The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention
Abstract
1. Introduction
2. Materials and Methods
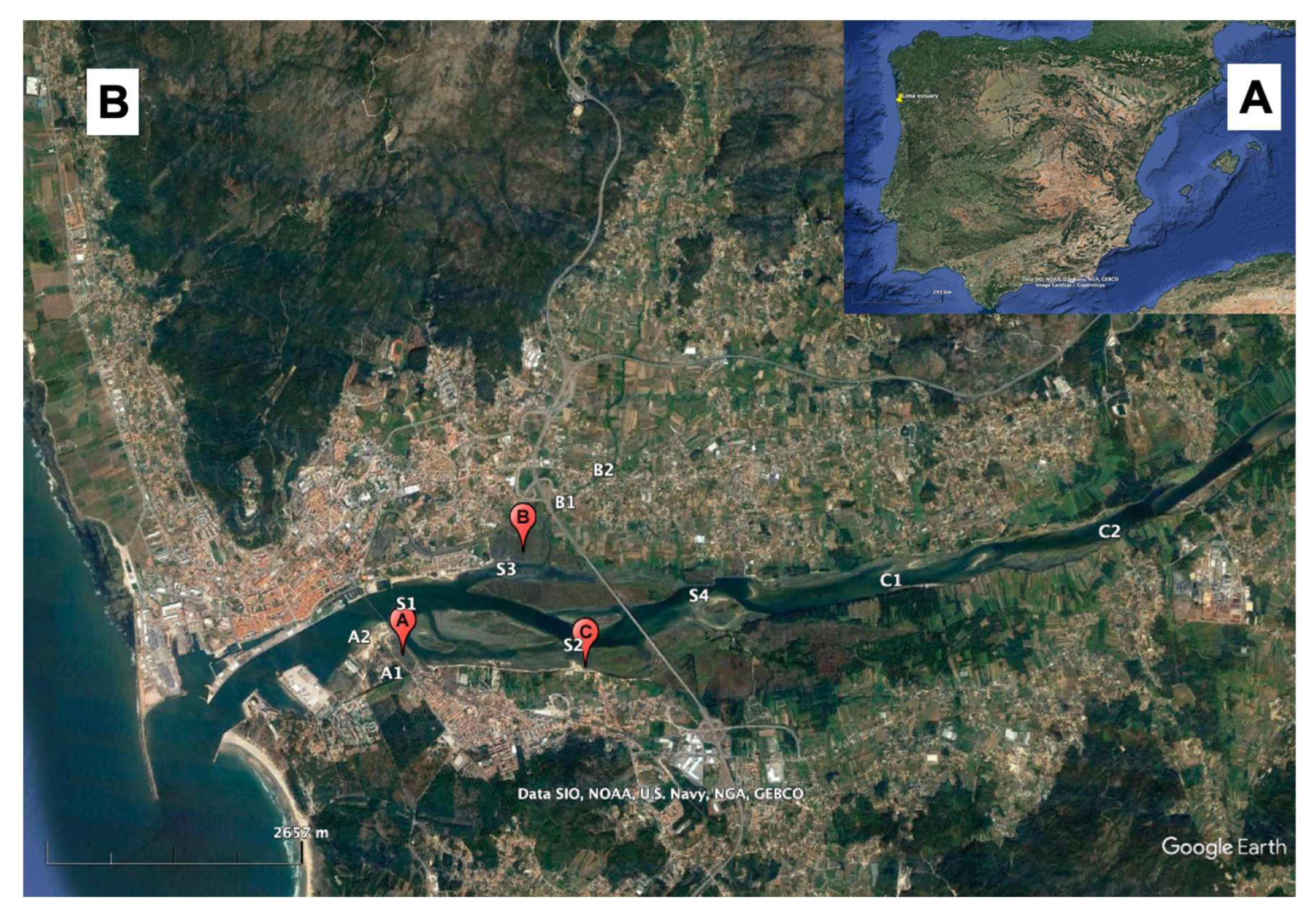
2.1. Sampling and Samples Preservation
- (1)
- Sra. das Areias (site A)—located in the south margin of the lower section of the Lima estuary. A small open water reservoir drains into this sampling station (A2);
- (2)
- Salinas (site B)—located in the north margin of the middle estuary. There is a freshwater stream close by, Ribeira de Portuzelo;
- (3)
- Canoagem (site C)—located in the south margin of the middle estuary.
