Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination
Abstract
1. Introduction
2. Chlorinated Ethenes
3. Microbial Transformation of Chlorinated Ethenes
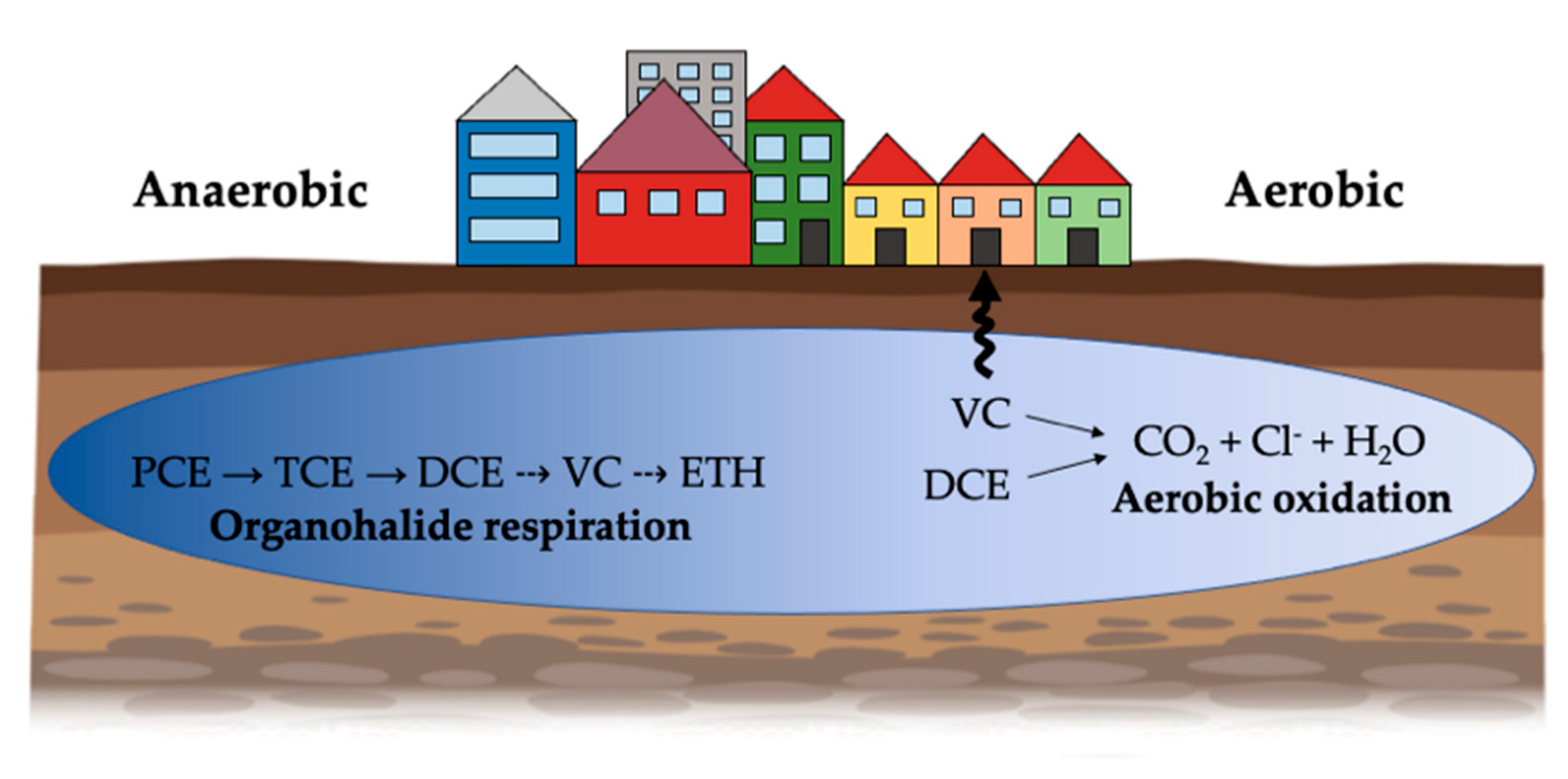
4. Organohalide Respiration
5. Aerobic Pathways of Lower Chlorinated Ethenes Biodegradation
5.1. DCE and VC Metabolic Degradation
5.2. Co-Metabolic Aerobic Degradation
6. Bio-Based Substrates for Stimulation of OHR Bacteria
7. Bioelectrochemical Systems
8. Environmental Halogenomics: Detection and Distribution of Halo-Bacteria
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 1,1-DCE | 1,1-trans-dichloroethene |
| 1,2-DCE | 1,2-trans-dichloroethene |
| AkMO | alkene monooxygenase |
| BES | bioelectrochemical systems |
| bvcA | vinyl chloride reductase |
| CEs | chloroethenes |
| cis-DCE | cis-dichloroethene |
| D-NAPL | dense non-aqueous phase liquid |
| EaCoMT | epoxyalkane:coenzyme M transferase |
| GSH | glutathione |
| GST | glutathione S-transferase |
| MMO | methanol monooxygenase |
| nZVI | nano zero valent iron |
| OHR | organohalide respiration |
| OHRB | Organohalide respiring bacteria |
| ORP | oxidative redox potential |
| PCE | tetrachloroethene |
| pceA | tetrachloroethene reductive dehalogenase |
| pMMO | particulate MMO |
| RDases | reductive dehalogenase homologous genes |
| sMMO | soluble MMO |
| TCE | trichloroethene |
| tceA | trichloroethene reductive dehalogenase |
| VC | vinyl chloride |
| vcrA | vinyl chloride reductase |
References
- Available online: https://www.fao.org/sustainable-development-goals/en/ (accessed on 20 September 2022).
- Hasan, M.M. Ground Water Making the Invisible Visible. Legal Lock J. 2021, 1, 69. [Google Scholar]
- Foster, S.; Gathu, J.; Eichholz, M.; Hirata, R. Climate Change: The utility groundwater role in supply security. Source 2020, 18, 50–54. [Google Scholar]
- Margat, J.; Van der Gun, J. Groundwater around the World: A Geographic Synopsis; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- UNESCO. The United Nations World Water Development Report 2015; UNESCO: Paris, France, 2015. [Google Scholar]
- Available online: https://www.eea.europa.eu/data-and-maps/indicators/progress-in-management-of-contaminated-sites-3/assessment (accessed on 20 September 2022).
