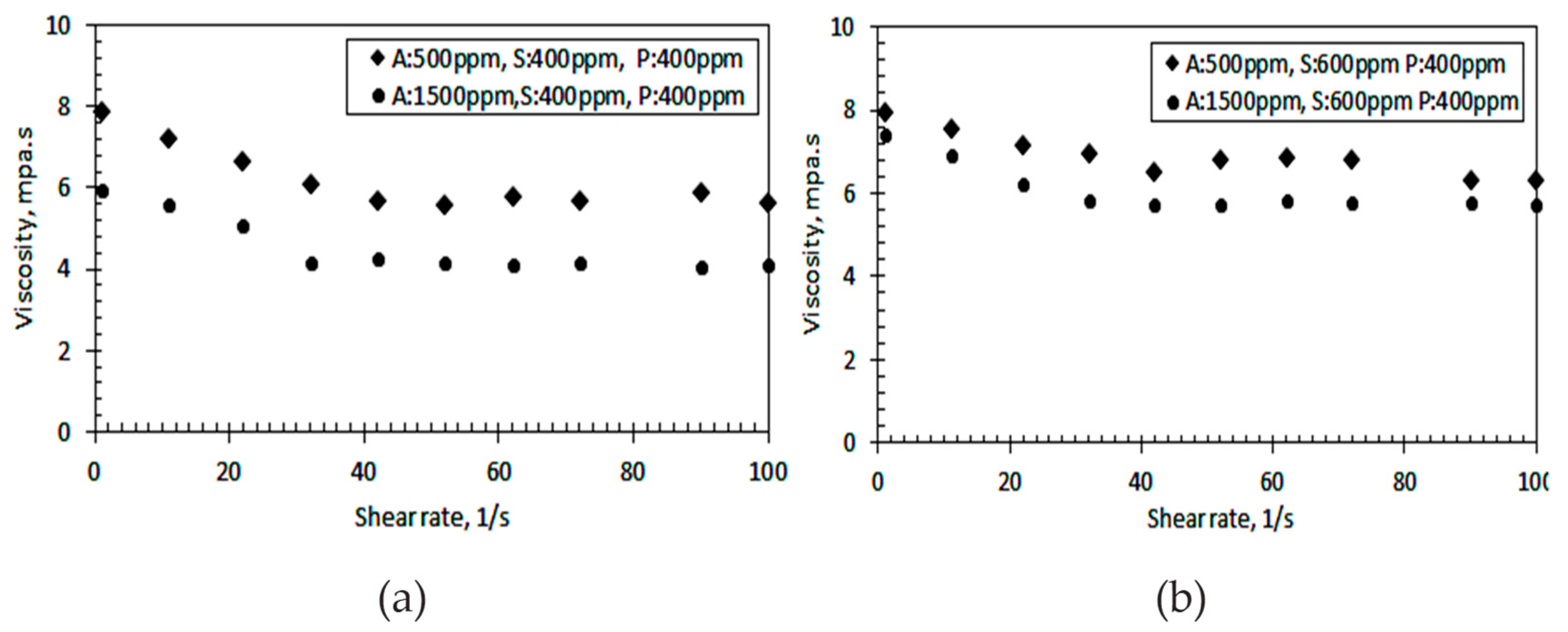
3.4. Viscosities of Residual Emulsion
3.5. Effect of ASP on Zeta Potential of Droplets
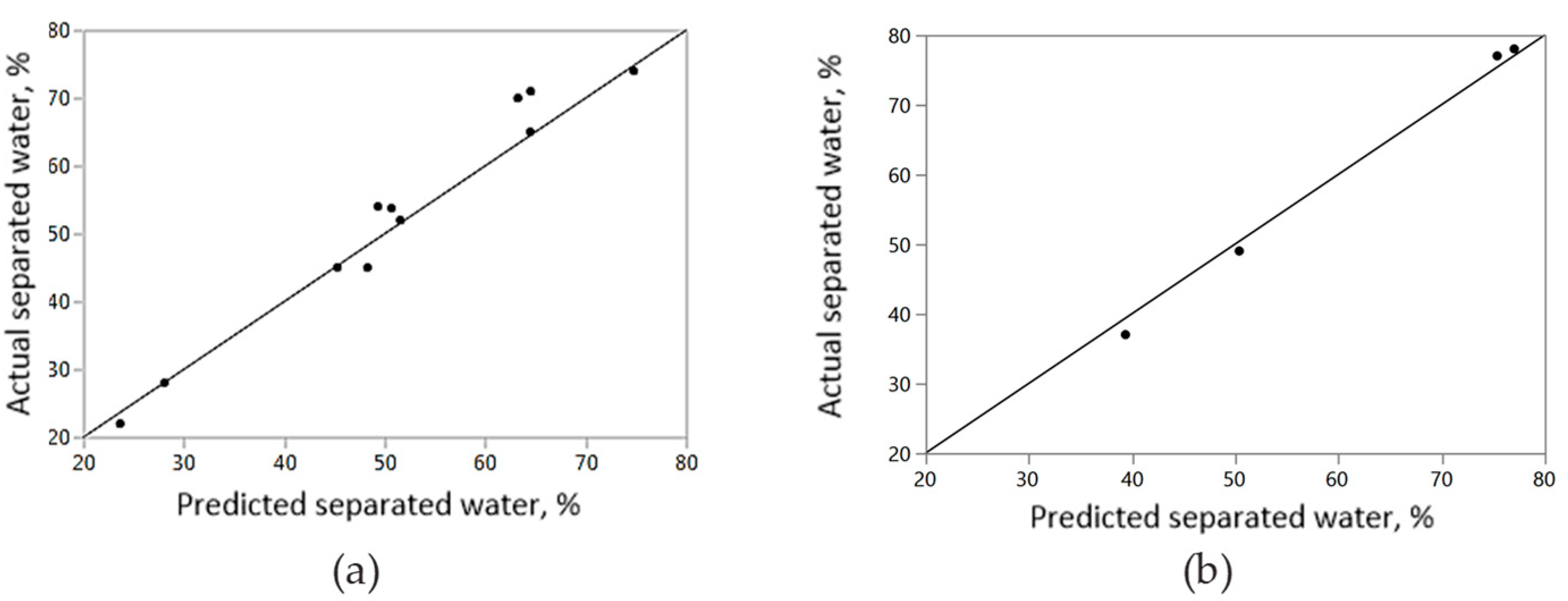
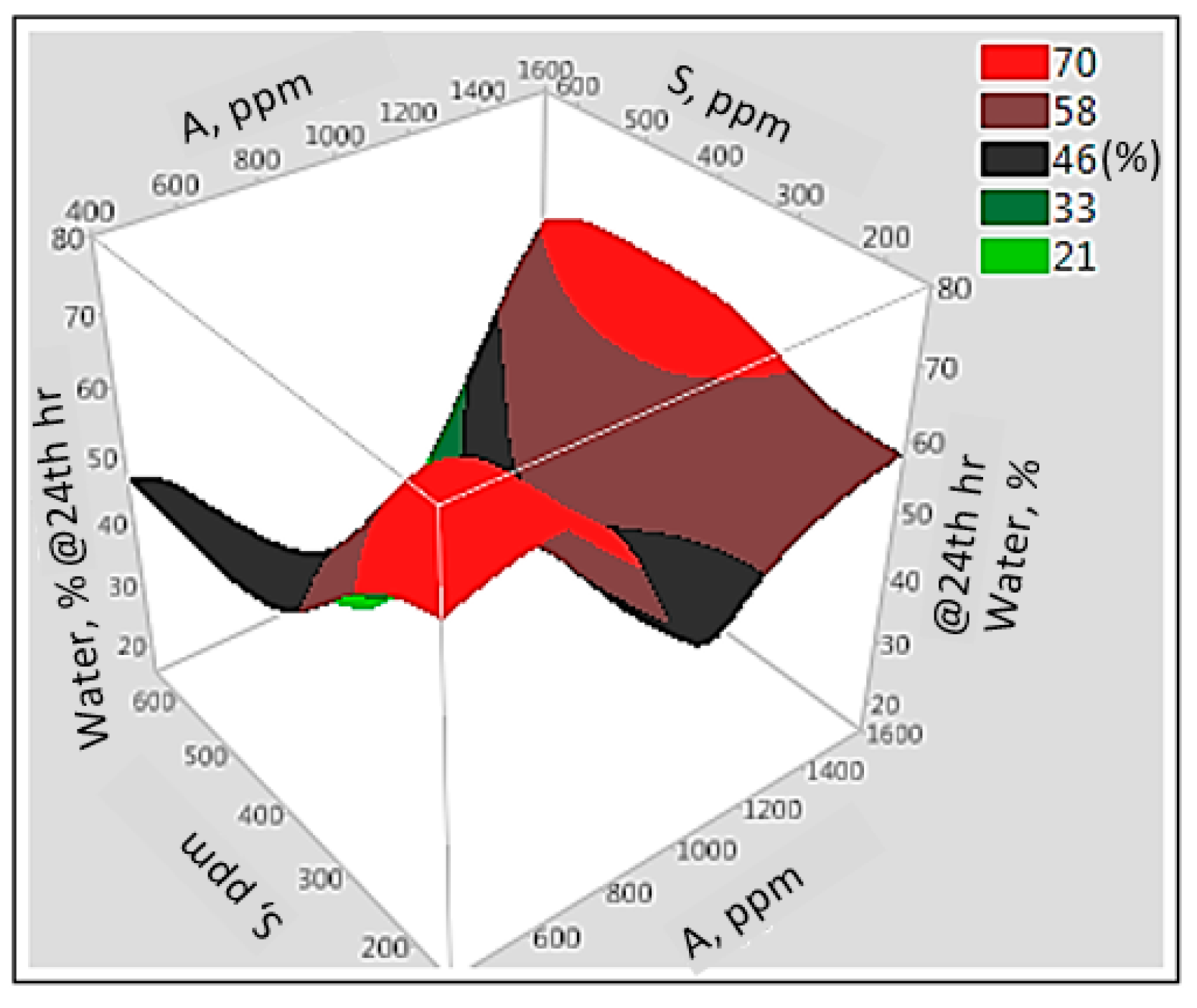
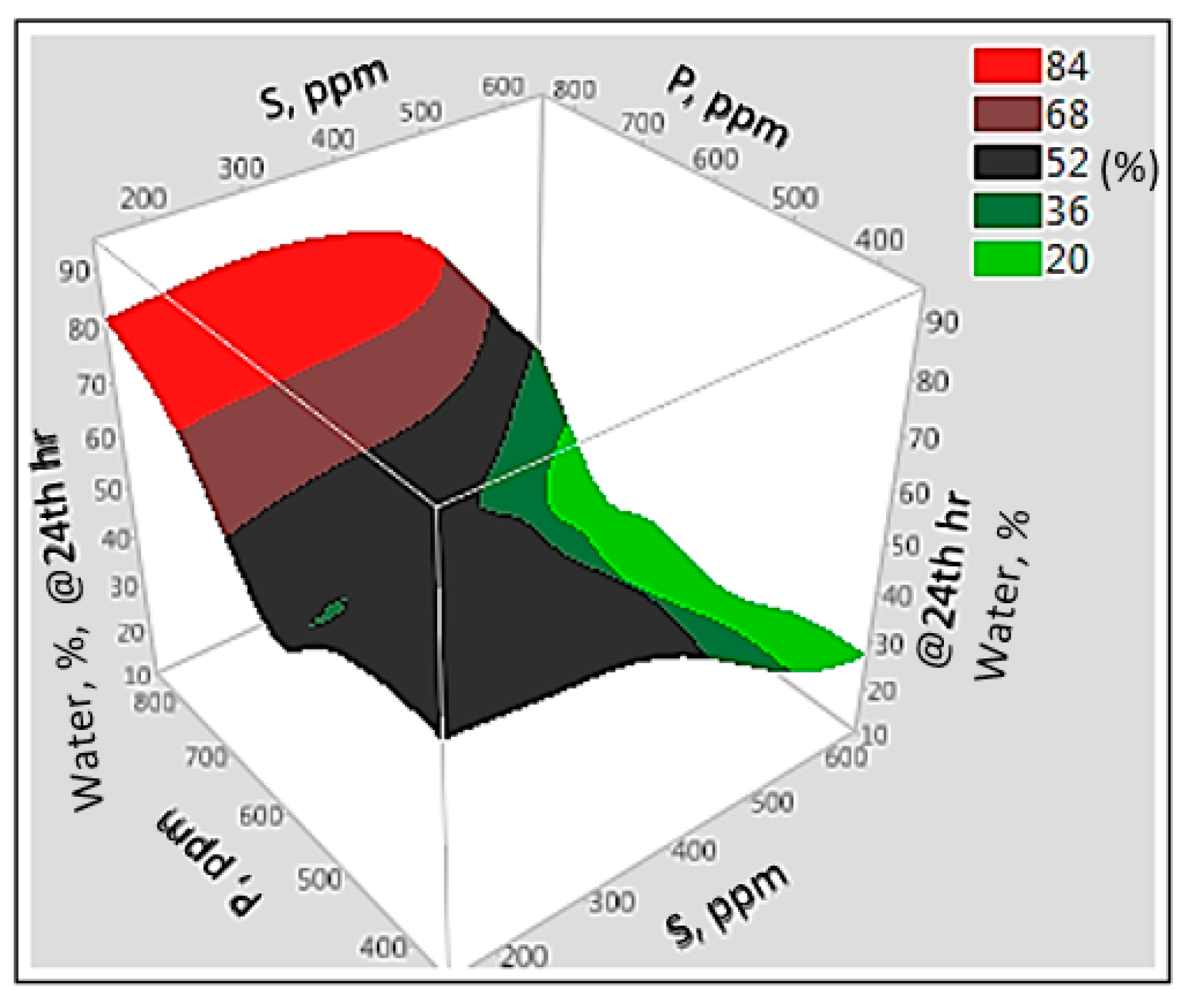
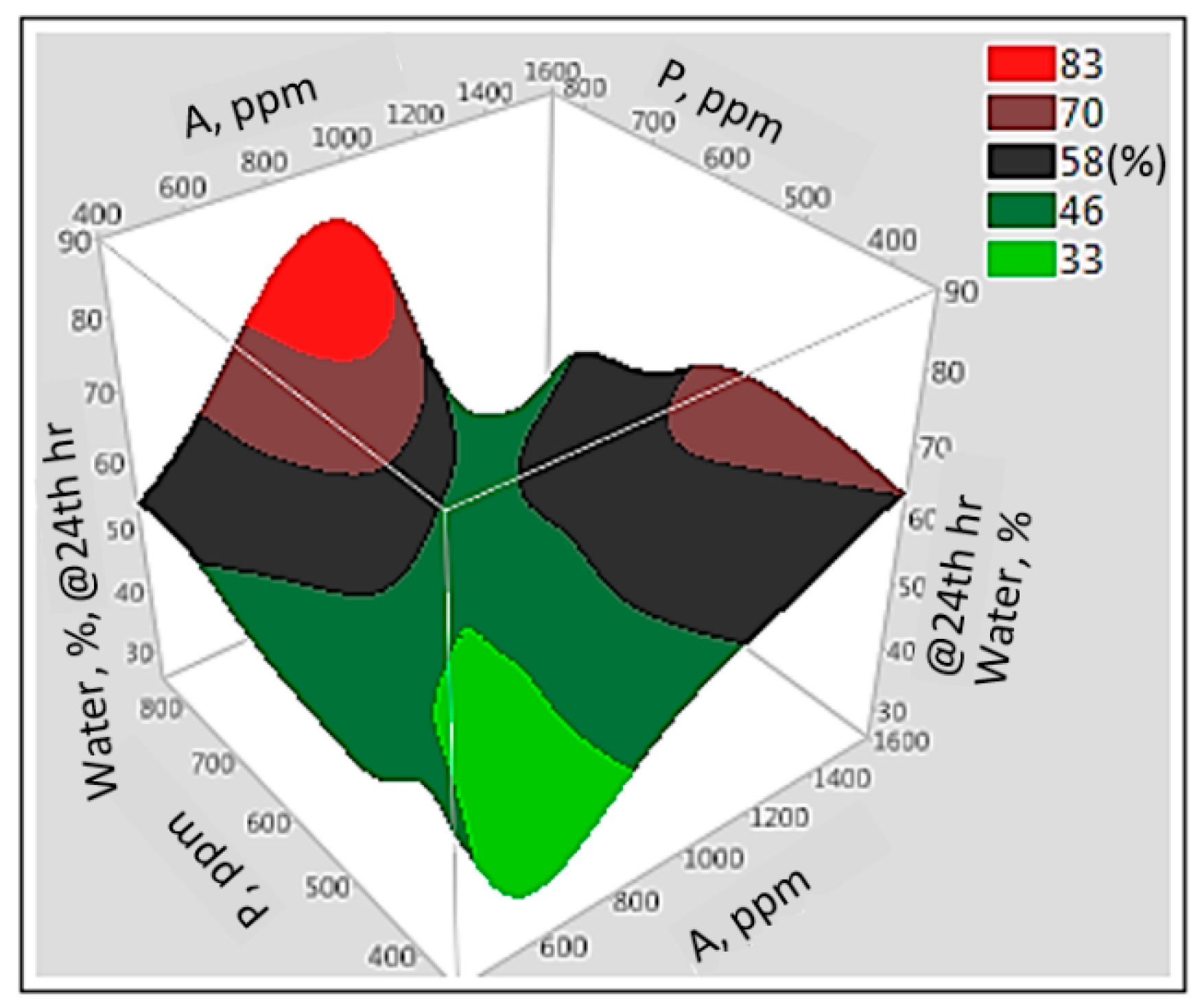
3.6. Interaction Effect of Parameters with Artificial Neural Network
3.7. Prediction Profiles of ASP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madhu, G.M.; Kumar, S.M.; Lourdu Antony Raj, M.A. Studies on Separation of Liquid-Liquid Dispersions: Diesel-Water System in Batch Settler. J. Dispers. Sci. Technol. 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Noïk, C.; Palermo, T.; Dalmazzone, C. Modeling of liquid/liquid phase separation: Application to petroleum emulsions. J. Dispers. Sci. Technol. 2013, 34, 1029–1042. [Google Scholar] [CrossRef]
- Kokal, S.L. Crude oil emulsions: A state-of-the-art review. SPE Prod. Facil. 2002, 20, 5–13. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilising foams and emulsions. Adv. Colloid. Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Sztukowski, D.M.; Yarranton, H.W. Oilfield solids and water-in-oil emulsion stability. J. Colloid. Interface Sci. 2005, 285, 821–833. [Google Scholar] [CrossRef]
- Aleem, W.; Mellon, N. Experimental Study on the Effect of Parameters on Sedimentation and Coalescing Profiles in Liquid-liquid Batch Settler. Procedia Eng. 2016, 148, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.H.; Van Den Broek, W.M.G.T.; Harris, C.K. Laboratory study investigating emulsion formation in the near-wellbore region of a high water-cut oil well. SPE J. 2001, 6, 71–79. [Google Scholar] [CrossRef]
