Antibacterial and Photocatalytic Dye Degradation Activities of Green Synthesized NiSe Nanoparticles from Hibiscus rosa-sinensis Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesized Materials
2.2. Preparation of Hibiscus rosa-sinensis Leaf Extract
2.3. Synthesis of NiSe NPs
2.4. Characterization of NiSe NPs
2.5. Bacterial Suspension
2.6. Photocatalytic Degradation Experiment
3. Results and Discussion
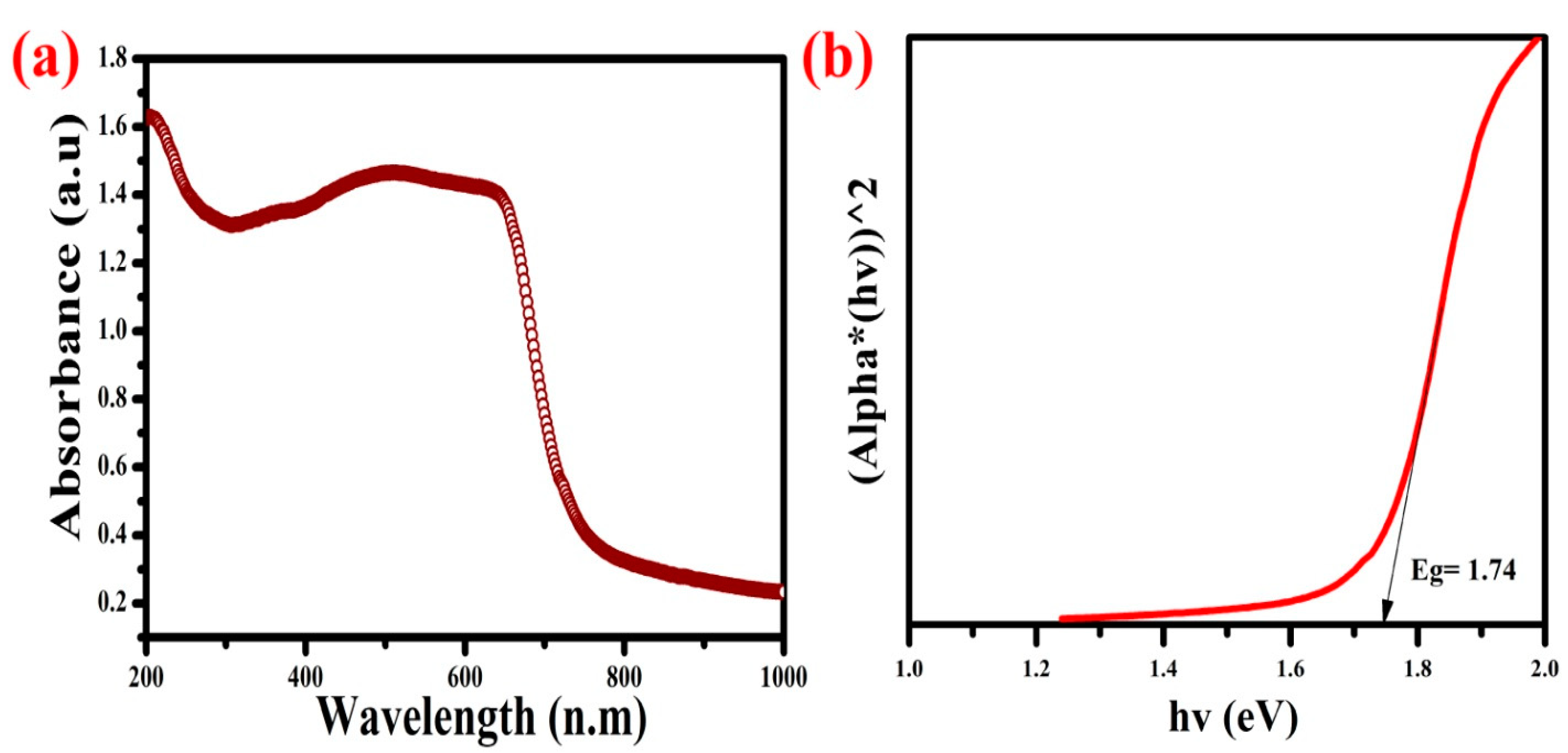
3.1. UV-Visible DRS Analysis
3.2. XRD Analysis
3.3. FTIR Analysis
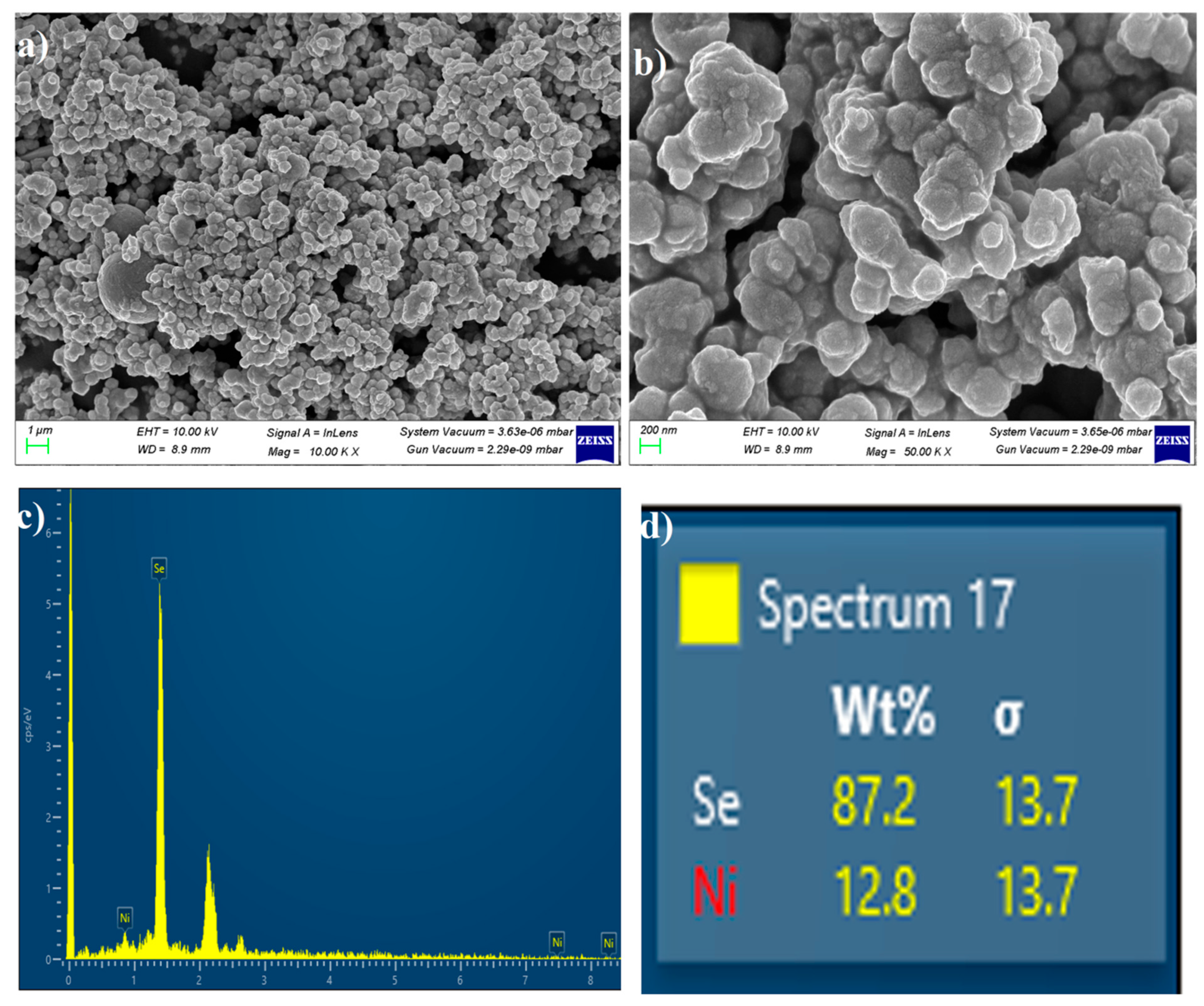
3.4. FE-SEM with EDX Analysis
3.5. TEM Analysis
3.6. XPS Analysis
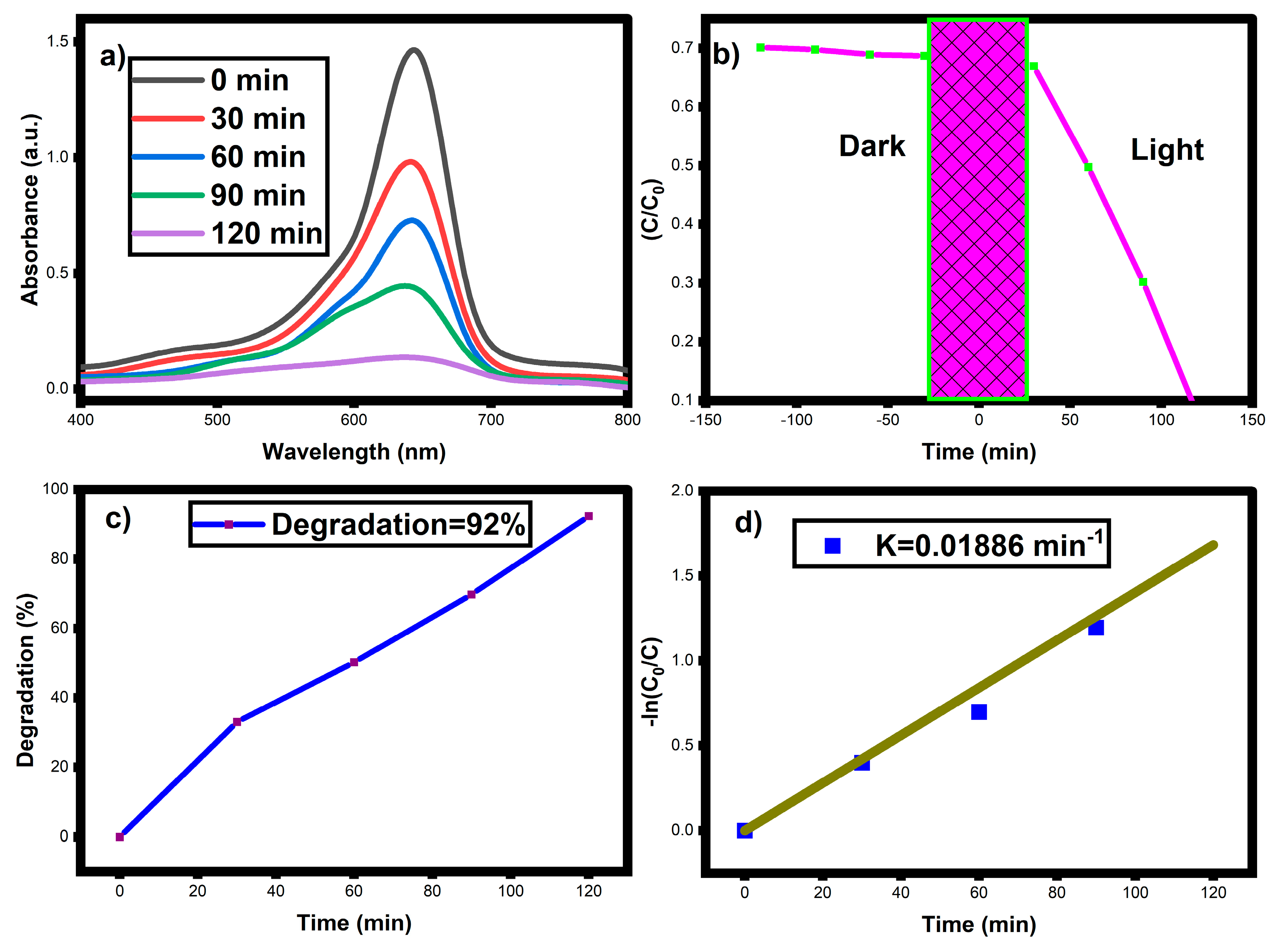
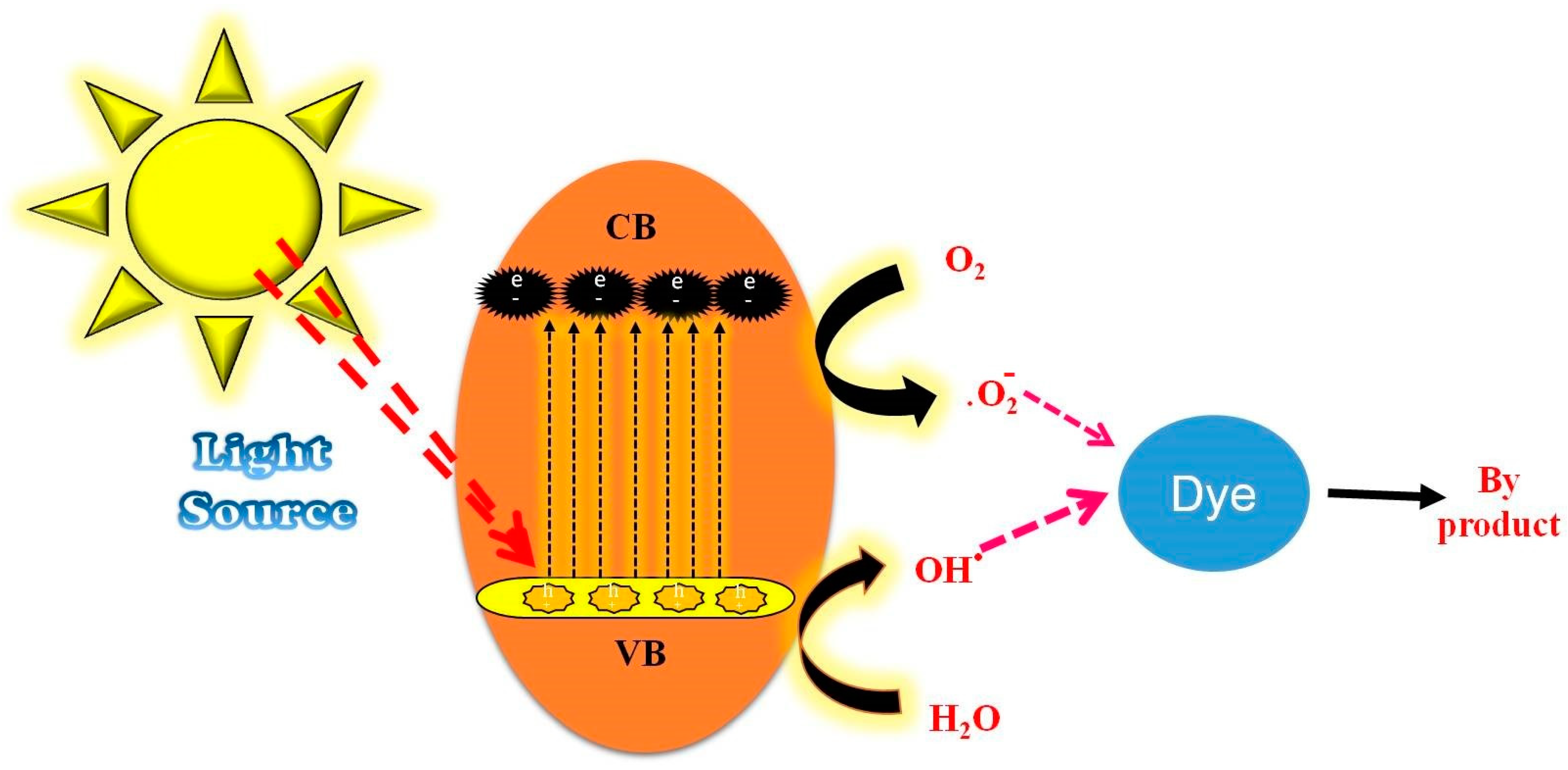
3.7. Photocatalytic Activity
3.8. Antimicrobial Activity
4. Conclusions
5. Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, M. (Ed.) Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Abualnaja, K.M.; El-Beltagi, H.S.; Ramadan, K.M.A.; Ashour, M. Dried Brown Seaweed’s Phytoremediation Potential for Methylene Blue Dye Removal from Aquatic Environments. Polymers 2022, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Curteanu, S.; Piuleac, C.G.; Godini, K.; Azaryan, G. Modeling of electrolysis process in wastewater treatment using different types of neural networks. Chem. Eng. J. 2011, 172, 267–276. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.; Ekama, G.A.; Brdjanovic, D. (Eds.) Biological Wastewater Treatment; IWA Publishing: London, UK, 2008. [Google Scholar]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total. Environ. 2020, 765, 142795. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total. Environ. 2019, 704, 135249. [Google Scholar] [CrossRef]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.; Hmoudah, M.; Broccoli, F.; Iesce, M.R.; Jung, O.-S.; Di Serio, M. Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation. Front. Chem. Eng. 2020, 2, 581487. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.-L. Application of physico-chemical pretreatment methods to enhance the sludge disintegration and subsequent anaerobic digestion: An up to date review. Rev. Environ. Sci. Bio. Technol. 2011, 10, 215–242. [Google Scholar] [CrossRef]
- Xiao, M.; Wang, Z.; Lyu, M.; Luo, B.; Wang, S.; Liu, G.; Cheng, H.-M.; Wang, L. Hollow nanostructures for photocatalysis: Advantages and challenges. Adv. Mater. 2019, 31, 1801369. [Google Scholar] [CrossRef] [PubMed]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Batistela, V.R.; Fogaça, L.Z.; Fávaro, S.L.; Caetano, W.; Fernandes-Machado NR, C.; Hioka, N. ZnO supported on zeolites: Photocatalyst design, microporosity and properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 20–27. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef]