- (1)
- Sra. das Areias (Site B): 3 samples (non-vegetated; J. maritimus; P. australis)
- (2)
- Salinas (Site B): 3 samples (non-vegetated; J. maritimus; P. australis)
- (3)
- Canoagem (Site C): 3 samples (non-vegetated; J. maritimus; S. maritima)
2.2. MPs Analysis
2.2.1. MPs Extraction
2.2.2. MPs Characterisation
2.3. Data Analysis
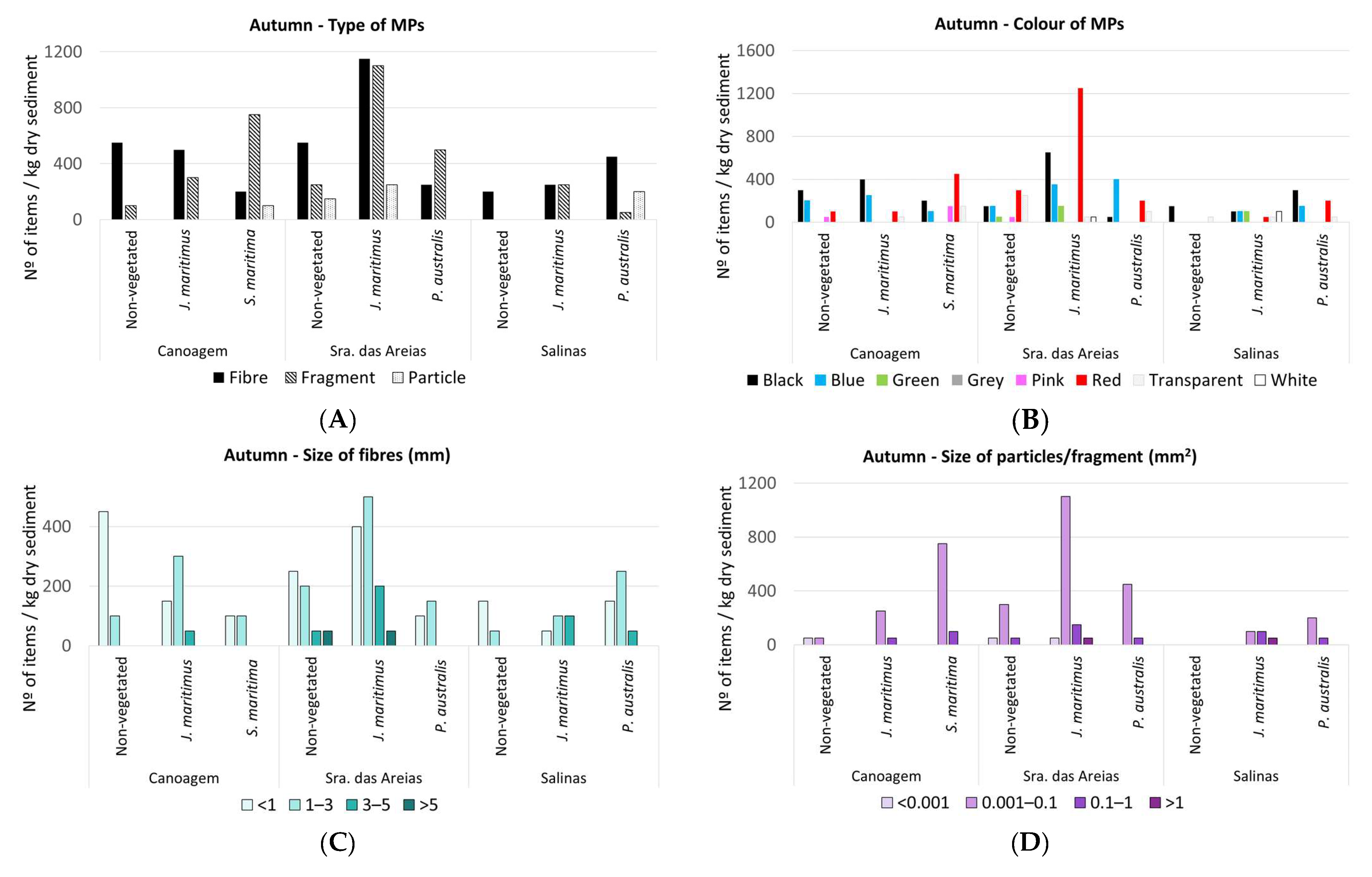
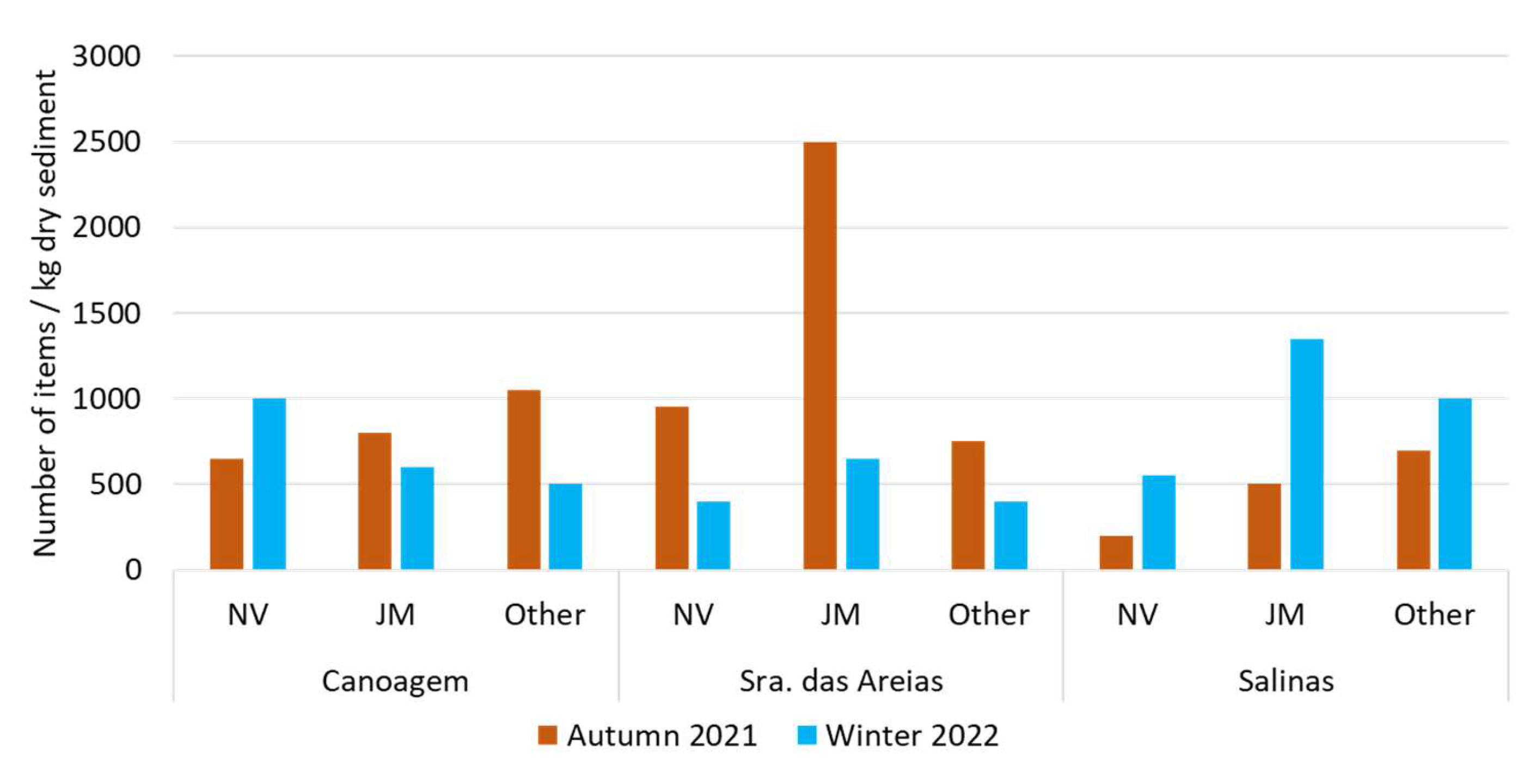
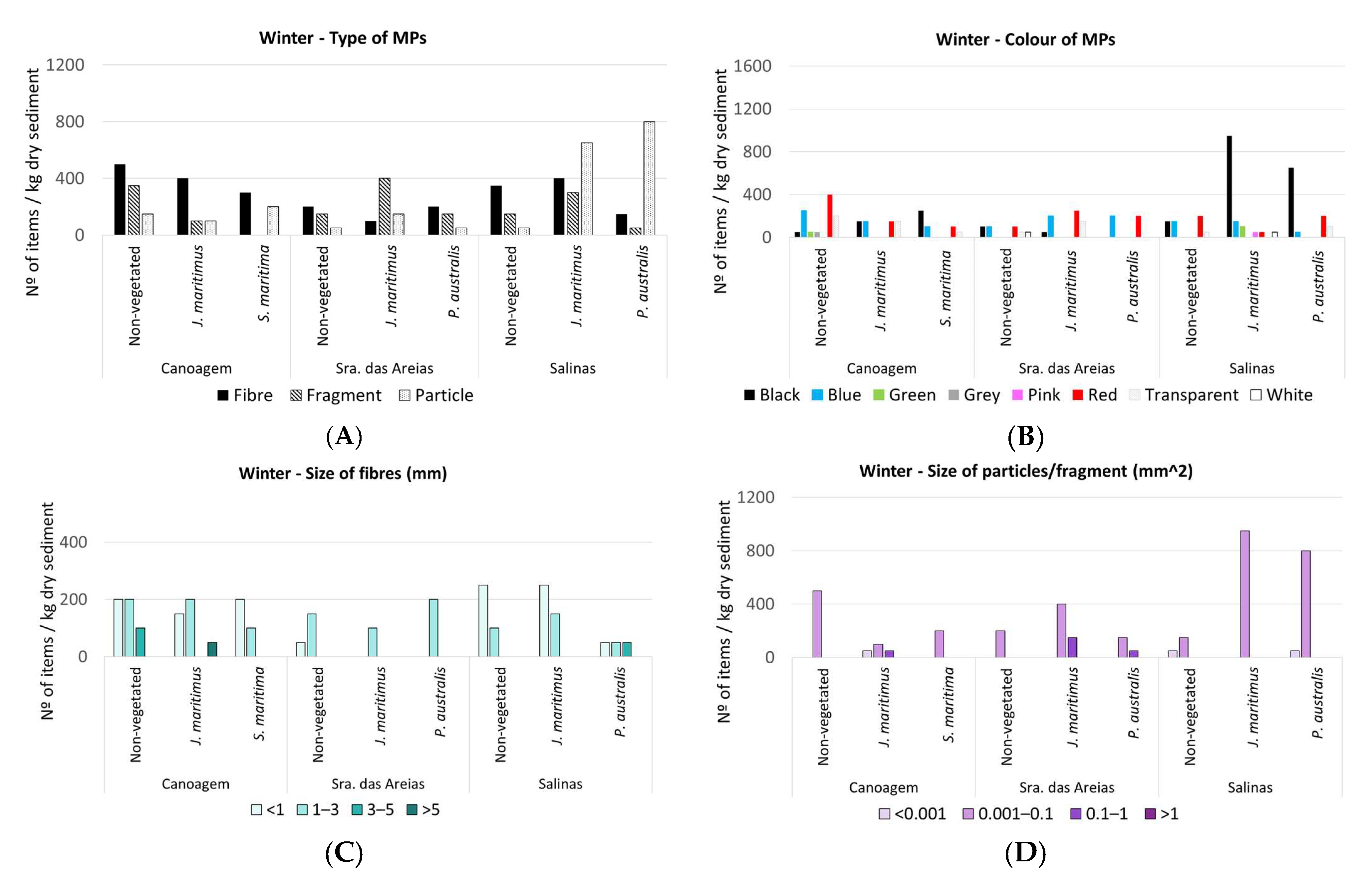
3. Results
3.1. MPs in Estuarine Sediments
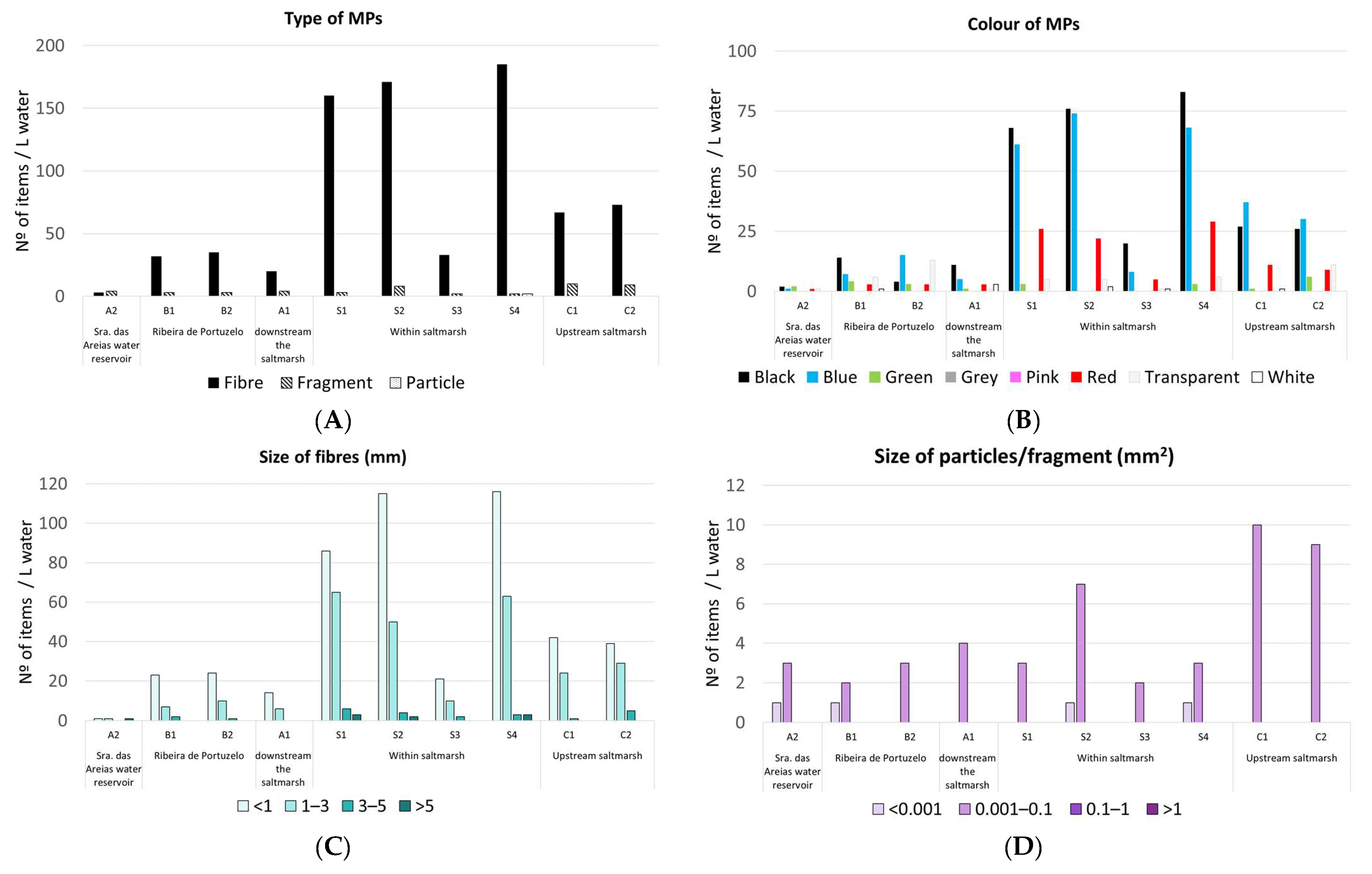
3.2. MPs in Estuarine Waters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velez, N.; Zardi, G.I.; Lo Savio, R.; McQuaid, C.D.; Valbusa, U.; Sabour, B.; Nicastro, K.R. A baseline assessment of beach macrolitter and microplastics along northeastern Atlantic shores. Mar. Pollut. Bull. 2019, 149, 110649. [Google Scholar] [CrossRef]
- Villarrubia-Gómez, P.; Cornell, S.E.; Fabres, J. Marine plastic pollution as a planetary boundary threat—The drifting piece in the sustainability puzzle. Mar. Policy 2018, 96, 213–220. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Year Book 2014: Emerging Issues in Our Global Environment—United Nations Environment Programme; United Nations Environment Programme: Nairobi, Kenya, 2014. [Google Scholar]
- Rodrigues, S.M.; Elliott, M.; Almeida, C.M.R.; Ramos, S. Microplastics and plankton: Knowledge from laboratory and field studies to distinguish contamination from pollution. J. Hazard. Mater. 2021, 417, 126057. [Google Scholar] [CrossRef] [PubMed]
- Maghsodian, Z.; Sanati, A.M.; Tahmasebi, S.; Shahriari, M.H.; Ramavandi, B. Study of microplastics pollution in sediments and organisms in mangrove forests: A review. Environ. Res. 2022, 208, 112725. