Chlorine-Resistant Loose Nanofiltration Membranes Fabricated via Interfacial Polymerization Using Sulfone Group-Containing Amine Monomer for Dye/Salt Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
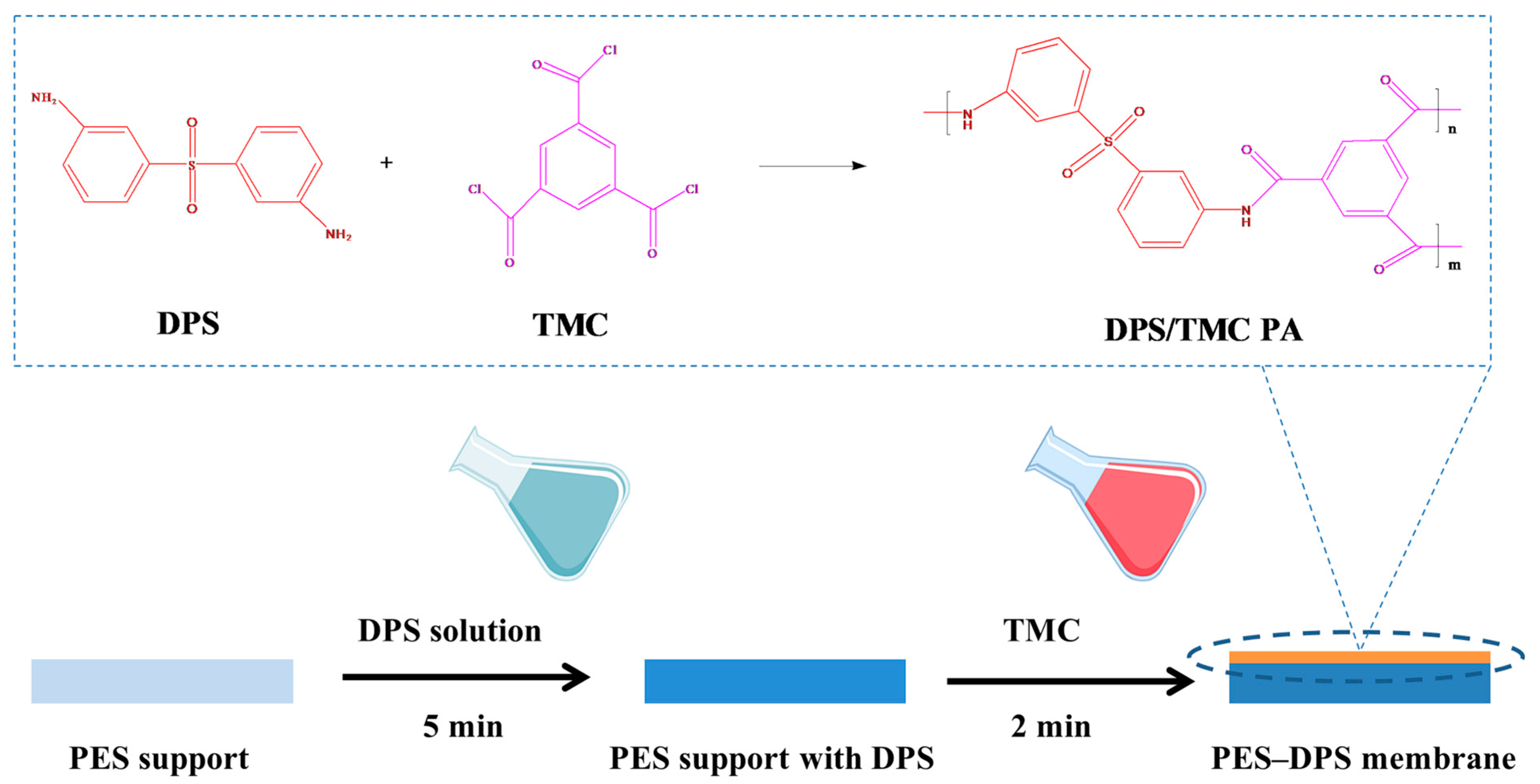
2.2. Fabrication of Loose TFC NF Membranes
2.3. Characterization of Membranes
2.4. Filtration Performance of the Membranes
2.5. Chlorine Resistance Performance of the Membranes
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Morphology of Membranes
3.1.2. Chemical Composition of the Membranes
3.1.3. Surface Charge of Membranes
3.2. Membrane Separation Performance
3.2.1. Pure Water Flux of the Membranes
3.2.2. Optimization of the Separation Performance
3.2.3. MWCO of the PES–DPS–1 Membrane
3.2.4. Solute Rejection by the PES–DPS–1 Membrane
3.2.5. Effect of Operating Pressure on the Separation Performance
3.2.6. Dye Desalination Performance of PES–DPS–1 Membrane
3.3. Membrane Chlorine Resistance Evaluations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NF | nanofiltration |
| DPS | 3,3′-diaminodiphenyl sulfone |
| IP | interfacial polymerization |
| POPs | porous organic polymers |
| TMC | trimesoyl chloride |
| APS | 2-aminophenol-4-sulfonic |
| PES | polyethersulfone |
| DI | deionized |
| FTIR | fourier transform infrared spectrometer |
| FESEM | field emission scanning electron microscope |
| Ra | average roughness |
| PEG | polyethylene glycol |
| PA–TFC | polyamide thin-film composite |
| PA | polyamide |
| CV | crystal violet |
| CR | congo red |
| G-250 | coomassie brilliant blue G-250 |
| DB71 | direct blue 71 |
| DR80 | direct red 80 |
| TFC | thin-film composite |
| XPS | X-ray photoelectron spectroscopy |
| AFM | atomic force microscope |
| PWF | pure water flux |
| MWCO | molecular weight cut-off |
References
- Naeim, S.; Emam, A.A.; Mobarak, M.F.; Hosny, R. Textile water treatment via adsorption of basic brown 1 dye on ZnO/PVC nanocomposite membrane through membrane adsorption process. Desalination Water Treat. 2021, 227, 58–67. [Google Scholar] [CrossRef]
- Mubarak, M.F.; Ragab, A.H.; Hosny, R.; Ahmed, I.A.; Ahmed, H.A.; El-Bahy, S.M.; El Shahawy, A. Enhanced Performance of Chitosan via a Novel Quaternary Magnetic Nanocomposite Chitosan/Grafted Halloysitenanotubes@ZnγFe3O4 for Uptake of Cr (III), Fe (III), and Mn (II) from Wastewater. Polymers 2021, 13, 2714. [Google Scholar] [CrossRef] [PubMed]
- El-Saeed, R.A.; Hosny, R.; Mubarak, M.F.; Abdou, M.M.; Shoueir, K.R. An innovative SiO2-pyrazole nanocomposite for Zn(II) and Cr(III) ions effective adsorption and anti-sulfate-reducing bacteria from the produced oilfield water. Arab. J. Chem. 2022, 15, 103949. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Lu, D.; Wu, W.; Ding, J.; Zhao, X.; Xiong, R.; Yang, M.; Wu, P.; Chen, F.; et al. Visible-light-driven water splitting from dyeing wastewater using Pt surface-dispersed TiO2-based nanosheets. J. Alloys Compd. 2017, 699, 183–192. [Google Scholar] [CrossRef]