- Sun, C.; Yang, J.; Xu, M.; Cui, Y.; Ren, W.; Zhang, J.; Zhao, H.; Liang, B. Recent intensification strategies of SnO2-based photocatalysts: A review. Chem. Eng. J. 2021, 427, 131564. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Transition metal selenides and diselenides: Hydrothermal fabrication, investigation of morphology, particle size and and their applications in photocatalyst. Adv. Colloid Interface Sci. 2021, 287, 102321. [Google Scholar] [CrossRef]
- Frame, F.A.; Carroll, E.C.; Larsen, D.S.; Sarahan, M.; Browning, N.D.; Osterloh, F.E. First demonstration of CdSe as a photocatalyst for hydrogen evolution from water under UV and visible light. Chem. Commun. 2008, 19, 2206–2208. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, H.; Xie, X.; Zhang, F.; Li, L. Preparation and photocatalytic activity of hollow ZnSe microspheres via Ostwald ripening. J. Alloys Compd. 2009, 473, 65–70. [Google Scholar] [CrossRef]
- Győri, Z.; Kónya, Z.; Kukovecz, Á. Visible light activation photocatalytic performance of PbSe quantum dot sensitized TiO2 Nanowires. Appl. Catal. B Environ. 2015, 179, 583–588. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Feng, Z.; Lan, F.; Teng, C.; Li, D.; Tang, J. A new HgSe photocatalyst for degradation of organic dyes under visible light irradiation. Mater. Lett. 2015, 161, 591–594. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.; Khan, H.; Ali, N.; Sartaj, S.; Malik, S.; Ali, N.; Khan, H.; Shah, S.; Bilal, M. Development and characterization of regenerable chitosan-coated nickel selenide nano-photocatalytic system for decontamination of toxic azo dyes. Int. J. Biol. Macromol. 2021, 182, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.A.; Kuperman, N.; Barnes, J.; Solanki, R. Magnetic Characterization of Cobalt Selenide and Nickel Selenide Thin Films. In Proceedings of the 2018 IEEE 13th Nanotechnology Materials and Devices Conference (NMDC), Portland, OR, USA, 14–17 October 2018; pp. 1–4. [Google Scholar]
- Hankare, P.; Jadhav, B.; Garadkar, K.; Chate, P.; Mulla, I.; Delekar, S. Synthesis and characterization of nickel selenide thin films deposited by chemical method. J. Alloys Compd. 2010, 490, 228–231. [Google Scholar] [CrossRef]
- Tito, G.S.; Abolanle, A.S.; Kuvarega, A.T.; Idris, A.O.; Mamba, B.B.; Feleni, U. Nickel selenide quantum dot applications in electrocatalysis and sensors. Electroanalysis 2020, 32, 2603–2614. [Google Scholar] [CrossRef]
- Du, L.; Du, W.; Ren, H.; Wang, N.; Yao, Z.; Shi, X.; Zhang, B.; Zai, J.; Qian, X. Honeycomb-like metallic nickel selenide nanosheet arrays as binder-free electrodes for high-performance hybrid asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 22527–22535. [Google Scholar] [CrossRef]
- Rahman, M.A.; Radhakrishnan, R. Microstructural properties and antibacterial activity of Ce doped NiO through chemical method. SN Appl. Sci. 2019, 1, 221. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, P.; Jo, S.; Pitipuech, N.; Sohn, J.I. Bifunctionality behavior of phase controlled nickel selenides in alkaline water electrolysis application. Electrochim. Acta 2020, 354, 136742. [Google Scholar] [CrossRef]