- Available online: https://www.eea.europa.eu/data-and-maps/data/wise-wfd-4 (accessed on 20 September 2022).
- Majone, M.; Verdini, R.; Aulenta, F.; Rossetti, S.; Tandoi, V.; Kalogerakis, N.; Agathos, S.; Puig, S.; Zanaroli, G.; Fava, F. In situ groundwater and sediment bioremediation: Barriers and perspectives at European contaminated sites. New Biotechnol. 2015, 32, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Battelle Memorial Institute. Permeable Reactive Barrier Cost and Performance Report; NAVFAC: Port Hueneme, CA, USA, 2012. [Google Scholar]
- Das, S.; Dash, H.R. Microbial bioremediation: A potential tool for restoration of contaminated areas. In Microbial Biodegradation and Bioremediation; Elsevier: Waltham, MA, USA, 2014; pp. 1–21. [Google Scholar]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, K.; Ekdahl, A.; Collen, J.; Pedersen, M. Marine algae-a source of trichloroethylene and perchloroethylene. Limnol. Oceanogr. 1995, 40, 1321–1326. [Google Scholar] [CrossRef]
- Field, J.A. Natural production of organohalide compounds in the environment. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–29. [Google Scholar]
- Keppler, F.; Borchers, R.; Pracht, J.; Rheinberger, S.; Scholer, H.F. Natural formation of vinyl chloride in the terrestrial environment. Environ. Sci. Technol. 2002, 36, 2479–2483. [Google Scholar] [CrossRef]
- Öberg, G.M. The Biogeochemistry of Chlorine in Soil. Handb. Environ. Chem. 2004, 3, 43–62. [Google Scholar] [CrossRef]
- McCarty, P.L. Groundwater contamination by chlorinated solvents: History, remediation technologies and strategies. In In Situ Remediation of Chlorinated Solvent Plumes; Springer: New York, NY, USA, 2010; pp. 1–28. [Google Scholar]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated solvents in groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef]
- Beamer, P.I.; Luik, C.E.; Abrell, L.; Camposc, S.; Martínez, M.E.; Sáez, A.E. Concentration of Trichloroethylene in Breast Milk and Household Water from Nogales, Arizona. Environ. Microbiol. 2012, 46, 9055–9061. [Google Scholar] [CrossRef]
- Huang, B.; Lei, C.; Wei, C.; Zeng, G. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef]
- USA EPA. Toxicity and Exposure Assessment for Children’s Health. Trichloroethylene—TEACH Chemical Summary. 2007. Available online: http://www.epa.gov/teach/chem_summ/TCE_summary.pdf. (accessed on 17 October 2022).
- IARC. Vinyl Chloride; International Agency for Research on Cancer: Ottawa, ON, Canada, 1987. [Google Scholar]
- IARC Monographs on the Identification of Carcinogenic Hazards to Humans. 2020, Volume 1–127. Available online: https://monographs.iarc.fr/list-of-classifications. (accessed on 5 September 2022).
- Lynge, E.; Anttila, A.; Hemminki, K. Organic solvents and cancer. Cancer Causes Control 1992, 55, 1353–1395. [Google Scholar]
- Bradley, P.M. History and ecology of chlororethene biodegradation: A review. Bioremediat. J. 2003, 7, 81–109. [Google Scholar] [CrossRef]
- Mattes, T.E.; Alexander, A.K.; Coleman, N.V. Aerobic biodegradation of the chloroethenes: Pathways, enzymes, ecology, and evolution. FEMS Microbiol. Rev. 2010, 34, 445–475. [Google Scholar] [CrossRef]
- Tiehm, A.; Schmidt, K.R. Sequential anaerobic/aerobic biodegradation of chloroethenes-aspects of field application. Curr. Opin. Biotechnol. 2011, 22, 415–421. [Google Scholar] [CrossRef]
- Anam, G.B.; Choi, J.; Ahn, Y. Reductive dechlorination of perchloroethene (PCE) and bacterial community changes in a continuous-flow, two-stage anaerobic column. Int. Biodeter. Biodegr. 2019, 138, 41–49. [Google Scholar] [CrossRef]
- Chen, S.K.; Yang, H.Y.; Huang, S.R.; Hung, J.M.; Lu, C.J.; Liu, M.H. Complete degradation of chlorinated ethenes and its intermediates through sequential anaerobic/aerobic biodegradation in simulated groundwater columns (complete degradation of chlorinated ethenes). IJEST 2020, 17, 4517–4530. [Google Scholar] [CrossRef]
- Leys, D.; Adrian, L.; Smidt, H. Organohalide respiration: Microbes breathing chlorinated molecules. Proc. R. Soc. B 2013, 368, 20120316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Atashgahi, S.; Bosma, T.N.; Peng, P.; Smidt, H. Organohalide respiration potential in marine sediments from Aarhus Bay. FEMS Microbiol. 2022, 98, fiac073. [Google Scholar] [CrossRef]
- Maymo-Gatell, X. “Dehalococcoides Ethenogenes” Strain 195: A Novel Eubacterium that Reductively Dechlorinates Tetrachloroethene (PCE) to Ethene; Cornell University: Ithaca, NY, USA, 1997. [Google Scholar]
- Yang, Y.; Higgins, S.A.; Yan, J.; Şimşir, B.; Chourey, K.; Iyer, R.; Hettich, R.L.; Baldwin, B.; Oglen, D.M.; Löffler, F.E. Grape pomace compost harbors organohalide-respiring Dehalogenimonas species with novel reductive dehalogenase genes. ISME J. 2017, 11, 2767–2780. [Google Scholar] [CrossRef]
- Chen, G.; Kara Murdoch, F.; Xie, Y.; Murdoch, R.W.; Cui, Y.; Yang, Y.; Yan, Y.; Key, T.A.; Löffler, F.E. Dehalogenation of Chlorinated Ethenes to Ethene by a Novel Isolate, “Candidatus Dehalogenimonas etheniformans”. Appl. Environ. Microbiol. 2022, e00443-22. [Google Scholar] [CrossRef]