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Piarulli, S.; Vanhove, B.; Comandini, P.; Scapinello, S.; Moens, T.; Vrielinck, H.; Sciutto, G.; Prati, S.; Mazzeo, R.; Booth, A.M.; et al. Do different habits affect microplastics contents in organisms? A trait-based analysis on salt marsh species. Mar. Pollut. Bull. 2020, 153, 110983. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.M.; Agostini, V.O.; Lima, A.R.A.; Ward, R.D.; Pinho, G.L.L. The fate of plastic litter within estuarine compartments: An overview of current knowledge for the transboundary issue to guide future assessments. Environ. Pollut. 2021, 279, 116908. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Rodrigues, S.M.; Pereira, R.; Silva, D.; Almeida, C.M.R. Floatables and Plastic Debris in Estuarine and Coastal Marine Environments. In Treatise on Estuarine and Coastal Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Unsworth, R.K.F.; Higgs, A.; Walter, B.; Cullen-Unsworth, L.C.; Inman, I.; Jones, B.L. Canopy Accumulation: Are Seagrass Meadows a Sink of Microplastics? Oceans 2021, 2, 162–178. [Google Scholar] [CrossRef]
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Mar. Pollut. Bull. 2019, 145, 569–582. [Google Scholar] [CrossRef]
- Qian, J.; Tang, S.; Wang, P.; Lu, B.; Li, K.; Jin, W.; He, X. From source to sink: Review and prospects of microplastics in wetland ecosystems. Sci. Total Environ. 2021, 758, 143633. [Google Scholar] [CrossRef]
- Malli, A.; Corella-Puertas, E.; Hajjar, C.; Boulay, A.-M. Transport mechanisms and fate of microplastics in estuarine compartments: A review. Mar. Pollut. Bull. 2022, 177, 113553. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Arias, A.H.; Ronda, A.C.; Piccolo, M.C. Continental microplastics: Presence, features, and environmental transport pathways. Sci. Total Environ. 2021, 799, 149447. [Google Scholar] [CrossRef]