- Divya, R.; Manikandan, N.; Vinitha, G. Synthesis and characterization of nickel doped zinc selenide nanospheres for nonlinear optical applications. J. Alloys Compd. 2019, 791, 601–612. [Google Scholar] [CrossRef]
- Wei, P.; Li, J.; Kang, H.; Yang, Y.; Hao, Z.; Chen, X.; Guo, D.; Liu, L. Facile synthesis and promising application of iron-doped nickel selenide nanoparticles as high-efficiency counter electrodes of dye-sensitized solar cells. Int. J. Energy Res. 2019, 44, 845–854. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Physica E Low Dimens. Syst. Nanostruct. 2010, 42, 1417. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnology 2015, 9, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.-Y.; Lam, S.-M.; Sin, J.-C. Green synthesis of magnetic Fe-doped ZnO nanoparticles via Hibiscus rosa-sinensis leaf extracts for boosted photocatalytic, antibacterial and antifungal activities. Mater. Lett. 2019, 242, 103–106. [Google Scholar] [CrossRef]
- Anuradha, C.T.; Raji, P. Effect of annealing temperature on antibacterial, antifungal and structural properties of bio-synthesized Co3O4 nanoparticles using Hibiscus Rosa-sinensis. Mater. Res. Express 2019, 6, 095063. [Google Scholar] [CrossRef]
- Lu, L.; Zhuang, Z.; Fan, M.; Liu, B.; Yang, Y.; Huang, J.; Da, X.; Mo, J.; Li, Q.; Lu, H. Green formulation of Ag nanoparticles by Hibiscus rosa-sinensis: Introducing a navel chemotherapeutic drug for the treatment of liver cancer. Arab. J. Chem. 2021, 15, 103602. [Google Scholar] [CrossRef]
- Buarki, F.; AbuHassan, H.; Al Hannan, F.; Henari, F.Z. Green Synthesis of Iron Oxide Nanoparticles Using Hibiscus rosa sinensis Flowers and Their Antibacterial Activity. J. Nanotechnol. 2022, 2022, 5474645. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. China Rose/Hibiscus rosa-sinensis Pollen-Mediated Phytosynthesis of Silver Nanoparticles and Their Catalytic Activity. J. Compos. Sci. 2022, 6, 322. [Google Scholar] [CrossRef]
- Mughilmathi; Sonali, J.M.I.; Kumar, P.S.; Archana, K.M.; Rajagopal, R.; Gayathri, K.V. Application of copper iodide (CuI) and natural dye extracted from Hibiscus rosa-sinensis onto cotton fabric: An integrated approach. Appl. Nanosci. 2022, 1–10. [Google Scholar] [CrossRef]
- Sampath, S.; Bhushan, M.; Saxena, V.; Pandey, L.M.; Singh, L.R. Green synthesis of Ag doped ZnO nanoparticles: Study of their structural, optical, thermal and antibacterial properties. Mater. Technol. 2022, 37, 2785–2794. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Thangavel, S.; Kannan, K.; Selvakumar, V.; Muthusamy, K.; Siddiqui, M.R.; Wabaidur, S.M.; Parvathiraja, C. Lawsonia inermis mediated synthesis of ZnO/Fe2O3 nanorods for photocatalysis–Biological treatment for the enhanced effluent treatment, antibacterial and antioxidant activities. Chem. Phys. Lett. 2022, 804, 139907. [Google Scholar] [CrossRef]