- Yan, N.; Masliyah, J.H. Effect of pH on adsorption and desorption of clay particles at oil–water interface. J. Colloid. Interface Sci. 1996, 181, 20–27. [Google Scholar] [CrossRef]
- Zerpa, L.E.; Queipo, N.V.; Pintos, S.; Salager, J.L. An optimization methodology of alkaline–surfactant–polymer flooding processes using field scale numerical simulation and multiple surrogates. J. Pet. Sci. Eng. 2005, 47, 197–208. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, S.; Mandal, A. Temperature-dependent phase behavior, particle size, and conductivity of middle-phase microemulsions stabilized by ethoxylated nonionic surfactants. J. Chem. Eng. Data 2012, 57, 3617–3623. [Google Scholar] [CrossRef]
- Jeelani, S.A.; Hartland, S. Effect of dispersion properties on the separation of batch liquid—liquid dispersions. Ind. Eng. Chem. Res. 1998, 37, 547–554. [Google Scholar] [CrossRef]
- Aleem, W.; Mellon, N. Model for the prediction of separation profile of oil-in-water emulsion. J. Dispers. Sci. Technol. 2018, 39, 8–17. [Google Scholar] [CrossRef]
- Frising, T.; Noïk, C.; Dalmazzone, C. The liquid/liquid sedimentation process: From droplet coalescence to technologically enhanced water/oil emulsion gravity separators: A review. J. Dispers. Sci. Technol. 2006, 27, 1035–1057. [Google Scholar] [CrossRef]
- Khan, J.A.; Al-Kayiem, H.H.; Aleem, W.; Saad, A.B. Influence of alkali-surfactant-polymer flooding on the coalescence and sedimentation of oil/water emulsion in gravity separation. J. Pet. Sci. Eng. 2019, 173, 640–649. [Google Scholar] [CrossRef]