- Cichocka, D.; Nikolausz, M.; Haest, P.J.; Nijenhuis, I. Tetrachloroethene conversion to ethene by a Dehalococcoides-containing enrichment culture from Bitterfeld. FEMS Microbiol. Ecol. 2010, 72, 297–310. [Google Scholar] [CrossRef]
- AFCEE. Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents; Department of Defense, Air Force Center for Environmental Excellence and the Environmental Security Technology Certification Program (ESTCP): Washington, DC, USA, 2004. [Google Scholar]
- Heimann, A.C.; Batstone, D.J.; Jakobsen, R. Methanosarcina spp. drive vinyl chloride dechlorination via interspecies hydrogen transfer. Appl. Environ. Microbiol. 2006, 72, 2942–2949. [Google Scholar] [CrossRef]
- Smidt, H.; de Vos, W.M. Anaerobic Microbial Dehalogenation. Annu. Rev. Microbiol. 2004, 58, 43–73. [Google Scholar] [CrossRef]
- Futagami, T.; Goto, M.; Furukawa, K. Biochemical and genetic bases of dehalorespiration. Chem. Rec. 2008, 8, 1–12. [Google Scholar] [CrossRef]
- Abe, Y.; Aravena, R.; Zopfi, J.; Parker, B.; Hunkeler, D. Evaluating the fate of chlorinated ethenes in streambed sediments by combining stable isotope, geochemical and microbial methods. J. Contam. Hydrol. 2009, 107, 10–21. [Google Scholar] [CrossRef]
- Hug, L.A.; Maphosa, F.; Leys, D.; Löffler, F.E.; Smidt, H.; Edwards, E.A.; Adrian, L. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Phil. Trans. R. Soc. B 2013, 368, 20120322. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Villalobos Solis, M.I.; Jin, H.; Chourey, K.; Li, X.; Yang, Y.; Yin, Y.; Hettich, R.L.; Loffler, F.E. Respiratory vinyl chloride reductive dechlorination to ethene in TceA-expressing Dehalococcoides mccartyi. Environ. Sci. Technol. 2021, 55, 4831–4841. [Google Scholar] [CrossRef]
- Men, Y.; Feil, H.; VerBerkmoes, N.C.; Shah, M.B.; Johnson, D.R.; Lee, P.K.; West, K.A.; Zinder, S.H.; Andersen, G.L.; Alvarez-Cohen, L. Sustainable syntrophic growth of Dehalococcoides ethenogenes strain 195 with Desulfovibrio vulgaris Hildenborough and Methanobacterium congolense: Global transcriptomic and proteomic analyses. ISME J. 2012, 6, 410–421. [Google Scholar] [CrossRef]
- Teng, Y.; Xu, Y.; Wang, X.; Christie, P. Function of biohydrogen metabolism and related microbial communities in environmental bioremediation. Front. Microbiol. 2019, 10, 106. [Google Scholar] [CrossRef]
- Ziv-El, M.; Delgado, A.G.; Yao, Y.; Kang, D.W.; Nelson, K.G.; Halden, R.U.; Krajmalnik-Brown, R. Development and characterization of DehaloR^2, a novel anaerobic microbial consortium performing rapid dechlorination of TCE to ethene. Appl. Microbiol. Biotechnol. 2011, 92, 1063–1071. [Google Scholar] [CrossRef]
- Maphosa, F.; van Passel, M.W.; de Vos, W.M.; Smidt, H. Metagenome analysis reveals yet unexplored reductive dechlorinating potential of Dehalobacter sp. E1 growing in co-culture with Sedimentibacter sp. Environ. Microbiol. Rep. 2012, 4, 604–616. [Google Scholar] [PubMed]
- Ziv-El, M.; Popat, S.C.; Parameswaran, P.; Kang, D.W.; Polasko, A.; Halden, R.U.; Rittmann, B.E.; Krajmalnik-Brown, R. Using electron balances and molecular techniques to assess trichoroethene-induced shifts to a dechlorinating microbial community. Biotechnol. Bioeng. 2012, 109, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ritalahti, K.M.; Wagner, D.D.; Löffler, F.E. Unexpected specificity of interspecies cobamide transfer from Geobacter spp. to organohalide-respiring Dehalococcoides mccartyi strains. Appl. Environ. Microbiol. 2012, 78, 6630–6636. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Im, J.; Yang, Y.; Löffler, F.E. Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120320. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Lee, P.K.; Harding, K.C.; Alvarez-Cohen, L. Characterization of four TCE-dechlorinating microbial enrichments grown with different cobalamin stress and methanogenic conditions. Appl. Microbiol. Biotechnol. 2013, 97, 6439–6450. [Google Scholar] [CrossRef]
- Adrian, L.; Löffler, F.E. Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; Volume 85. [Google Scholar]
- Richardson, R.E. Organohalide-respiring bacteria as members of microbial communities: Catabolic food webs and biochemical interactions. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 309–341. [Google Scholar]
- Fennell, D.E.; Gossett, J.M.; Zinder, S.H. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 1997, 31, 918–926. [Google Scholar] [CrossRef]
- Matteucci, F.; Ercole, C.; Del Gallo, M. A study of chlorinated solvent contamination of the aquifers of an industrial area in central Italy: A possibility of bioremediation. Front. Microbiol. 2015, 6, 924. [Google Scholar] [CrossRef]
- Yang, Y.; McCarty, P.L. Comparison between donor substrates for biologically enhanced tetrachloroethene DNAPL dissolution. Environ. Sci. Technol. 2002, 36, 3400–3404. [Google Scholar] [CrossRef]
- Dolfing, J. Energetic considerations in organohalide respiration. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–48. [Google Scholar]
- Lin, W.H.; Chien, C.C.; Lu, C.W.; Hou, D.; Sheu, Y.T.; Chen, S.C.; Kao, C.M. Growth inhibition of methanogens for the enhancement of TCE dechlorination. Sci. Total Environ. 2021, 787, 147648. [Google Scholar] [CrossRef]
- Yang, Y.; McCarty, P.L. Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ. Sci. Technol. 1998, 32, 3591–3597. [Google Scholar] [CrossRef]