- Mansour, A.T.; Alprol, A.E.; Khedawy, M.; Abualnaja, K.M.; Shalaby, T.A.; Rayan, G.; Ramadan, K.M.A.; Ashour, M. Green Synthesis of Zinc Oxide Nanoparticles Using Red Seaweed for the Elimination of Organic Toxic Dye from an Aqueous Solution. Materials 2022, 15, 5169. [Google Scholar] [CrossRef] [PubMed]
- Tito, G.S.; Kuvarega, A.T.; Mamba, B.B.; Feleni, U. Electrochemical Detection of Nevirapine Using Banana Peel Extract Functionalised Nickel Selenide Quantum Dots. Electrocatalysis 2022, 14, 393–405. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, A.; Lai, W.-C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Al-Hada, N.M.; Kasmani, R.M.; Kasim, H.; Al-Ghaili, A.M.; Saleh, M.A.; Baqiah, H.; Al-Asbahi, B.A.; Yang, J.; Noorazlan, A.M.; Li, Q.; et al. Up-scalable synthesis of size-controlled NiSe nanoparticles using single step technique. J. Mater. Res. Technol. 2022, 18, 4918–4929. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, Y.; Liang, H.; Zhao, B.; Jin, Y.; Liu, J.; Li, Z. Bimetal selenide NiSe/ZnSe heterostructured nanoparticals decorated porous g-C3N4 nanosheets to boost H2 evolution and urea synthesis. Appl. Surf. Sci. 2022, 602, 154329. [Google Scholar] [CrossRef]
- Zeng, R.; Cheng, C.; Xing, F.; Zou, Y.; Ding, K.; Huang, C. Dual vacancies induced local polarization electric field for high-performance photocatalytic H2 production. Appl. Catal. B Environ. 2022, 316, 121680. [Google Scholar] [CrossRef]
- Jia, J.; Zheng, L.; Li, K.; Zhang, Y.; Xie, H. Two-electron transfer mechanism from 3D/3D nickel selenide/MoS2 heterostructure accelerates photocatalytic hydrogen evolution and tetracycline hydrochloride removal. Chem. Eng. J. 2022, 429, 132432. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Liao, J.; Wang, L.; Ju, Y.; Wang, X.; Wei, J.; Hu, N.; Xu, R.; Yang, L. NiSe@ Ni12P5 hierarchical nanorod arrays coupled on nickel-copper foam for highly efficient urea oxidation. Appl. Surf. Sci. 2023, 607, 155041. [Google Scholar] [CrossRef]
- Xu, X.; Wang, R.; Chen, S.; Trukhanov, A.; Wu, Y.; Shao, L.; Heng, L.; Sun, Z. Interface engineering of hierarchical P-doped NiSe/2H-MoSe 2 nanorod arrays for efficient hydrogen evolution. Inorg. Chem. Front. 2022, 9, 5507–5516. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Xie, M.; Zhao, S.; Li, Z.; Liu, Q. Interfacially engineered induced nickel-based heterostructures as efficient catalysts for Li-O2 batteries. Electrochim. Acta 2023, 437, 141476. [Google Scholar] [CrossRef]
- Manickam, S.; Kuzhandaivel, H.; Selvaraj, Y.; Franklin, M.C.; Nallathambi, K.S. One-pot synthesis of TEA functionalized and NiSe embedded rGO nanocomposites for supercapacitor application. Dalton Trans. 2022, 51, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.A.; Yazdi, M.S.; Noghani, M.T.; Moghanian, A.; Hosseini, S.A. Synthesis and optimization of Mo–Ni–Se@ NiSe core-shell nanostructures as efficient and durable electrocatalyst for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2022, 47, 34455–34470. [Google Scholar] [CrossRef]