- Paduani, M. Microplastics as novel sedimentary particles in coastal wetlands: A review. Mar. Pollut. Bull. 2020, 161, 111739. [Google Scholar] [CrossRef] [PubMed]
- Näkki, P.; Setälä, O.; Lehtiniemi, M. Bioturbation transports secondary microplastics to deeper layers in soft marine sediments of the northern Baltic Sea. Mar. Pollut. Bull. 2017, 119, 255–261. [Google Scholar] [CrossRef]
- Yao, W.; Di, D.; Wang, Z.; Liao, Z.; Huang, H.; Mei, K.; Dahlgren, R.A.; Zhang, M.; Shang, X. Micro- and macroplastic accumulation in a newly formed Spartina alterniflora colonized estuarine saltmarsh in southeast China. Mar. Pollut. Bull. 2019, 149, 110636. [Google Scholar] [CrossRef]
- Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P. Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef]
- Fraser, M.A.; Chen, L.; Ashar, M.; Huang, W.; Zeng, J.; Zhang, C.; Zhang, D. Occurrence and distribution of microplastics and polychlorinated biphenyls in sediments from the Qiantang River and Hangzhou Bay, China. Ecotoxicol. Environ. Saf. 2020, 196, 110536. [Google Scholar] [CrossRef] [PubMed]
- Lloret, J.; Pedrosa-Pamies, R.; Vandal, N.; Rorty, R.; Ritchie, M.; McGuire, C.; Chenoweth, K.; Valiela, I. Salt marsh sediments act as sinks for microplastics and reveal effects of current and historical land use changes. Environ. Adv. 2021, 4, 100060. [Google Scholar] [CrossRef]
- Ouyang, X.; Duarte, C.M.; Cheung, S.-G.; Tam, N.F.-Y.; Cannicci, S.; Martin, C.; Lo, H.S.; Lee, S.Y. Fate and Effects of Macro- and Microplastics in Coastal Wetlands. Environ. Sci. Technol. 2022, 56, 2386–2397. [Google Scholar] [CrossRef]