- Nedjhioui, M.; Moulai-Mostefa, N.; Morsli, A.; Bensmaili, A. Combined effects of polymer/surfactant/oil/alkali on physical chemical properties. Desalination 2005, 185, 543–550. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Raney, O.G.; Poindexter, M.K.; Nguyen, D.T.; Hera, J. Separation of Produced Emulsions from Surfactant Enhanced Oil Recovery Processes. Energy Fuels. 2010, 25, 555–561. [Google Scholar] [CrossRef]
- Tan, X.; Fenniri, H.; Gray, M.R. Water enhances the aggregation of model asphaltenes in solution via hydrogen bonding. Energy Fuels. 2009, 23, 3687–3693. [Google Scholar] [CrossRef]
- Arhuoma, M.; Daoyong, Y.; Mingzhe, D.; Heng, L.; Idem, R. Numerical simulation of displacement mechanisms for enhancing heavy oil recovery during alkaline flooding. Energy Fuels. 2009, 23, 5995–6002. [Google Scholar] [CrossRef]
- Kokal, S.; Wingrove, M. Emulsion separation index: From laboratory to field case studies. In Proceedings of the SPE Annual Technical Conference and Exhibition, SPE-63165-MS. Dallas, TX, USA, 1–4 October 2000. [Google Scholar]
- Zhang, F.; Liu, G.; Ma, J.; Ouyang, J.; Yi, X.; Su, H. Main challenges in demulsifier research and application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 167, p. 012068. [Google Scholar]
- Othman, M.; Chong, M.B.O.; Sai, R.M.; Zainal, S.; Yaacob, A.A.; Zakaria, M.S. Meeting the challenges in alkaline surfactant pilot project implementation at Angsi field, offshore Malaysia. In Proceedings of the SPE Offshore Europe Oil and Gas Conference and Exhibition, Society of Petroleum Engineers. Aberdeen, UK, 4–7 September 2007. [Google Scholar]