- Cao, Y.; Lei, F.; Li, Y.; Fu, Y.; Zhao, J.; Qiu, S.; Zhang, Z. Interface engineering in NiSe2/Ni2Co/CoSe2 heterostructures encapsulated in hollow carbon shells for high-rate Li–Se batteries. Nanoscale 2022, 14, 13227–13235. [Google Scholar] [CrossRef] [PubMed]
- Balaji, D.; Arunachalam, P.; Duraimurugan, K.; Madhavan, J.; Theerthagiri, J.; Al-Mayouf, A.M.; Choi, M.Y. Highly efficient Ni0.5Fe0.5Se2/MWCNT electrocatatalyst for hydrogen evolution reaction in acid media. Int. J. Hydrogen Energy 2020, 45, 7838–7847. [Google Scholar] [CrossRef]
- Hussain, R.A.; Hussain, I. Fabrication and applications of nickel selenide. J. Solid State Chem. 2019, 277, 316–328. [Google Scholar] [CrossRef]
- Khan, B.A.; Hussain, R.; Shah, A.; Mahmood, A.; Shah, M.Z.U.; Ismail, J.; Rahman, S.U.; Sajjad, M.; Assiri, M.A.; Imran, M.; et al. NiSe2 nanocrystals intercalated rGO sheets as a high-performance asymmetric supercapacitor electrode. Ceram. Int. 2022, 48, 5509–5517. [Google Scholar] [CrossRef]
- Wang, Y.; Torres, J.A.; Shviro, M.; Carmo, M.; He, T.; Ribeiro, C. Photocatalytic materials applications for sustainable agriculture. Prog. Mater. Sci. 2022, 130, 100965. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.-U.; Tahir, M.B.; Riaz, K.N.; Iqbal, T.; Rafique, M.; Hussain, S.; Raza, W.; Aslam, I.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. Int. J. Environ. Anal. Chem. 2020, 102, 434–442. [Google Scholar] [CrossRef]
- Mahdavi, K.; Zinatloo-Ajabshir, S.; Yousif, Q.A.; Salavati-Niasari, M. Enhanced photocatalytic degradation of toxic contaminants using Dy2O3-SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach. Ultrason. Sonochem. 2021, 82, 105892. [Google Scholar] [CrossRef] [PubMed]
- Ravele, M.P.; Oyewo, O.A.; Ramaila, S.; Mavuru, L.; Onwudiwe, D.C. Facile synthesis of copper oxide nanoparticles and their applications in the photocatalytic degradation of acyclovir. Results Eng. 2022, 14, 100479. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, T.; Wang, X.; Pan, H.; Xie, Z.; Lu, H.; Pan, K.; Xie, Y. Enhancement of the triiodide reduction reaction by doping molybdenum in NiSe hierarchical microspheres: A theoretical and experimental study. Inorg. Chem. Front. 2022, 10, 460–467. [Google Scholar] [CrossRef]
- Gan, Y.; Li, Z.; Ye, Y.; Dai, X.; Nie, F.; Yin, X.; Ren, Z.; Wu, B.; Cao, Y.; Cai, R.; et al. Doping Mo into NiFe LDH/NiSe Heterostructure to Enhance Oxygen Evolution Activity by Synergistically Facilitating Electronic Modulation and Surface Reconstruction. ChemSusChem 2022, 15, e202201205. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-Y.; Zhang, X.; Han, X.; Zhou, C.; Lu, S.; Lang, J.-P.; Gu, H. Nanostructured catalyst assembled from CNTs, NiSe2 nanoparticles, and 2D Ni-MOF nanosheets for electrocatalytic hydrogen evolution reaction. CrystEngComm 2022, 24, 8503–8508. [Google Scholar] [CrossRef]
