Feasibility of Time-Dependent Amplitude in Pulse-Compressed Broadband Acoustic Signals for Determining the Dorsal Orientation of Fish
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment with Fish Observed in Dorsal Aspects
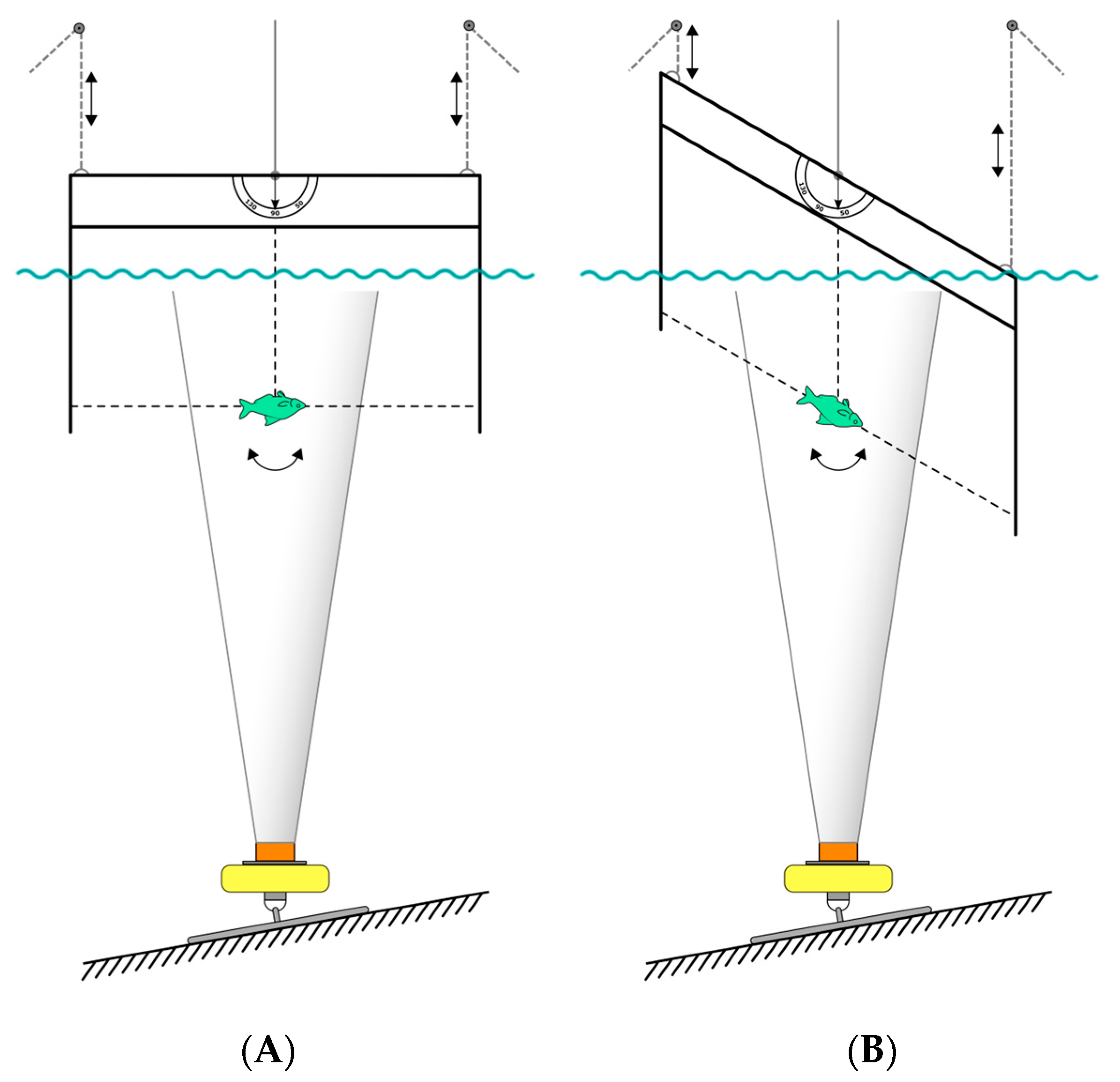
2.1.1. Experimental Site
2.1.2. Hydroacoustic Description and Settings
2.1.3. Experimental Procedure
2.2. Data Processing and Analysis
2.2.1. Extraction of Amplitude Echo Envelopes
2.2.2. Extraction of Amplitude Echo Descriptors
2.2.3. Modeling of Amplitude Echo Envelopes and Descriptors
- is the response (such as the measured amplitude) for fish i, angle a, time t (within a pulse) and replicate r;
- is the (unknown) intercept parameter;
- is the individual-specific random effect (distribution of which depends on unknown variance parameter );
- is an (unknown) smooth component (function of one variable), implemented as a thin-plate spline [28];
- is an (unknown) smooth component (function of two variables), implemented as a factor smooth interactive term (called “factor smooth interactions”, [25]). This term formalizes the interaction between (within-pulse) time and angle. Specifically, it allows for time profile deformation in relation to the body angle. This is a key term with respect to the main purpose of the study;
- is a (theoretical) residual with unknown variance parameter .