- Robinson, C.; Barry, D.A.; McCarty, P.L.; Gerhard, J.I.; Kouznetsova, I. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Sci. Total Environ. 2009, 407, 4560–4573. [Google Scholar] [CrossRef]
- Schmidt, K.R.; Gaza, S.; Voropaev, A.; Ertl, S.; Tiehm, A. Aerobic biodegradation of trichloroethene without auxiliary substrates. Water Res. 2014, 59, 112–118. [Google Scholar] [CrossRef]
- Jennings, L.K.; Chartrand, M.M.G.; Lacrampe-Couloume, G.; Lollar, B.S.; Spain, J.C.; Gossett, J.M. Proteomic and transcriptomic analyses reveal genes upregulated by cis-dichloroethene in Polaromonas sp. Strain JS666. Appl. Environ. Microbiol. 2009, 75, 3733–3744. [Google Scholar] [CrossRef]
- Coleman, N.V.; Mattes, T.E.; Gossett, J.M.; Spain, J.C. Biodegradation of cis-dichloroethene as the sole carbon source by a β-Proteobacterium. Appl. Environ. Microbiol. 2002, 68, 2726–2730. [Google Scholar] [CrossRef]
- Mattes, T.E.; Alexander, A.K.; Richardson, P.M.; Munk, A.C.; Han, C.S.; Stothard, P.; Coleman, N.V. The genome of Polaromonas sp. strain JS666: Insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Appl. Environ. Microbiol. 2008, 74, 6405–6416. [Google Scholar] [CrossRef]
- Coleman, N.V.; Spain, J.C. Distribution of the Coenzyme M Pathway of Epoxide Metabolism among Ethene- and Vinyl Chloride-Degrading Mycobacterium Strains. Appl. Environ. Microbiol. 2003, 69, 6041–6046. [Google Scholar] [CrossRef]
- Xing, Z.; Su, X.; Zhang, X.; Zhang, L.; Zhao, T. Direct aerobic oxidation (DAO) of chlorinated aliphatic hydrocarbons: A review of key DAO bacteria, biometabolic pathways and in-situ bioremediation potential. Environ. Int. 2022, 162, 107165. [Google Scholar] [CrossRef] [PubMed]
- Mattes, T.E.; Coleman, N.V.; Spain, J.C.; Gossett, J.M. Physiological and molecular genetic analyses of vinyl chloride and ethene biodegradation in Nocardioides sp. strain JS614. Arch. Microbiol. 2005, 183, 95–106. [Google Scholar] [CrossRef]
- Coleman, N.V.; Mattes, T.E.; Gossett, J.M.; Spain, J.C. Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl. Environ. Microbiol. 2002, 68, 6162–6171. [Google Scholar] [CrossRef]
- Jin, Y.O.; Cheung, S.; Coleman, N.V.; Mattes, T.E. Association of missense mutations in epoxyalkane coenzyme M transferase with adaptation of Mycobacterium sp. Strain JS623 to growth on vinyl chloride. Appl. Environ. Microbiol. 2010, 76, 3413–3419. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Wilson, F.P.; Yu, K.; Lintner, C.; Cupples, A.M.; Mattes, T.E. Integrated methodological approach reveals microbial diversity and functions in aerobic groundwater microcosms adapted to vinyl chloride. FEMS Microbiol. Ecol. 2018, 94, fiy124. [Google Scholar] [CrossRef] [PubMed]
- Hartmans, S.; De Bont, J.; Tramper, J.; Luyben, K.C.A. Bacterial degradation of vinyl chloride. Biotechnol. Lett. 1985, 7, 383–388. [Google Scholar] [CrossRef]
- Malachowsky, K.; Phelps, T.; Teboli, A.; Minnikin, D.; White, D. Aerobic mineralization of trichloroethylene, vinyl chloride and aromatic compounds by Rhodococcus species. Appl. Environ. Microb. 1994, 60, 542–548. [Google Scholar] [CrossRef]
- Verce, M.F.; Ulrich, R.L.; Freedman, D.L. Characterization of an isolate that uses vinyl chloride as a growth substrate under aerobic conditions. Appl. Environ. Microbiol. 2000, 66, 3535–3542. [Google Scholar] [CrossRef]
- Verce, M.F.; Ulrich, R.L.; Freedman, D.L. Transition from cometabolic to growth-linked biodegradation of vinyl chloride by a Pseudomonas sp. isolated on ethene. Environ. Sci. Technol. 2001, 35, 4242–4251. [Google Scholar] [CrossRef]
- Danko, A.S.; Luo, M.; Bagwell, C.E.; Brigmon, R.L.; Freedman, D.L. Involvement of linear plasmids in aerobic biodegradation of vinyl chloride. Appl. Environ. Microbiol. 2004, 70, 6092–6097. [Google Scholar] [CrossRef]
- Elango, V.K.; Liggenstoffer, A.S.; Fathepure, B.Z. Biodegradation of vinyl chloride and cis-dichloroethene by a Ralstonia sp. strain TRW-1. Appl. Microbiol. Biotechnol. 2006, 72, 1270–1275. [Google Scholar] [CrossRef]
- Paes, F.; Liu, X.; Mattes, T.E.; Cupples, A.M. Elucidating carbon uptake from vinyl chloride using stable isotope probing and Illumina sequencing. Appl. Microbiol. Biotechnol. 2015, 99, 7735–7743. [Google Scholar] [CrossRef]
- Gupta, R.S.; Lo, B.; Son, J. Phylogenomic and comparative genomic studies robustly support division of the genus Mycobacterium into an emended genus Mycobacterium and four novel genera. Front. Microbiol. 2018, 9, 67. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.M.; Ewald, J.M.; Zhao, W.; Rectanus, H.; Fan, D.; Durant, N.; Pound, M.; Mattes, T.E. Natural biodegradation of vinyl chloride and cis-dichloroethene in aerobic and suboxic conditions. ESPR 2022, 1–14. [Google Scholar] [CrossRef]
- Fullerton, H.; Rogers, R.; Freedman, D.L.; Zinder, S.H. Isolation of an aerobic vinyl chloride oxidizer from anaerobic groundwater. Biodegradation 2014, 25, 893–901. [Google Scholar] [CrossRef]
- Zhao, H.P.; Schmidt, K.R.; Tiehm, A. Inhibition of aerobic metabolic cis-1,2-dichloroethene biodegradation by other chloroethenes. Water Res. 2010, 44, 2276–2282. [Google Scholar] [CrossRef]