- Xin, Y.; Nie, S.; Pan, S.; Miao, C.; Mou, H.; Wen, M.; Xiao, W. Electrospinning fabrication of Sb-SnSb/TiO2@ CNFs composite nanofibers as high-performance anodes for lithium-ion batteries. J. Colloid Interface Sci. 2022, 630, 403–414. [Google Scholar] [CrossRef]
- Bao, W.; Yang, C.; Ai, T.; Zhang, J.; Zhou, L.; Yan, L.; Wei, X.; Zou, X.; Wang, Y. Modulating interfacial charge distribution of NiSe nanoarrays with NiFe-LDH nanosheets for boosting oxygen evolution reaction. Fuel 2023, 332, 126227. [Google Scholar] [CrossRef]
- Chang, K.; Tran, D.T.; Wang, J.; Kim, N.H.; Lee, J.H. A 3D hierarchical network derived from 2D Fe-doped NiSe nanosheets/carbon nanotubes with enhanced OER performance for overall water splitting. J. Mater. Chem. A 2021, 10, 3102–3111. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Liu, Z.; Gao, S.; Zhang, G.; Hou, Y.; Wen, G.; Zhang, L.; Gu, H.; Zhang, R. A unique two-phase heterostructure with cubic NiSe2 and orthorhombic NiSe2 for enhanced lithium ion storage and electrocatalysis. Dalton Trans. 2022, 51, 12829–12838. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Pang, X.; Wang, X.; Xu, H.; Zhang, C.; Zhang, Q.; Zhou, Y.; Tian, L. Facile fabrication of hydrangea-like NiSe/FeSe2 nanostructures towards efficient water oxidation. J. Saudi Chem. Soc. 2022, 26, 101469. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Suganthy, N.; Shanmugam, S.; Brindhadevi, K.; Sabour, A.; Alshiekheid, M.; Chi, N.T.L.; Pugazhendhi, A.; Shanmuganathan, R. Biosynthesis of zirconium nanoparticles (ZrO2 NPs) by Phyllanthus niruri extract: Characterization and its photocatalytic dye degradation activity. Food Chem. Toxicol. 2022, 168, 113340. [Google Scholar] [CrossRef] [PubMed]
- Shelke, H.D.; Machale, A.R.; Survase, A.A.; Pathan, H.M.; Lokhande, C.D.; Lokhande, A.C.; Shaikh, S.F.; Rana, A.U.H.S.; Palaniswami, M. Multifunctional Cu2SnS3 Nanoparticles with Enhanced Photocatalytic Dye Degradation and Antibacterial Activity. Materials 2022, 15, 3126. [Google Scholar] [CrossRef]
- Tantubay, K.; Das, P.; Baskey, M. Hydrogen peroxide–assisted photocatalytic dye degradation over reduced graphene oxide integrated ZnCr2O4 nanoparticles. Environ. Sci. Pollut. Res. 2021, 29, 17309–17318. [Google Scholar] [CrossRef] [PubMed]
- Jasrotia, R.; Kumari, N.; Kumar, R.; Naushad, M.; Dhiman, P.; Sharma, G. Photocatalytic degradation of environmental pollutant using nickel and cerium ions substituted Co0.6Zn0.4Fe2O4 nanoferrites. Earth Syst. Environ. 2021, 5, 399–417. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J.-Q. A new 3D 8-connected Cd(ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The extra-large calixarene-based MOFs-derived hierarchical composites for photocatalysis of dye: Facile syntheses and contribution of carbon species. J. Alloys Compd. 2021, 897, 163178. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Liu, S.; Lu, C.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalton Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef]