- is the response (AMAX or EL) for fish i, angle a and replicate r;
- is the (unknown) intercept parameter;
- is the individual-specific random effect (whose distribution depends on unknown variance parameter );
- is an (unknown) parameter (slope);
- is an (unknown) smooth component (function of one variable), implemented as a thin-plate spline [28]. This is a key term with respect to the main purpose of the study (when focusing on characteristics derived from the profile).
2.2.4. Software
3. Results
3.1. Interaction between Fish Tilt Angle and Amplitude Echo Envelope
3.2. Interaction between Fish Tilt Angle and Amplitude Echo Descriptors
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds, J.; MacLennan, D.N. Fisheries Acoustics: Theory and Practice, 2nd ed.; Simmonds, J., MacLennan, D., Eds.; Blackwell Publishing: New York, NY, USA, 2005; ISBN 978-0-470-99529-7. [Google Scholar]
- Demer, D.A.; Andersen, L.N.; Bassett, C.; Berger, L.; Chu, D.; Condiotty, J.; Cutter, G.R., Jr.; Hutton, B.; Korneliussen, R.; Bouffant, N.L.; et al. 2016 USA–Norway EK80 Workshop Report: Evaluation of a Wideband Echosounder for Fisheries and Marine Ecosystem Science. ICES Coop. Res. Rep. (CRR) 2017, 336, 79. [Google Scholar] [CrossRef]
- Turin, G. An Introduction to Matched Filters. IEEE Trans. Inf. Theory 1960, 6, 311–329. [Google Scholar] [CrossRef]
- Ehrenberg, J.E.; Torkelson, T.C. FM Slide (Chirp) Signals: A Technique for Significantly Improving the Signal-to-Noise Performance in Hydroacoustic Assessment Systems. Fish. Res. 2000, 47, 193–199. [Google Scholar] [CrossRef]
- Stanton, T.K.; Reeder, D.B.; Jech, J.M. Inferring Fish Orientation from Broadband-Acoustic Echoes. ICES J. Mar. Sci. 2003, 60, 524–531. [Google Scholar] [CrossRef]
- Zakharia, M.E.; Magand, F.; Hetroit, F.; Diner, N. Wideband Sounder for Fish Species Identification at Sea. ICES J. Mar. Sci. 1996, 53, 203–208. [Google Scholar] [CrossRef]
- Bassett, C.; De Robertis, A.; Wilson, C.D. Broadband Echosounder Measurements of the Frequency Response of Fishes and Euphausiids in the Gulf of Alaska. ICES J. Mar. Sci. 2018, 75, 1131–1142. [Google Scholar] [CrossRef]
- Blanluet, A.; Doray, M.; Berger, L.; Romagnan, J.-B.; Bouffant, N.L.; Lehuta, S.; Petitgas, P. Characterization of Sound Scattering Layers in the Bay of Biscay Using Broadband Acoustics, Nets and Video. PLoS ONE 2019, 14, e0223618. [Google Scholar] [CrossRef]
- Gugele, S.M.; Widmer, M.; Baer, J.; DeWeber, J.T.; Balk, H.; Brinker, A. Differentiation of Two Swim Bladdered Fish Species Using next Generation Wideband Hydroacoustics. Sci. Rep. 2021, 11, 10520. [Google Scholar] [CrossRef]
- Horne, J.; Jech, J.M. Multi-Frequency Estimates of Fish Abundance: Constraints of Rather High Frequencies. ICES J. Mar. Sci. 1999, 56, 184–199. [Google Scholar] [CrossRef][Green Version]
- Fässler, S.M.M.; Santos, R.; García-Núñez, N.; Fernandes, P.G. Multifrequency Backscattering Properties of Atlantic Herring (Clupea harengus) and Norway Pout (Trisopterus esmarkii). Can. J. Fish. Aquat. Sci. 2007, 64, 362–374. [Google Scholar] [CrossRef]
- Jaffe, J.S.; Roberts, P.L.D. Estimating Fish Orientation from Broadband, Limited-Angle, Multiview, Acoustic Reflections. J. Acoust. Soc. Am. 2011, 129, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Kubilius, R.; Macaulay, G.J.; Ona, E. Remote Sizing of Fish-like Targets Using Broadband Acoustics. Fish. Res. 2020, 228, 105568. [Google Scholar] [CrossRef]
- Kubilius, R.; Bergès, B.; Macaulay, G.J. Remote Acoustic Sizing of Tethered Fish Using Broadband Acoustics. Fish. Res. 2023, 260, 106585. [Google Scholar] [CrossRef]
- Frouzova, J.; Kubecka, J.; Balk, H.; Frouz, J. Target Strength of Some European Fish Species and Its Dependence on Fish Body Parameters. Fish. Res. 2005, 75, 86–96. [Google Scholar] [CrossRef]
- Miyanohana, Y.; Ishii, K.; Furusawa, M. Dorsal Aspect Target Strength Functions of Eight Species of Fish at Four Frequencies. Tech. Rep. Natl. Res. Inst. Fish. Eng. Fish. Boat Instrum. 1990, 189, 317–324. [Google Scholar]