- Dolinová, I.; Štrojsová, M.; Černík, M.; Němeček, J.; Macháčková, J.; Ševců, A. Microbial degradation of chloroethenes: A review. ESPR 2017, 24, 13262–13283. [Google Scholar] [CrossRef]
- Lange, C.C.; Wackett, L.P. Oxidation of aliphatic olefins by toluene dioxygenase: Enzyme rates and product identification. J. Bacteriol. 1997, 179, 3858–3865. [Google Scholar] [CrossRef]
- Doughty, D.M.; Sayavedra-Soto, L.A.; Arp, D.J.; Bottomley, P.J. Effects of dichloroethene isomers on the induction and activity of butane monooxygenase in the alkane-oxidizing bacterium ‘Pseudomonas butanovora’. Appl. Environ. Microbiol. 2005, 71, 6054–6059. [Google Scholar] [CrossRef]
- Ryoo, D.; Shim, H.; Canada, K.; Barbieri, P.; Wood, T.K. Aerobic degradation of tetrachloroethylene by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1, Nat. Biotechnol. 2000, 18, 775–778. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Azizmohseni, F.; Golmohamad, F. Characterization and kinetic study of PAH–degrading Sphingopyxis ummariensis bacteria isolated from a petrochemical wastewater treatment plant. Adv. Environ. Sci. Technol. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Frascari, D.; Pinelli, D.; Nocentini, M.; Baleani, E.; Cappelletti, M.; Fedi, S. A kinetic study of chlorinated solvent cometabolic biodegradation by propane-grown Rhodococcus sp. PB1. Biochem. Eng. J. 2008, 42, 139–147. [Google Scholar] [CrossRef]
- Zalesak, M.; Ruzicka, J.; Vicha, R.; Dvorackova, M. Cometabolic degradation of dichloroethenes by Comamonas testosteroni RF2. Chemosphere 2017, 186, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.P.; Jiménez, L.; Rosario, I.; Hazen, T.C.; Sayler, G.S. Characterization of the methanotrophic bacterial community present in a trichloroethylene-contaminated subsurface groundwater site. Appl. Environ. Microbiol. 1993, 59, 2380–2387. [Google Scholar] [CrossRef]
- Yoon, S.; Im, J.; Bandow, N.; Dispirito, A.A.; Semrau, J.D. Constitutive expression of pMMO by Methylocystis strain SB2 when grown on multi-carbon substrates: Implications for biodegradation of chlorinated ethenes. Environ. Microbiol. Rep. 2011, 3, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Keeney, D.R.; Lim, D.H.; Dispirito, A.A.; Semrau, J.D. Mixed pollutant degradation by Methylosinus trichosporium OB3b expressing either soluble or particulate methane monooxygenase: Can the tortoise beat the hare. Appl. Environ. Microbiol. 2006, 72, 7503–7509. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Knief, C.; Dunfield, P.F. Methylocella species are facultatively methanotrophic. J. Bacteriol. 2005, 187, 4665–4670. [Google Scholar] [CrossRef]
- Dunfield, P.F.; Belova, S.E.; Vorob’ev, A.V.; Cornish, S.L.; Dedysh, S.N. Methylocapsa aurea sp. nov., a facultatively methanotrophic bacterium possessing a particulate methane monooxygenase. Int. J. Syst. Evol. Microbiol. 2010, 60, 2659–2664. [Google Scholar] [CrossRef]
- Im, J.; Lee, S.W.; Yoon, S.; DiSpirito, A.A.; Semrau, J.D. Characterization of a novel facultative Methylocystis species capable of growth on methane, ethanol, and acetate. Environ. Microbiol. Rep. 2010. [Google Scholar] [CrossRef]
- Freedman, D.L.; Danko, A.S.; Verce, M.F. Substrate interactions during aerobic biodegradation of methane, ethene, vinyl chloride and 1,2-dichloroethenes. Water Sci. Technol. 2001, 43, 333–340. [Google Scholar] [CrossRef]
- Findlay, M.; Smoler, D.F.; Fogel, S.; Mattes, T.E. Aerobic vinyl chloride metabolism in groundwater microcosms by methanotrophic and etheneotrophic bacteria. Environ. Sci. Technol. 2016, 50, 3617–3625. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wu, J.H. Microbiome composition resulting from different substrates influences trichloroethene dechlorination performance. J. Environ. Manag. 2022, 303, 114145. [Google Scholar] [CrossRef]
- Conrad, M.E.; Brodie, E.L.; Radtke, C.W.; Bill, M.; Delwiche, M.E.; Lee, M.H.; Swift, D.L.; Colwell, F.S. Field evidence for co-metabolism of trichloroethene stimulated by addition of electron donor to groundwater. Environ. Sci. Technol. 2010, 44, 4697–4704. [Google Scholar] [CrossRef]
- Steffan, R.J.; Schaefer, C.E. Current and future bioremediation applications: Bioremediation from a practical and regulatory perspective. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 517–540. [Google Scholar]
- Blazquez-Palli, N.; Rosell, M.; Varias, J.; Bosch, M.; Soler, A.; Vicent, T.; Marco-Urrea, E. Multi-method assessment of the intrinsic biodegradation potential of an aquifer contaminated with chlorinated ethenes at an industrial area in Barcelona (Spain). Environ. Pollut. 2019, 244, 165–173. [Google Scholar] [CrossRef]
- Li, J.; Hu, A.; Bai, S.; Yang, X.; Sun, Q.; Liao, X.; Yu, C.P. Characterization and performance of lactate-feeding consortia for reductive dechlorination of trichloroethene. Microorganisms 2021, 9, 751. [Google Scholar] [CrossRef]
- Tomita, R.; Yoshida, N.; Meng, L. Formate: A promising electron donor to enhance trichloroethene-to-ethene dechlorination in Dehalococcoides-augmented groundwater ecosystems with minimal bacterial growth. Chemosphere 2022, 307, 136080. [Google Scholar] [CrossRef]