- Martin, C.; Almahasheer, H.; Duarte, C.M. Mangrove forests as traps for marine litter. Environ. Pollut. 2019, 247, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Frias, J.; Sobral, P. Microplastics on the Portuguese coast. Mar. Pollut. Bull. 2018, 131, 294–302. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental status of (micro)plastics contamination in Portugal. Ecotoxicol. Environ. Saf. 2020, 200, 110753. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Microplastics contamination along the coastal waters of NW Portugal. Case Stud. Chem. Environ. Eng. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Ramos, S.; Ré, P.; Bordalo, A.A. Recruitment of flatfish species to an estuarine nursery habitat (Lima estuary, NW Iberian Peninsula). J. Sea Res. 2010, 64, 473–486. [Google Scholar] [CrossRef]
- Ramos, S.; Cabral, H.; Elliott, M. Do fish larvae have advantages over adults and other components for assessing estuarine ecological quality? Ecol. Indic. 2015, 55, 74–85. [Google Scholar] [CrossRef]
- Rocha, A.C.S.; Teixeira, C.; Almeida, C.M.R.; Basto, M.C.P.; Reis-Henriques, M.A.; Guimarães, L.; Ferreira, M. Assessing contamination from maritime trade and transportation on Iberian waters: Impact on Platichthys flesus. Environ. Sustain. Indic. 2021, 9, 100098. [Google Scholar] [CrossRef]
- Montenegro, I.P.F.M.; Mucha, A.P.; Reis, I.; Rodrigues, P.; Almeida, C.M.R. Effect of petroleum hydrocarbons in copper phytoremediation by a salt marsh plant (Juncus maritimus) and the role of autochthonous bioaugmentation. Environ. Sci. Pollut. Res. 2016, 23, 19471–19480. [Google Scholar] [CrossRef] [PubMed]
- Mal, T.K.; Narine, L. The biology of Canadian weeds. 129. Phragmites australis (Cav.) Trin. ex Steud. Can. J. Plant Sci. 2004, 84, 365–396. [Google Scholar] [CrossRef]
- Rivoira, L.; Castiglioni, M.; Rodrigues, S.M.; Freitas, V.; Bruzzoniti, M.C.; Ramos, S.; Almeida, C.M.R. Microplastic in marine environment: Reworking and optimisation of two analytical protocols for the extraction of microplastics from sediments and oysters. MethodsX 2020, 7, 101116. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Adaptation of a laboratory protocol to quantity microplastics contamination in estuarine waters. Methods X 2019, 6, 740–749. [Google Scholar] [CrossRef]