- Tavangar, T.; Karimi, M.; Rezakazemi, M.; Reddy, K.R.; Aminabhavi, T.M. Textile waste, dyes/inorganic salts separation of cerium oxide-loaded loose nanofiltration polyethersulfone membranes. Chem. Eng. J. 2020, 385, 123787. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Song, P. Free Radical Graft Copolymerization Strategy To Prepare Catechin-Modified Chitosan Loose Nanofiltration (NF) Membrane for Dye Desalination. ACS Sustain. Chem. Eng. 2018, 6, 4253–4263. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Li, R.; Mai, Z.; Peng, D.; Xu, S.; Wang, J.; Zhu, J.; Zhang, Y. In situ formation of porous organic polymer-based thin polyester membranes for loose nanofiltration. J. Membr. Sci. 2022, 644, 120074. [Google Scholar] [CrossRef]
- Liu, L.; Zuo, X.; He, J.; Zhou, Y.; Xiong, J.; Ma, C.; Chen, Z.; Yu, S. Fabrication and characterization of 2-aminophenol-4-sulfonic acid-integrated polyamide loose nanofiltration membrane. J. Membr. Sci. 2021, 640, 119867. [Google Scholar] [CrossRef]
- Xu, M.; Feng, X.; Liu, Z.; Han, X.; Zhu, J.; Wang, J.; Van der Bruggen, B.; Zhang, Y. MOF laminates functionalized polyamide self-cleaning membrane for advanced loose nanofiltration. Sep. Purif. Technol. 2021, 275, 119150. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, R.; Uliana, A.A.; Liu, Y.; de Donnea, D.; Zhang, X.; Xu, D.; Gao, Q.; Jin, P.; Liu, Y.; et al. Separation of textile wastewater using a highly permeable resveratrol-based loose nanofiltration membrane with excellent anti-fouling performance. Chem. Eng. J. 2022, 434, 134705. [Google Scholar] [CrossRef]
- Glater, J.; Zachariah, M.R. A Mechanistic Study of Halogen Interaction with Polyamide Reverse-Osmosis Membranes. In Reverse Osmosis and Ultrafiltration; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1985; pp. 345–358. [Google Scholar]
- Kim, Y.K.; Lee, S.Y.; Kim, D.H.; Lee, B.S.; Nam, S.Y.; Rhim, J.W. Preparation and characterization of thermally crosslinked chlorine resistant thin film composite polyamide membranes for reverse osmosis. Desalination 2010, 250, 865–867. [Google Scholar] [CrossRef]
- Kang, G.-D.; Gao, C.-J.; Chen, W.-D.; Jie, X.-M.; Cao, Y.-M.; Yuan, Q. Study on hypochlorite degradation of aromatic polyamide reverse osmosis membrane. J. Membr. Sci. 2007, 300, 165–171. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Wang, L.; Yu, S.; Gao, C. Improving fouling resistance and chlorine stability of aromatic polyamide thin-film composite RO membrane by surface grafting of polyvinyl alcohol (PVA). Desalination 2015, 367, 11–20. [Google Scholar] [CrossRef]