- Khan, J.A.; Al-Kayiem, H.H.; Aris, M.S. Stabilization of Produced Crude Oil Emulsion in the Presence of ASP. In Proceedings of the SPE Asia Pacific Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 11–13 August 2015; p. SPE-174671-MS. [Google Scholar]
- Aleem, W.; Mellon, N.; Khan, J.A.; Al-Kayiem, H.H. Experimental investigation and mathematical modeling of oil/water emulsion separation effectiveness containing alkali-surfactant-polymer. J. Dispers. Sci. Technol. 2021, 42, 1286–1298. [Google Scholar] [CrossRef]
- Al-Kayiem, H.H.; Khan, J.A. Evaluation of Alkali/Surfactant/Polymer Flooding on Separation and Stabilization of Water/Oil Emulsion by Statistical Modeling. Energy Fuels 2017, 31, 9290–9301. [Google Scholar] [CrossRef]
- Hajduk, Z. High accuracy FPGA activation function implementation for neural networks. Neurocomputing 2017, 247, 59–61. [Google Scholar] [CrossRef]
- Ibrahim, Z.B.; Manap, A.A.A.; Hamid, P.A.; Yee, V.H.; Hong, L.P.; Wyatt, K. Laboratory aspect of chemical EOR processes evaluation for Malaysian oilfields. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 11–13 September 2006. [Google Scholar]
- Ghadami, N.; Das, A.K.; Tunio, K.H.; Sabzabadi, A. Sensitivity analysis and optimization of alkaline-surfactant flooding in a thin clastic reservoir. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar]
- Hirasaki, G.J.; Miller, C.A.; Puerto, M. Recent advances in surfactant EOR. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Shupe, R.D. Surfactant Oil Recovery Process Usable in High Temperature Formations Containing High Salinity Water. U.S. Patent 4,088,189, 9 May 1978. [Google Scholar]