- Perumal, K.; Muthuramalingam, S. Global sources, abundance, size, and distribution of microplastics in marine sediments—A critical review. Estuar. Coast. Shelf Sci. 2022, 264, 107702. [Google Scholar] [CrossRef]
- Tramoy, R.; Gasperi, J.; Colasse, L.; Tassin, B. Transfer dynamic of macroplastics in estuaries—New insights from the Seine estuary: Part 1. Long term dynamic based on date-prints on stranded debris. Mar. Pollut. Bull. 2020, 152, 110894. [Google Scholar] [CrossRef]
- Hope, J.A.; Coco, G.; Ladewig, S.M.; Thrush, S.F. The distribution and ecological effects of microplastics in an estuarine ecosystem. Environ. Pollut. 2021, 288, 117731. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef] [PubMed]
- Scircle, A.; Cizdziel, J.V.; Tisinger, L.; Anumol, T.; Robey, D. Occurrence of Microplastic Pollution at Oyster Reefs and Other Coastal Sites in the Mississippi Sound, USA: Impacts of Freshwater Inflows from Flooding. Toxics 2020, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Saha, M.; Rathore, C.; Suneel, V.; Ray, D.; Naik, A.; Unnikrishnan, K.; Dhivya, M.; Daga, K. Spatial and seasonal variation of microplastics and possible sources in the estuarine system from central west coast of India. Environ. Pollut. 2021, 288, 117665. [Google Scholar] [CrossRef]
- Ramos, S.; Cowen, R.K.; Paris, C.; Ré, P.; Bordalo, A.A. Environmental forcing and larval fish assemblage dynamics in the Lima River estuary (northwest Portugal). J. Plankton Res. 2006, 28, 275–286. [Google Scholar] [CrossRef]