- Oh, W.-C.; Ullah, K.; Zhu, L.; Meng, Z.-D.; Ye, S.; Sarkar, S. Photocatalytic properties under visible light with graphene based platinium selenide nanocomposites synthesized by microwave assisted method. Mater. Sci. Semicond. Process. 2014, 25, 34–42. [Google Scholar] [CrossRef]
- Beena, V.; Ajitha, S.; Rayar, S.L.; Parvathiraja, C.; Kannan, K.; Palani, G. Enhanced Photocatalytic and Antibacterial Activities of ZnSe Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4390–4401. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, A.; Ali, N.; Khan, S.; Uddin, S.; Malik, S.; Ali, N.; Khan, H.; Khan, H.; Bilal, M. Photocatalytic degradation of crystal violet dye under sunlight by chitosan-encapsulated ternary metal selenide microspheres. Environ. Sci. Pollut. Res. 2020, 28, 8074–8087. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.; E Iram, N.; Khan, M.S.; Baig, U. Facile Synthesis, Characterization and Photocatalytic Activity of Band Gap Engineered Cobalt Selenide Nanoparticles. Arab. J. Sci. Eng. 2016, 41, 2377–2384. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Shalan, A.E.; Afifi, M.; El-Desoky, M.M.; Lanceros-Méndez, S. Silver-doped cadmium selenide/graphene oxide-filled cellulose acetate nanocomposites for photocatalytic degradation of malachite green toward wastewater treatment. ACS Omega 2021, 6, 23129–23138. [Google Scholar] [CrossRef]
- Ashiq, M.N.; Irshad, S.; Ehsan, M.F.; Rehman, S.; Farooq, S.; Najam-Ul-Haq, M.; Zia, A. Visible-light active tin selenide nanostructures: Synthesis, characterization and photocatalytic activity. New J. Chem. 2017, 41, 14689–14695. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Xiong, X.; Chen, C.; Zeng, J.; Kong, Z.; Wang, H.; Xi, J.; Yuan, Y.-J.; Ji, Z. High photocatalytic and photoelectrochemical performances of the CuSe/MoSe2 2D/2D face-to-face heterojunction photocatalyst. J. Alloys Compd. 2021, 870, 159540. [Google Scholar] [CrossRef]
- Patidar, D.; Yadav, A.; Paul, D.R.; Sharma, A.; Nehra, S. Nanohybrids cadmium selenide-reduced graphene oxide for improving photo-degradation of methylene blue. Phys. E Low-Dimens. Syst. Nanostructures 2019, 114, 113560. [Google Scholar] [CrossRef]
- Ullah, K.; Ye, S.; Jo, S.-B.; Zhu, L.; Cho, K.-Y.; Oh, W.-C. Optical and photocatalytic properties of novel heterogeneous PtSe2–graphene/TiO2 nanocomposites synthesized via ultrasonic assisted techniques. Ultrason. Sonochem. 2014, 21, 1849–1857. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Pham, D.K.; Tran, N.Q.; Dang, L.H.; Nguyen, N.H.; Nguyen, T.V.; Nguyen, T.H.; Luong, T.B. Comparative Studies of Blue-Emitting Zinc Selenide Nanocrystals Doped with Ag, Cu, and Mg towards Medical Applications. Crystals 2022, 12, 625. [Google Scholar] [CrossRef]