- Hartland, S.; Jeelani, S. Prediction of sedimentation and coalescence profiles in a decaying batch dispersion. Chem. Eng. Sci. 1988, 43, 2421–2429. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, J.A.; Ullah, H.; Al-Kayiem, H.H.; Irawan, S.; Irfan, M.; Glowacz, A.; Liu, H.; Glowacz, W.; Rahman, S. De-emulsification and gravity separation of micro-emulsion produced with enhanced oil recovery chemicals flooding. Energies 2021, 14, 2249. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Wang, F.; Gu, Y. Comparison of Strong-Alkali and Weak-Alkali ASP-Flooding Field Tests in Daqing Oilfield. SPE Prod. Oper. 2017, 33, 353–362. [Google Scholar]
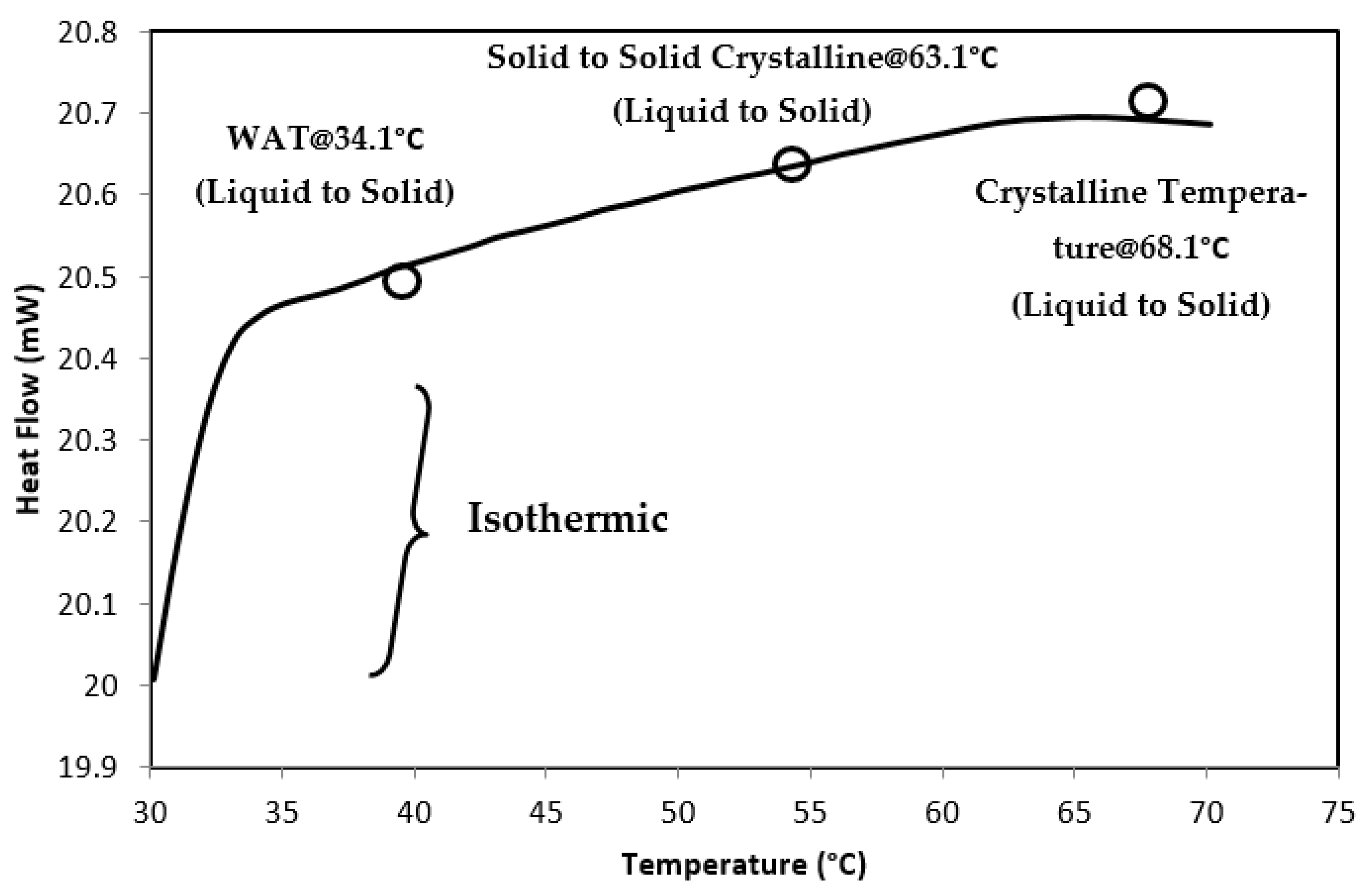
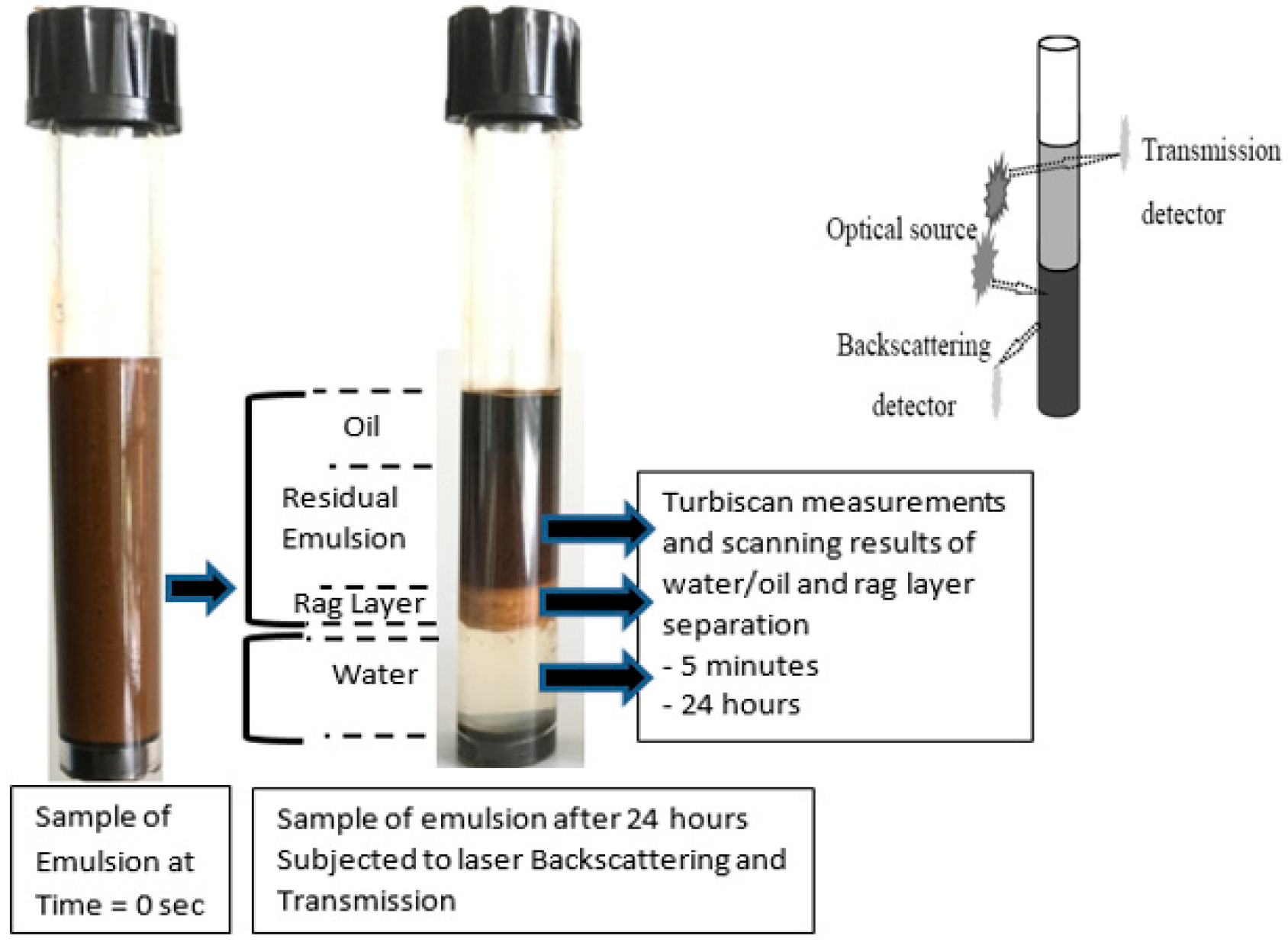


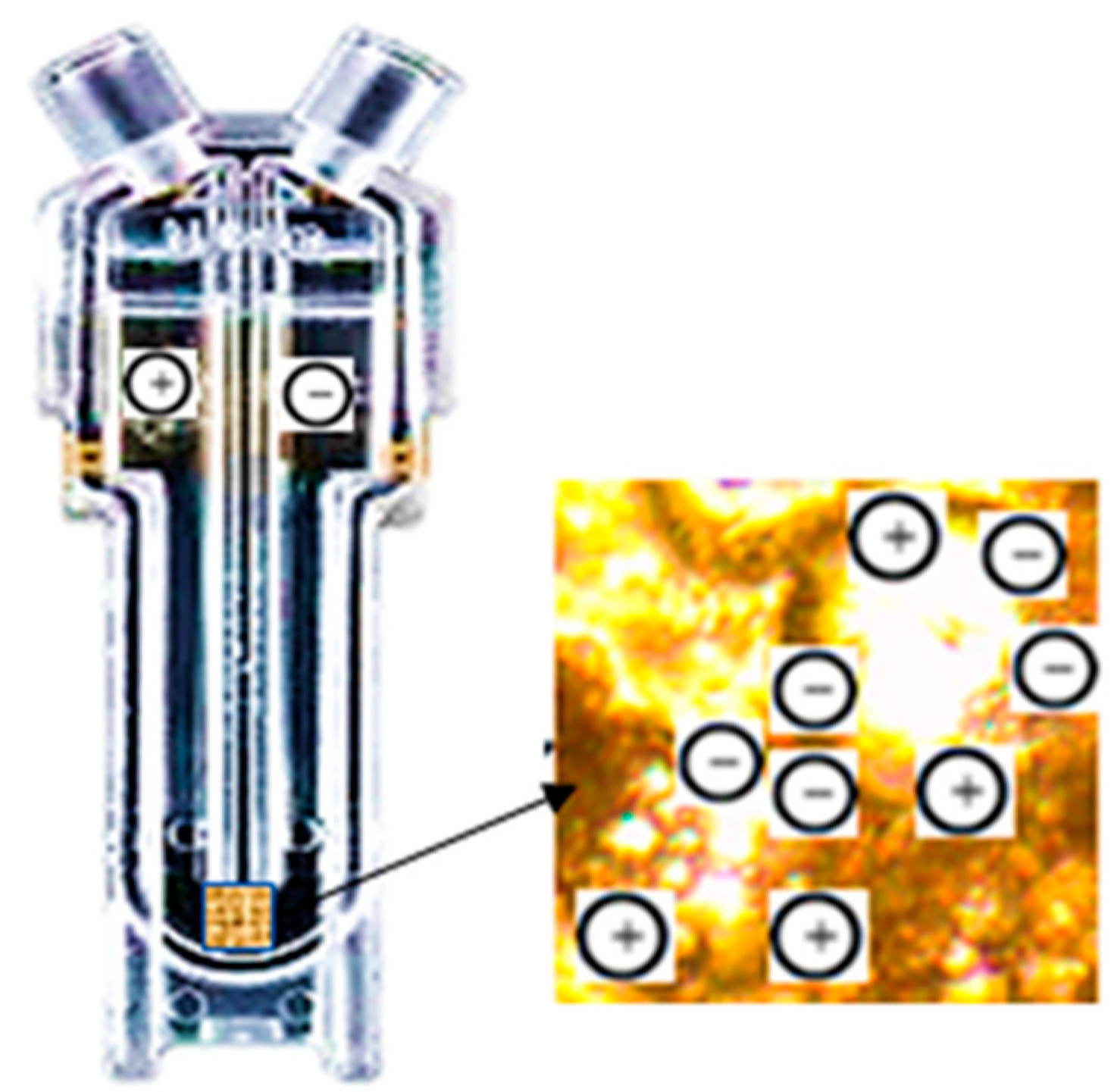
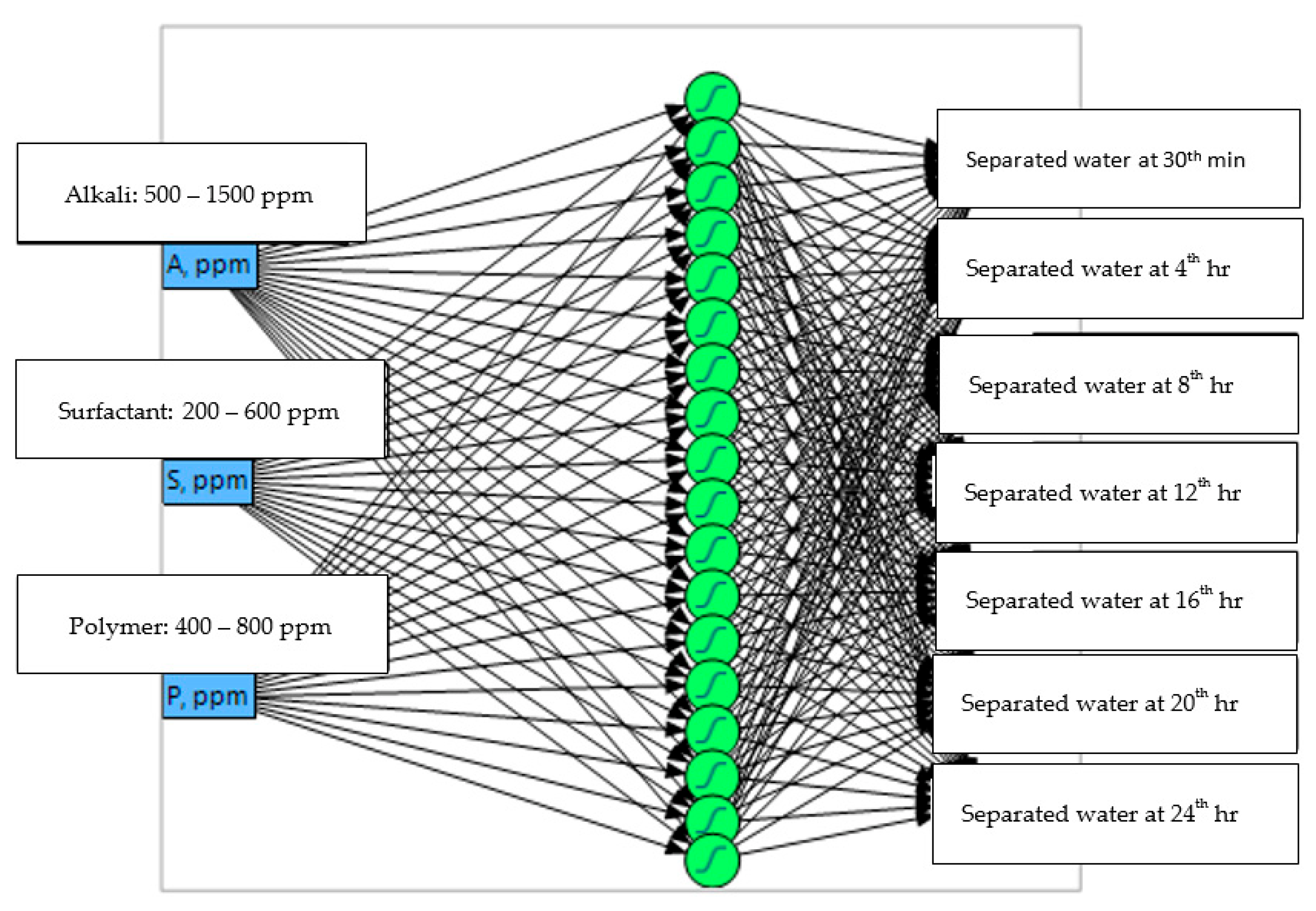




| Samples | Miri | Angsi | Penara | Dulang |
|---|---|---|---|---|
| Wax Appearance Temperature (°C) | 36.1 | 34.1 | 37 | 34.1 |
| Solid-to-Solid Crystalline (°C) | 59 | 63.1 | 47 | 50 |
| Crystallization Temperature (°C) | 63.1 | 68.1 | 51.1 | 55 |
| Samples | Miri | Angsi | Penara | Dulang |
|---|---|---|---|---|
| Wax Content (wt%) | 28.90 | 25.83 | 33.65 | 27.35 |
| Samples | Miri | Angsi | Penara | Dulang |
|---|---|---|---|---|
| Cumulative Mol Percent (from C20 to C40) | 16.83 | 15.21 | 26.74 | 25.86 |
| Crude Oil | Reservoir Brine | ||
|---|---|---|---|
| Property | Value | Chemicals | g/L |