- Defontaine, S.; Sous, D.; Tesan, J.; Monperrus, M.; Lenoble, V.; Lanceleur, L. Microplastics in a salt-wedge estuary: Vertical structure and tidal dynamics. Mar. Pollut. Bull. 2020, 160, 111688. [Google Scholar] [CrossRef]
- van Emmerik, T.; Schwarz, A. Plastic debris in rivers. WIREs Water 2020, 7, e1398. [Google Scholar] [CrossRef]
- Kowalski, N.; Reichardt, A.M.; Waniek, J.J. Sinking rates of microplastics and potential implications of their alteration by physical, biological, and chemical factors. Mar. Pollut. Bull. 2016, 109, 310–319. [Google Scholar] [CrossRef]
- Ivar do Sul, J.A.; Costa, M.F.; Silva-Cavalcanti, J.S.; Araújo, M.C.B. Plastic debris retention and exportation by a mangrove forest patch. Mar. Pollut. Bull. 2014, 78, 252–257. [Google Scholar] [CrossRef]
- Helcoski, R.; Yonkos, L.T.; Sanchez, A.; Baldwin, A.H. Wetland soil microplastics are negatively related to vegetation cover and stem density. Environ. Pollut. 2020, 256, 113391. [Google Scholar] [CrossRef]
- Han, N.; Zhao, Q.; Ao, H.; Hu, H.; Wu, C. Horizontal transport of macro- and microplastics on soil surface by rainfall induced surface runoff as affected by vegetations. Sci. Total Environ. 2022, 831, 154989. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Fórián, S.; Szatmári, G.; Sipos, G. Spatial distribution of microplastics in the fluvial sediments of a transboundary river—A case study of the Tisza River in Central Europe. Sci. Total Environ. 2021, 785, 147306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, H.; Xiong, X.; Zhao, E.; Wang, K.; Wu, C. Urban natural wetland as a sink for microplastics: A case from Lalu Wetland in Tibet, China. Sci. Total Environ. 2022, 828, 154399. [Google Scholar] [CrossRef] [PubMed]
| Sediment Samples | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Canoagem | Sra. Das Areias | Salinas | |||||||