- Bootchanont, A.; Wechprasit, T.; Isran, N.; Theangsunthorn, J.; Chaosuan, N.; Chanlek, N.; Kidkhunthod, P.; Yimnirun, R.; Jiamprasertboon, A.; Eknapakul, T.; et al. Correlation of the antibacterial activity and local structure in Zn- and Mn-doped hydroxyapatites by Rietveld refinement and the first-principles method. Materialia 2022, 26, 101586. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Priyadarshi, R.; Biswas, D.; Rhim, J.W. Recent advances in metal sulfide nanoparticle-added bionanocomposite films for food packaging applications. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, Y.; Wu, G.; Zhou, L.; Wei, S. Synthesis of sub-1 nm 2D transitional metal oxides via anion exchange-bonds cleavage cascade reactions. Chem. Eng. J. 2022, 437, 135230. [Google Scholar] [CrossRef]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]






| Si. No | Material | Dye | Dye Volume | Catalyst Load | Light Source | Efficiency | Ref |
|---|---|---|---|---|---|---|---|
| 1. | PbSe NPs | Rh-B | 50 mL | 50 mg | 50 W- halogen light | 82%/30 min | [82] |
| 2. | CuSe 2-x | MB | 50 mL | 0.1 g | Sunlight | 93.4% | [83] |
| 3. | PtSe2/graphene | MB | 80 mL | 10 mg | Visible light source | 95%/180 min | [84] |
| 4. | ZnSe | MO | 100 mL | 10 mg | XENON LAMP | 80%/120 min | [85] |
| 5. | BISe-CM | CV | 20 mL | 0.2 g | Natural Sunlight | 98%/150 min | [86] |
| 6. | CoSe | Rh-B | 50 mL | 50 μg/mL | UV light | 98%/120 min | [87] |
| 7. | Ag-CdSe/GO@CA | Mg | 30 mL | 120 mg | 300 W mercury lamp light s | 97%/25 min | [88] |
| 8. | SnSe | MB | 100 mL | 0.10 g | Sunlight | 98.4%/60 min | [89] |
| 9. | CuSe/MoSe2 | MB | 100 mL | 20 mg | 250 W high-pressure mercury lamp | 95%/120 min | [90] |
| 10. | CdSe-rGO | MB | 80 mL | 30 mg | 8 W-Hg lamps | 70%/210 min | [91] |
| 11. | PtSe2–graphene/TiO2 | Rh-B | 80 mL | 10 mg | UV light | 70%/180 min | [92] |
| 12. | NiSe | MB | 50 mL | 50 mg | Visible light | 92%/120 min | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayutham, L.; Parvathiraja, C.; Anitha, D.C.; Mahalakshmi, K.; Jenila, M.; Gupta, J.K.; Wabaidur, S.M.; Siddiqui, M.R.; Aftab, S.; Lai, W.-C. Antibacterial and Photocatalytic Dye Degradation Activities of Green Synthesized NiSe Nanoparticles from Hibiscus rosa-sinensis Leaf Extract. Water 2023, 15, 1380. https://doi.org/10.3390/w15071380
Velayutham L, Parvathiraja C, Anitha DC, Mahalakshmi K, Jenila M, Gupta JK, Wabaidur SM, Siddiqui MR, Aftab S, Lai W-C. Antibacterial and Photocatalytic Dye Degradation Activities of Green Synthesized NiSe Nanoparticles from Hibiscus rosa-sinensis Leaf Extract. Water. 2023; 15(7):1380. https://doi.org/10.3390/w15071380
Chicago/Turabian StyleVelayutham, Lakshmi, C. Parvathiraja, Dhivya Christo Anitha, K. Mahalakshmi, Mary Jenila, Jeetendra Kumar Gupta, Saikh Mohammad Wabaidur, Masoom Raza Siddiqui, Sikandar Aftab, and Wen-Cheng Lai. 2023. "Antibacterial and Photocatalytic Dye Degradation Activities of Green Synthesized NiSe Nanoparticles from Hibiscus rosa-sinensis Leaf Extract" Water 15, no. 7: 1380. https://doi.org/10.3390/w15071380