| Density, g/cc @ 60 °C | 0.79 | CaCl2(H2O)2 | 0.8153 |
| Viscosity, cP @60 °C | 0.4 | MgCl2(H2O)6 | 0.7517 |
| API | 42 | NaCl | 9.2734 |
| TAN, mg KOH g−1 | 0.19 | SrCl2·6H2O | 0.0296 |
| Asphaltenes Content (wt%) | 0.1 | KCl | 0.4238 |
| Wax Content (wt%) | 25.83 | NaHCO3 | 7.1593 |
| Chemical | Property | Specification/Value |
|---|---|---|
| Alkali | Name | Sodium carbonate |
| Formula | Na2CO3 | |
| Chemical structure |  | |
| Molar mass | 106.0 g/mol | |
| Density | 2.54 g/mL | |
| Melting point | 851 °C | |
| Surfactant | Name | Alpha Olefin Sulfonate |
| Structural formula | RCH (OH) (CH2)n-SO3Na | |
| Active Matter Content (%) | 92 | |
| PH Value (1% aq. solution) | 9.5–11.5 | |
| Density | 2.54 g/mL | |
| Appearance | Clear and Bright | |
| Polymer | Name | Hydrolyzed Polyacrylicamide (HPAM) |
| Structural formula | 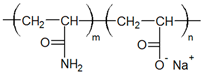 | |
| Hydrolysis in mole,% | 25–30 | |
| Molecular weight (million Dalton) | 16 | |
| Density | 1.189 g/mL at 25 °C | |
| Water Solubility | SOLUBLE | |
| Viscosity (mPa-S), at concentration, 1000 mg/L, at 60 ℃ | 10.5 |
| EOR Chemicals | Breakthrough in Separator | Reservoir Brine, Vol% | Crude Oil, Vol% |
|---|---|---|---|
| Alkali, Vol% | 5–15% | 40% | 60% |
| Surfactant, Vol% | 20–40% | ||
| Polymer, Vol% | 60–70% |
| Training | Validation | ||
|---|---|---|---|
| Measures | Value | Measures | Value |
| R2 | 0.95 | R2 | 0.99 |
| RMSE | 3.39 | RMSE | 1.66 |
| Mean Abs Dev. | 2.51 | Mean Abs Dev. | 1.585 |
| Log Likelihood | 31.71 | Log Likelihood | 7.71 |
| SSE | 138.66 | SSE | 11.09 |
| Sum Freq. | 11 | Sum Freq. | 4 |