- Sheu, Y.T.; Tsang, D.C.; Dong, C.D.; Chen, C.W.; Luo, S.G.; Kao, C.M. Enhanced bioremediation of TCE-contaminated groundwater using gamma poly-glutamic acid as the primary substrate. J. Clean. Prod. 2018, 178, 108–118. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Summer, D.; Schöftner, P.; Wimmer, B.; Pastar, M.; Kostic, T.; Sessitsch, A.; Gerzabeck, M.H.; Reichenauer, T.G. Synergistic effects of microbial anaerobic dechlorination of perchloroethene and nano zero-valent iron (nZVI)–A lysimeter experiment. New Biotechnol. 2020, 57, 34–44. [Google Scholar] [CrossRef]
- Matturro, B.; Pierro, L.; Frascadore, E.; Petrangeli Papini, M.; Rossetti, S. Microbial community changes in a chlorinated solvents polluted aquifer over the field scale treatment with poly-3-hydroxybutyrate as amendment. Front. Microbiol. 2018, 9, 1664. [Google Scholar] [CrossRef]
- Bertolini, M.; Zecchin, S.; Beretta, G.P.; De Nisi, P.; Ferrari, L.; Cavalca, L. Effectiveness of permeable reactive bio-barriers for bioremediation of an organohalide-polluted aquifer by natural-occurring microbial community. Water 2021, 13, 2442. [Google Scholar] [CrossRef]
- Masut, E.; Battaglia, A.; Ferioli, L.; Legnani, A.; Cruz Viggi, C.; Tucci, M.; Resitano, M.; Milani, A.; de Laurentiis, C.; Matturro, B.; et al. A microcosm treatability study for evaluating wood mulch-based amendments as electron donors for trichloroethene (TCE) reductive dechlorination. Water 2021, 13, 1949. [Google Scholar] [CrossRef]
- Öztürk, Z.; Tansel, B.; Katsenovich, Y.; Sukop, M.; Laha, S. Highly organic natural media as permeable reactive barriers: TCE partitioning and anaerobic degradation profile in eucalyptus mulch and compost. Chemosphere 2012, 89, 665–671. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.E.; Ervin, J.; Zhou, J.; Sorensen, D.L.; Dupont, R.R. Biostimulation and bioaugmentation to enhance reductive dechlorination of tce in a long-term flow through column study. Ground Water Monit. Remediat. 2015, 35, 76–88. [Google Scholar] [CrossRef]
- Mora, R.H.; Macbeth, T.W.; MacHarg, T.; Gundarlahalli, J.; Holbrook, H.; Schiff, P. Enhanced bioremediation using whey powder for a trichloroethene plume in a high-sulfate, fractured granitic aquifer. Remediat. J. J. Environ. Cleanup Costs Technol. Tech. 2008, 18, 7–30. [Google Scholar] [CrossRef]
- Robles, A.; Yellowman, T.L.; Joshi, S.; Mohana Rangan, S.; Delgado, A.G. Microbial chain elongation and subsequent fermentation of elongated carboxylates as H2-producing processes for sustained reductive dechlorination of chlorinated ethenes. Environ. Sci. Technol. 2021, 55, 10398–10410. [Google Scholar] [CrossRef]
- De Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium chain carboxylic acids from complex organic feedstocks by mixed culture fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef]
- Lohner, S.T.; Becker, D.; Mangold, K.M.; Tiehm, A. Sequential reductive and oxidative biodegradation of chloroethenes stimulated in a coupled bioelectro-process. Environ. Sci. Technol. 2011, 45, 6491–6497. [Google Scholar] [CrossRef]
- Verdini, R.; Aulenta, F.; De Tora, F.; Lai, A.; Majone, M. Relative contribution of set cathode potential and external mass transport on TCE dechlorination in a continuous-flow bioelectrochemical reactor. Chemosphere 2015, 136, 72–78. [Google Scholar] [CrossRef]
- Wang, X.; Aulenta, F.; Puig, S.; Esteve-Núnez, A.; He, Y.; Mu, Y.; Rabaey, K. Microbial electrochemistry for bioremediation. ESE 2020, 1, 100013. [Google Scholar] [CrossRef]
- Lovley, D.R. Happy together: Microbial communities that hook up to swap electrons. ISME J. 2017, 11, 327–336. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Guo, J.; Sun, G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2021, 47, 1707–1714. [Google Scholar] [CrossRef]
- Zheng, T.; Li, J.; Ji, Y.; Zhang, W.; Fang, Y.; Xin, F.; Dong, W.; Wei, P.; Ma, J.; Jiang, M. Progress and prospects of bioelectrochemical systems: Electron transfer and its applications in the microbial metabolism. Front. Bioeng. Biotechnol. 2020, 8, 10. [Google Scholar] [CrossRef]
- Rollefson, J.B.; Stephen, C.S.; Tien, M.; Bond, D.R. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J. Bacteriol. 2011, 193, 1023–1033. [Google Scholar] [CrossRef]
- Kouzuma, A.; Meng, X.Y.; Kimura, N.; Hashimoto, K.; Watanabe, K. Disruption of the putative cell surface polysaccharide biosynthesis gene SO3177 in Shewanella oneidensis MR-1 enhances adhesion to electrodes and current generation in microbial fuel cells. Appl. Environ. Microbiol. 2010, 76, 4151–4157. [Google Scholar] [CrossRef]
- Lai, A.; Aulenta, F.; Mingazzini, M.; Palumbo, M.T.; Papini, M.P.; Verdini, R.; Majone, M. Bioelectrochemical approach for reductive and oxidative dechlorination of chlorinated aliphatic hydrocarbons (CAHs). Chemosphere 2017, 169, 351–360. [Google Scholar] [CrossRef]
- Aulenta, F.; Tocca, L.; Verdini, R.; Reale, P.; Majone, M. Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: Effect of cathode potential on rate, selectivity, and electron transfer mechanisms. Environ. Sci. Technol. 2011, 45, 8444–8451. [Google Scholar] [CrossRef]
- Aulenta, F.; Canosa, A.; Roma, L.D.; Reale, P.; Panero, S.; Rossetti, S.; Majone, M. Influence of mediator immobilization on the electrochemically assisted microbial dechlorination of trichloroethene (TCE) and cis-dichloroethene (cis-DCE). J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 864–870. [Google Scholar] [CrossRef]