| Non- Vegetated | Vegetated Juncus maritimus | Vegetated Spartina maritima | Non-Vegetated | Vegetated Juncus maritimus | Vegetated Phragmites australis | Non-Vegetated | Vegetated Juncus maritimus | Vegetated Phragmites australis | |
| November 2021 (autumn sampling) | 2 Fragments red and pink PE | 2 Fragments blue PS 1 Fibre blue – PS | 3 Fragments transparent and blue PE | * | 1 Fragment blue PP 1 Fragment white PS | 1 Fragment blue – PP 1 Fragment blue PE | * | 2 Fibres black and blue PE 1 Fragment white PS | 2 Fragments blue and transparent PE 1 Fibre Blue PS |
| February 2022 (winter sampling) | 1 Fragment transparent PE | 2 Fragments transparent and blue PS 1 Fibre Blue PS | 3 Fragments transparent and white PS | * | 2 Fragments red and blue PE 1 Fragment transparent PP | 1 Fragment red PE 1 Fibre red PE | 2 Fragments white and transparent PE | 2 Fragments transparent PE 1 Fragment green PP | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, C.M.R.; Sáez-Zamacona, I.; Silva, D.M.; Rodrigues, S.M.; Pereira, R.; Ramos, S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water 2023, 15, 1382. https://doi.org/10.3390/w15071382
Almeida CMR, Sáez-Zamacona I, Silva DM, Rodrigues SM, Pereira R, Ramos S. The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water. 2023; 15(7):1382. https://doi.org/10.3390/w15071382
Chicago/Turabian StyleAlmeida, C. Marisa R., Iraide Sáez-Zamacona, Diogo M. Silva, Sabrina M. Rodrigues, Rúben Pereira, and Sandra Ramos. 2023. "The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention" Water 15, no. 7: 1382. https://doi.org/10.3390/w15071382
APA StyleAlmeida, C. M. R., Sáez-Zamacona, I., Silva, D. M., Rodrigues, S. M., Pereira, R., & Ramos, S. (2023). The Role of Estuarine Wetlands (Saltmarshes) in Sediment Microplastics Retention. Water, 15(7), 1382. https://doi.org/10.3390/w15071382