- Xue, J.; Jiao, Z.; Bi, R.; Zhang, R.; You, X.; Wang, F.; Zhou, L.; Su, Y.; Jiang, Z. Chlorine-resistant polyester thin film composite nanofiltration membranes prepared with β-cyclodextrin. J. Membr. Sci. 2019, 584, 282–289. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.-Y.; Suzuki, T. Positron Annihilation Spectroscopic Evidence to Demonstrate the Flux-Enhancement Mechanism in Morphology-Controlled Thin-Film-Composite (TFC) Membrane. Environ. Sci. Technol. 2005, 39, 1764–1770. [Google Scholar] [CrossRef]
- Wu, B.; Weng, X.-D.; Wang, N.; Yin, M.-J.; Zhang, L.; An, Q.-F. Chlorine-resistant positively charged polyamide nanofiltration membranes for heavy metal ions removal. Sep. Purif. Technol. 2021, 275, 119264. [Google Scholar] [CrossRef]
- Lounder, S.J.; Asatekin, A. Fouling- and Chlorine-Resistant Nanofiltration Membranes Fabricated from Charged Zwitterionic Amphiphilic Copolymers. ACS Appl. Polym. Mater. 2022, 4, 7998–8008. [Google Scholar] [CrossRef]
- Jin, P.; Chergaoui, S.; Zheng, J.; Volodine, A.; Zhang, X.; Liu, Z.; Luis, P.; Van der Bruggen, B. Low-pressure highly permeable polyester loose nanofiltration membranes tailored by natural carbohydrates for effective dye/salt fractionation. J. Hazard. Mater. 2022, 421, 126716. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Al-Mhyawi, S.R.; Mubarak, M.F.; Hosny, R.; Amine, M.; Abdelraheem, O.H.; Zayed, M.A.; Ragab, A.H.; El Shahawy, A. Enhanced Nanofiltration Process of Thin Film Composite Membrane Using Dodecyl Phenol Ethoxylate and Oleic Acid Ethoxylate for Oilfield Calcite Scale Control. Membranes 2021, 11, 855. [Google Scholar] [CrossRef]
- Mi, Y.F.; Wang, N.; Qi, Q.; Yu, B.; Peng, X.D.; Cao, Z.H. A loose polyamide nanofiltration membrane prepared by polyether amine interfacial polymerization for dye desalination. Sep. Purif. Technol. 2020, 248, 117079. [Google Scholar] [CrossRef]
- Feng, X.; Liu, D.; Ye, H.; Peng, D.; Wang, J.; Han, S.; Zhang, Y. High-flux polyamide membrane with improved chlorine resistance for efficient dye/salt separation based on a new N-rich amine monomer. Sep. Purif. Technol. 2021, 278, 119533. [Google Scholar] [CrossRef]
- Fathy, M.; Hosny, R.; Keshawy, M.; Gaffer, A. Green synthesis of graphene oxide from oil palm leaves as novel adsorbent for removal of Cu(II) ions from synthetic wastewater. Graphene Technol. 2019, 4, 33–40. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Kochameshki, M.G. The performance of polyethersulfone nanocomposite membrane in the removal of industrial dyes. Polymer 2021, 224, 123693. [Google Scholar] [CrossRef]
- Ye, C.-C.; An, Q.-F.; Wu, J.-K.; Zhao, F.-Y.; Zheng, P.-Y.; Wang, N.-X. Nanofiltration membranes consisting of quaternized polyelectrolyte complex nanoparticles for heavy metal removal. Chem. Eng. J. 2019, 359, 994–1005. [Google Scholar] [CrossRef]