- Zeppilli, M.; Matturro, B.; Dell’Armi, E.; Cristiani, L.; Papini, M.P.; Rossetti, S.; Majone, M. Reductive/oxidative sequential bioelectrochemical process for Perchloroethylene (PCE) removal: Effect of the applied reductive potential and microbial community characterization. J. Environ. Chem. Eng. 2021, 9, 104657. [Google Scholar] [CrossRef]
- Lohner, S.T.; Tiehm, A. Application of electrolysis to stimulate microbial reductive PCE dechlorination and oxidative VC biodegradation. Environ. Sci. Technol. 2009, 43, 7098–7104. [Google Scholar] [CrossRef]
- Aulenta, F.; Verdini, R.; Zeppilli, M.; Zanaroli, G.; Fava, F.; Rossetti, S.; Majone, M. Electrochemical stimulation of microbial cis-dichloroethene (cis-DCE) oxidation by an ethene-assimilating culture. New Biotechnol. 2013, 30, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Dell’Armi, E.; Cristiani, L.; Petrangeli Papini, M.; Majone, M. Reductive/oxidative sequential bioelectrochemical process for perchloroethylene removal. Water 2019, 11, 2579. [Google Scholar] [CrossRef]
- Strycharz, S.M.; Woodard, T.L.; Johnson, J.P.; Nevin, K.P.; Sanford, R.A.; Leoffler, F.E.; Lovley, D.R. Graphite electrode as a sole electron donor for reductive dechlorination of tetrachloroethene by Geobacter lovleyi. Appl. Environ. Microbiol. 2008, 74, 5943–5947. [Google Scholar] [CrossRef] [PubMed]
- Leitao, P.; Rossetti, S.; Nouws, H.P.A.; Danko, A.S.; Majone, M.; Aulenta, F. Bioelectrochemically-assisted reductive dechlorination of 1,2-dichloroethane by a Dehalococcoides-enriched microbial culture. Bioresour. Technol. 2015, 195, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.R.; Rajeev, P.; Jain, A.; Pirbadian, S.; Okamoto, A.; Gralnick, J.A.; El-Naggar, M.Y.; Nealson, K.H. Tracking electron uptake from a cathode into Shewanella cells: Implications for energy acquisition from solid-substrate electron donors. MBio 2018, 9, e02203-17. [Google Scholar] [CrossRef]
- Chen, F.; Liang, B.; Li, Z.L.; Yang, J.Q.; Huang, C.; Lyu, M.; Yuan, Y.; Nan, J.; Wang, A.J. Bioelectrochemical assisted dechlorination of tetrachloroethylene and 1,2-dichloroethane by acclimation of anaerobic sludge. Chemosphere 2019, 227, 514–521. [Google Scholar] [CrossRef]
- Wang, S.; He, J.; Shen, C.; Manefield, M.J. Editorial: Organohalide respiration: New findings in metabolic mechanisms and bioremediation applications. Front. Microbiol. 2019, 10, 526. [Google Scholar] [CrossRef]
- Meng, L.; Yoshida, N.; Li, Z. Soil microorganisms facilitated the electrode-driven trichloroethene dechlorination to ethene by Dehalococcoides species in a bioelectrochemical system. Environ. Res. 2022, 209, 112801. [Google Scholar] [CrossRef]
- Chen, F.; Li, Z.L.; Yang, J.Q.; Liang, B.; Lin, X.Q.; Nan, J.; Wang, A.J. Effects of different carbon substrates on performance, microbiome community structure and function for bioelectrochemical-stimulated dechlorination of tetrachloroethylene. J. Chem. Eng. 2018, 352, 730–736. [Google Scholar] [CrossRef]
- Dell’Armi, E.; Rossi, M.M.; Taverna, L.; Petrangeli Papini, M.; Zeppilli, M. Evaluation of the bioelectrochemical approach and different electron donors for biological trichloroethylene reductive dechlorination. Toxics 2022, 10, 37. [Google Scholar] [CrossRef]
- Cecconet, D.; Sabba, F.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. In situ groundwater remediation with bioelectrochemical systems: A critical review and future perspectives. Environ. Int. 2020, 137, 105550. [Google Scholar] [CrossRef]
- Palma, E.; Daghio, M.; Franzetti, A.; Petrangeli Papini, M.; Aulenta, F. The bioelectric well: A novel approach for in situ treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2018, 11, 112–118. [Google Scholar]
- Zanini, A.; Ghirardi, M.; Emiliani, R. A multidisciplinary approach to evaluate the effectiveness of natural attenuation at a contaminated site. Hydrology 2021, 8, 101. [Google Scholar] [CrossRef]
- Hunkeler, D.; Aravena, R.; Berry-Spark, K.; Cox, E. Assessment of degradation pathways in an aquifer with mixed chlorinated hydrocarbon contamination using stable isotope analysis. Environ. Sci. Technol. 2005, 39, 5975–5981. [Google Scholar] [CrossRef]
- Wen, L.L.; Li, Y.; Zhu, L.; Zhao, H.P. Influence of non-dechlorinating microbes on trichloroethene reduction based on vitamin B12 synthesis in anaerobic cultures. Environ. Pollut. 2020, 259, 113947. [Google Scholar] [CrossRef]
- Wu, Y.J.; Liu, P.W.G.; Hsu, Y.S.; Whang, L.M.; Lin, T.F.; Hung, W.N.; Cho, K.C. Application of molecular biological tools for monitoring efficiency of trichloroethylene remediation. Chemosphere 2019, 233, 697–704. [Google Scholar] [CrossRef]
- Jin, D.; Zhang, F.; Shi, Y.; Kong, X.; Xie, Y.; Du, X.; Li, Y.; Zhang, R. Diversity of bacteria and archaea in the groundwater contaminated by chlorinated solvents undergoing natural attenuation. Environ. Res. 2020, 185, 109457. [Google Scholar] [CrossRef]