| Sample | Alkali, ppm | Surfactant, ppm | Polymer, ppm | Separated Water, % |
|---|---|---|---|---|
| Effect of the alkali | 500 | 400 | 600 | 22 |
| 1500 | 33 | |||
| Effect of the surfactant | 1000 | 200 | 600 | 61 |
| 600 | 35 | |||
| Effect of the polymer | 1000 | 400 | 400 | 39 |
| 600 | 66 |
| Meas. Pts. | Shear Rate | Viscosity at Low Alkali, A = 500 ppm | Viscosity at High Alkali, A = 1500 ppm |
|---|---|---|---|
| [1/s] | [mPa-s] | [mPa-s] | |
| 1 | 1 | 7.90 | 6.00 |
| 2 | 11 | 7.20 | 5.60 |
| 3 | 22 | 6.70 | 5.10 |
| 4 | 32 | 6.10 | 4.20 |
| 5 | 42 | 5.70 | 4.30 |
| 6 | 52 | 5.60 | 4.20 |
| 7 | 62 | 5.80 | 4.10 |
| 8 | 72 | 5.70 | 4.20 |
| 9 | 90 | 5.90 | 4.10 |
| 10 | 100 | 5.60 | 4.10 |
| Meas. Pts. | Shear Rate | Viscosity A = 500 ppm | Viscosity A = 1500 ppm |
|---|---|---|---|
| [1/s] | [mPa-s] | [mPa-s] | |
| 1 | 1 | 8.00 | 7.40 |
| 2 | 11 | 7.60 | 6.90 |
| 3 | 22 | 7.20 | 6.20 |
| 4 | 32 | 7.00 | 5.80 |
| 5 | 42 | 6.50 | 5.70 |
| 6 | 52 | 6.80 | 5.80 |
| 7 | 62 | 6.90 | 5.80 |
| 8 | 72 | 6.80 | 5.80 |
| 9 | 90 | 6.30 | 5.80 |
| 10 | 100 | 6.30 | 5.70 |
| ASP Sample, ppm | Zeta Potential, mV | ASP Sample, ppm | Zeta Potential, mV | ASP Sample, ppm | Zeta Potential, mV |
|---|---|---|---|---|---|
| A = 500 | −30 | A = 500 | −31.5 | A = 500 | (Emulsion without ASP) −13.66 |
| S = 200 | S = 400 | S = 200 | |||
| P = 700 | P = 600 | P = 700 | |||
| A = 1000 | −27.5 | A = 1000 | −31.5 | A = 1000 | |
| S = 200, | S = 400 | S = 200 | |||
| P = 700 | P = 600 | P = 700 | |||
| A = 1500 | −21 | A = 1500 | −25 | A = 1500 | |
| S = 200 | S = 400 | S = 200 | |||