- Nightingale, E.R., Jr. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Lu, X.; Gabinet, U.R.; Ritt, C.L.; Feng, X.; Deshmukh, A.; Kawabata, K.; Kaneda, M.; Hashmi, S.M.; Osuji, C.O.; Elimelech, M. Relating Selectivity and Separation Performance of Lamellar Two-Dimensional Molybdenum Disulfide (MoS2) Membranes to Nanosheet Stacking Behavior. Environ. Sci. Technol. 2020, 54, 9640–9651. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Dong, S.-Q.; Qin, W.; Xue, Y.-X.; Wang, Q.; Zhang, J.; Liu, H.-Y.; Zhang, H.; Wang, W.; Wei, J.-F. Fabrication of highly permeable CS/NaAlg loose nanofiltration membrane by ionic crosslinking assisted layer-by-layer self-assembly for dye desalination. Sep. Purif. Technol. 2022, 284, 120202. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Ban, M.; Song, P.; Song, X.; Khan, B. Chelation–assisted in situ self-assembly route to prepare the loose PAN–based nanocomposite membrane for dye desalination. J. Membr. Sci. 2018, 566, 168–180. [Google Scholar] [CrossRef]
- Wang, X.-L.; Qin, W.; Wang, L.-X.; Zhao, K.-Y.; Wang, H.-C.; Liu, H.-Y.; Wei, J.-F. Desalination of dye utilizing carboxylated TiO2/calcium alginate hydrogel nanofiltration membrane with high salt permeation. Sep. Purif. Technol. 2020, 253, 117475. [Google Scholar] [CrossRef]
- Bera, A.; Trivedi, J.S.; Jewrajka, S.K.; Ghosh, P.K. In situ manipulation of properties and performance of polyethyleneimine nanofiltration membranes by polyethylenimine-dextran conjugate. J. Membr. Sci. 2016, 519, 64–76. [Google Scholar] [CrossRef]
- Ye, W.; Lin, J.; Borrego, R.; Chen, D.; Sotto, A.; Luis, P.; Liu, M.; Zhao, S.; Tang, C.Y.; Van der Bruggen, B. Advanced desalination of dye/NaCl mixtures by a loose nanofiltration membrane for digital ink-jet printing. Sep. Purif. Technol. 2018, 197, 27–35. [Google Scholar] [CrossRef]
- Peydayesh, M.; Mohammadi, T.; Bakhtiari, O. Effective treatment of dye wastewater via positively charged TETA-MWCNT/PES hybrid nanofiltration membranes. Sep. Purif. Technol. 2018, 194, 488–502. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Ji, Y.L.; Weng, X.D.; Mi, Y.F.; Ye, C.C.; An, Q.F.; Gao, C.J. High-Flux Positively Charged Nanocomposite Nanofiltration Membranes Filled with Poly(dopamine) Modified Multiwall Carbon Nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 6693–6700. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van der Bruggen, B. High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts. Environ. Sci. Nano 2019, 6, 2958–2967. [Google Scholar] [CrossRef]
- Liu, S.; Wu, C.; Hou, X.; She, J.; Liu, S.; Lu, X.; Zhang, H.; Gray, S. Understanding the chlorination mechanism and the chlorine-induced separation performance evolution of polypiperazine-amide nanofiltration membrane. J. Membr. Sci. 2019, 573, 36–45. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, X.; Xing, J.; Wang, T.; Xu, D.; Bai, L.; Luo, X.; Wang, W.; Li, G.; Liang, H. In-situ covalently bonded supramolecular-based protective layer for improving chlorine resistance of thin-film composite nanofiltration membranes. Desalination 2020, 474, 114197. [Google Scholar] [CrossRef]