- Garza-Rubalcava, U.; Hatzinger, P.B.; Schanzle, D.; Lavorgna, G.; Hedman, P.; Jackson, W.A. Improved assessment and performance monitoring of a biowall at a chlorinated solvent site using high-resolution passive sampling. J. Contam. Hydrol. 2022, 246, 103962. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Zhang, M.; Kawabe, Y.; Katayama, T. Effects of ferrous iron supplementation on reductive dechlorination of tetrachloroethene and on methanogenic microbial community. FEMS Microbiol. Ecol. 2021, 97, fiab069. [Google Scholar] [CrossRef]
- Lo, K.H.; Lu, C.W.; Chien, C.C.; Sheu, Y.T.; Lin, W.H.; Chen, S.C.; Kao, C.M. Cleanup chlorinated ethene-polluted groundwater using an innovative immobilized Clostridium butyricum column scheme: A pilot-scale study. J. Environ. Manag. 2022, 311, 114836. [Google Scholar] [CrossRef]
- Hellal, J.; Joulian, C.; Urien, C.; Ferreira, S.; Denonfoux, J.; Hermon, L.; Vuilleumier, S.; Imfeld, G. Chlorinated ethene biodegradation and associated bacterial taxa in multi-polluted groundwater: Insights from biomolecular markers and stable isotope analysis. Sci. Total Environ. 2021, 763, 142950. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.O.; Mattes, T.E. A Quantitative PCR Assay for aerobic, vinyl chloride- and ethene-assimilating microorganisms in groundwater. Environ. Sci. Technol. 2010, 44, 9036–9041. [Google Scholar] [CrossRef] [PubMed]
- Mattes, T.E.; Jin, Y.O.; Livermore, J.; Pearl, M.; Liu, X. Abundance and activity of vinyl chloride (VC)-oxidizing bacteria in a dilute groundwater VC plume biostimulated with oxygen and ethene. Appl. Microbiol. Biotechnol. 2015, 99, 9267–9276. [Google Scholar] [CrossRef] [PubMed]
- Atashgahi, S.; Maphosa, F.; Dogan, E.; Smidt, H.; Springael, D.; Dejonghe, W. Small-scale oxygen distribution determines the vinyl chloride biodegradation pathway in surficial sediments of riverbed hyporheic zones. FEMS Microbiol. Ecol. 2012, 84, 133–142. [Google Scholar] [CrossRef]
- Liang, Y.; Cook, L.J.; Mattes, T.E. Temporal abundance and activity trends of vinyl chloride (VC)-degrading bacteria in a dilute VC plume at Naval Air Station Oceana. Environ. Sci. Pollut. Res. 2017, 24, 13760–13774. [Google Scholar] [CrossRef]
- Richards, P.M.; Liang, Y.; Johnson, R.L.; Mattes, T.E. Cryogenic soil coring reveals coexistence of aerobic and anaerobic vinyl chloride degrading bacteria in a chlorinated ethene contaminated aquifer. Water Res. 2019, 157, 281–291. [Google Scholar] [CrossRef]
- Němeček, J.; Marková, K.; Špánek, R.; Antoš, V.; Kozubek, P.; Lhotský, O.; Černík, M. Hydrochemical conditions for aerobic/anaerobic biodegradation of chlorinated ethenes—A multi-site assessment. Water 2020, 12, 322. [Google Scholar] [CrossRef]





| Compound | Appearance | Water Solubility at 25 °C (g L−1) a | Density at 20 °C (g cm−3) a | Vapor Pressure at 20 °C (kPa) a | Autoignition Temperature (°C) a | Carcinogenicity b | Law Limits (µg L−1) Directive 2000/60/EC | |
|---|---|---|---|---|---|---|---|---|
| Vinyl chloride (VC) (C2H3Cl) | Colorless gas | Slightly soluble | 0.91 | 516.95 | 472° | Group 1 (2012) | 0.5 | |
| cis-dichloroethene (cis-DCE) (C2H2Cl2) | Colorless liquid | 1–5 | 1.28 | 26.66 | 460° | N | - | |
| trans-dichloroethene (trans-DCE) (C2H2Cl2) | 1,1-trans-DCE | Colorless liquid | 2.5 | 1.213 | 66.5 | 460° | Group 3 (1999) | 0.05 |
| 1,2-trans-DCE | Colorless liquid | <1.0 | 1.25 | 53.33 | 460° | N | 60 | |
| Trichloroethene (TCE) (C2HCl3) | Colorless liquid | 1.280 | 1.46 | 7.8 | >410° | Group 1 (2014) | 1.5 | |
| Tetrachloroethene (PCE) (C2Cl4) | Colorless liquid | 0.15 | 1.63 | 1.9 | >650° | Group 2A (2014) | 1.1 | |
| Genus | Species/Strains | Isolation Sources | References |
|---|---|---|---|
| Mycobacterium | aurum strain L1 | Contaminated soil | [69] |
| strains JS60 | Contaminated groundwater | [66] | |
| strains JS61 | Activated sludge | [66] | |
| strains JS616 | Sediment of industrial site | [66] | |
| strains JS617 | Activated carbon of pump and treatment plant | [66] | |
| Rhodococcus | rhodochrous | PCE degrading enrichment culture | [70] |
| Nocardioides | sp. strain JS614 | Soil of industrial site | [66] |
| Pseudomonas | aeruginosa strain DL1 | Activated sludge | [71] |
| aeruginosa strain MF1 | VC degrading enrichment culture | [72] | |
| putida strain AJ | Hazardous waste site | [73] | |
| Ochrobactrum | sp. strain TD | Hazardous waste site | [73] |
| Ralstonia | sp. strain TRW-1 | Chloroethene degrading enrichment culture | [74] |
| Brevundimonas | sp. | Contaminated groundwater | [75] |
| Rhodoferax | sp. | Contaminated groundwater | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertolini, M.; Zecchin, S.; Cavalca, L. Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination. Water 2023, 15, 1406. https://doi.org/10.3390/w15071406
Bertolini M, Zecchin S, Cavalca L. Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination. Water. 2023; 15(7):1406. https://doi.org/10.3390/w15071406
Chicago/Turabian StyleBertolini, Martina, Sarah Zecchin, and Lucia Cavalca. 2023. "Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination" Water 15, no. 7: 1406. https://doi.org/10.3390/w15071406
APA StyleBertolini, M., Zecchin, S., & Cavalca, L. (2023). Sequential Anaerobic/Aerobic Microbial Transformation of Chlorinated Ethenes: Use of Sustainable Approaches for Aquifer Decontamination. Water, 15(7), 1406. https://doi.org/10.3390/w15071406