| P = 700 | P = 600 | P = 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irfan, M.; Khan, J.A.; Al-Kayiem, H.H.; Waqas, S.; Aleem, W.; Rozali, N.E.M.; Qamar, S.; Ghanim, A.A.J.; Ginter-Kramarczyk, D.; Legutko, S.; et al. Characteristics of Malaysian Crude Oils and Measurement of ASP Flooded Water in Oil Emulsion Stability and Viscosity in Primary Separator. Water 2023, 15, 1290. https://doi.org/10.3390/w15071290
Irfan M, Khan JA, Al-Kayiem HH, Waqas S, Aleem W, Rozali NEM, Qamar S, Ghanim AAJ, Ginter-Kramarczyk D, Legutko S, et al. Characteristics of Malaysian Crude Oils and Measurement of ASP Flooded Water in Oil Emulsion Stability and Viscosity in Primary Separator. Water. 2023; 15(7):1290. https://doi.org/10.3390/w15071290
Chicago/Turabian StyleIrfan, Muhammad, Javed Akbar Khan, Hussain H. Al-Kayiem, Sharjeel Waqas, Waqas Aleem, Nor Erniza Mohammad Rozali, Sabih Qamar, Abdulnour Ali Jazem Ghanim, Dobrochna Ginter-Kramarczyk, Stanislaw Legutko, and et al. 2023. "Characteristics of Malaysian Crude Oils and Measurement of ASP Flooded Water in Oil Emulsion Stability and Viscosity in Primary Separator" Water 15, no. 7: 1290. https://doi.org/10.3390/w15071290
APA StyleIrfan, M., Khan, J. A., Al-Kayiem, H. H., Waqas, S., Aleem, W., Rozali, N. E. M., Qamar, S., Ghanim, A. A. J., Ginter-Kramarczyk, D., Legutko, S., Kruszelnicka, I., & Rahman, S. (2023). Characteristics of Malaysian Crude Oils and Measurement of ASP Flooded Water in Oil Emulsion Stability and Viscosity in Primary Separator. Water, 15(7), 1290. https://doi.org/10.3390/w15071290