- Fan, X.; Dong, Y.; Su, Y.; Zhao, X.; Li, Y.; Liu, J.; Jiang, Z. Improved performance of composite nanofiltration membranes by adding calcium chloride in aqueous phase during interfacial polymerization process. J. Membr. Sci. 2014, 452, 90–96. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Chen, H.; Liu, Q.; Wang, J.; Wang, T. Preparation of polyamide membranes with improved chlorine resistance by bis-2,6-N,N-(2-hydroxyethyl) diaminotoluene and trimesoyl chloride. Desalination 2013, 331, 16–25. [Google Scholar] [CrossRef]
- Geng, X.; Wang, J.; Ding, Y.; Zhang, W.; Wang, Y.; Liu, F. Poly(vinyl alcohol)/polydopamine hybrid nanofiltration membrane fabricated through aqueous electrospraying with excellent antifouling and chlorine resistance. J. Membr. Sci. 2021, 632, 119385. [Google Scholar] [CrossRef]
- Gao, C.; Lu, X.; Bao, Z. Polysulfone amide (PSA) asymmetric RO membrane. Desalination 1991, 83, 271–278. [Google Scholar] [CrossRef]
- Iborra, M.I.; Lora, J.; Alcaina, M.I.; Arnal, J.M. Effect of oxidation agents on reverse osmosis membrane performance to brackish water desalination. Desalination 1997, 108, 83–89. [Google Scholar] [CrossRef]

















| Dyes | Molecular Weight (Da) | Absorption Wavelength (nm) | Charge (±) | Molecular Structure |
|---|---|---|---|---|
| Crystal Violet | 373.53 | 590 | +1 |  |
| Congo Red | 696.66 | 497 | −2 |  |
| Coomassie Brilliant Blue G-250 | 825.97 | 595 | −2 |  |
| Direct Blue 71 | 1029.87 | 594 | −4 |  |
| Direct Red 80 | 1373.07 | 528 | −6 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Zheng, K.; Jin, Y.; Zhou, S. Chlorine-Resistant Loose Nanofiltration Membranes Fabricated via Interfacial Polymerization Using Sulfone Group-Containing Amine Monomer for Dye/Salt Separation. Water 2023, 15, 1456. https://doi.org/10.3390/w15081456
Huang L, Zheng K, Jin Y, Zhou S. Chlorine-Resistant Loose Nanofiltration Membranes Fabricated via Interfacial Polymerization Using Sulfone Group-Containing Amine Monomer for Dye/Salt Separation. Water. 2023; 15(8):1456. https://doi.org/10.3390/w15081456
Chicago/Turabian StyleHuang, Longwei, Ke Zheng, Yuting Jin, and Shaoqi Zhou. 2023. "Chlorine-Resistant Loose Nanofiltration Membranes Fabricated via Interfacial Polymerization Using Sulfone Group-Containing Amine Monomer for Dye/Salt Separation" Water 15, no. 8: 1456. https://doi.org/10.3390/w15081456
APA StyleHuang, L., Zheng, K., Jin, Y., & Zhou, S. (2023). Chlorine-Resistant Loose Nanofiltration Membranes Fabricated via Interfacial Polymerization Using Sulfone Group-Containing Amine Monomer for Dye/Salt Separation. Water, 15(8), 1456. https://doi.org/10.3390/w15081456