- De Robertis, A.; Schell, C.; Jaffe, J.S. Acoustic Observations of the Swimming Behavior of the Euphausiid Euphausia Pacifica Hansen. ICES J. Mar. Sci. 2003, 60, 885–898. [Google Scholar] [CrossRef][Green Version]
- Genin, A.; Jaffe, J.S.; Reef, R.; Richter, C.; Franks, P.J.S. Swimming against the Flow: A Mechanism of Zooplankton Aggregation. Science 2005, 308, 860–862. [Google Scholar] [CrossRef]
- Tušer, M.; Kubečka, J.; Frouzová, J.; Jarolím, O. Fish Orientation along the Longitudinal Profile of the Římov Reservoir during Daytime: Consequences for Horizontal Acoustic Surveys. Fish. Res. 2009, 96, 23–29. [Google Scholar] [CrossRef]
- Burwen, D.L.; Nealson, P.A.; Fleischman, S.J.; Mulligan, T.J.; Horne, J.K. The Complexity of Narrowband Echo Envelopes as a Function of Fish Side-Aspect Angle. ICES J. Mar. Sci. 2007, 64, 1066–1074. [Google Scholar] [CrossRef]
- Demer, D.A.; Berger, L.; Bernasconi, M.; Bethke, E.; Boswell, K.; Chu, D.; Domokos, R.; Dunford, A.; Fässler, S.; Gauthier, S.; et al. Calibration of Acoustic Instruments. ICES Coop. Res. Rep. (CRR) 2015, 326, 136. [Google Scholar] [CrossRef]
- Oppenheim, A.V.; Schafer, R.W. Discrete-Time Signal Processing, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1989; ISBN 978-0-13-216292-0. [Google Scholar]
- Tušer, M.; Frouzová, J.; Balk, H.; Muška, M.; Mrkvička, T.; Kubečka, J. Evaluation of Potential Bias in Observing Fish with a DIDSON Acoustic Camera. Fish. Res. 2014, 155, 114–121. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-0-412-34390-2. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017; ISBN 978-1-315-37027-9. [Google Scholar]
- De Boor, C. A Practical Guide to Splines; Springer: New York, NY, USA, 2001; Volume 27, ISBN 978-0-387-95366-3. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company: Boston, MA, USA, 1977; ISBN 978-0-201-07616-5. [Google Scholar]
- Wood, S.N. Thin Plate Regression Splines. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2003, 65, 95–114. [Google Scholar] [CrossRef]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing Parameter and Model Selection for General Smooth Models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation; 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 12 March 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wood, S.N.; Goude, Y.; Shaw, S. Generalized Additive Models for Large Data Sets. J. R. Stat. Society. Ser. C (Appl. Stat.) 2015, 64, 139–155. [Google Scholar] [CrossRef]
- Ho, T.K. Random Decision Forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–16 August 1995; Volume 1, pp. 278–282. [Google Scholar]
- Au, W.W.L.; Benoit-Bird, K.J. Acoustic Backscattering by Hawaiian Lutjanid Snappers. II. Broadband Temporal and Spectral Structure. J. Acoust. Soc. Am. 2003, 114, 2767–2774. [Google Scholar] [CrossRef]
- Lavery, A.C.; Bassett, C.; Lawson, G.L.; Jech, J.M. Exploiting Signal Processing Approaches for Broadband Echosounders. ICES J. Mar. Sci. 2017, 74, 2262–2275. [Google Scholar] [CrossRef]
- Henderson, M.J.; Horne, J.K.; Towler, R.H. The Influence of Beam Position and Swimming Direction on Fish Target Strength. ICES J. Mar. Sci. 2008, 65, 226–237. [Google Scholar] [CrossRef]
- Foote, K.G. Importance of the Swimbladder in Acoustic Scattering by Fish: A Comparison of Gadoid and Mackerel Target Strengths. J. Acoust. Soc. Am. 1980, 67, 2084–2089. [Google Scholar] [CrossRef]
- Macaulay, G.J. Anatomically Detailed Acoustic Scattering Models of Fish. Bioacoustics 2002, 12, 275–277. [Google Scholar] [CrossRef]
- Beregi, A.; Székely, C.; Békési, L.; Szabó, J.; Molnár, V.; Molnár, K. Radiodiagnostic Examination of the Swimbladder of Some Fish Species. Acta. Vet. Hung. 2001, 49, 87–98. [Google Scholar] [CrossRef]



| ID | Standard Length [mm] | Total Length [mm] | Weight [g] |
|---|---|---|---|
| bream5 | 165 | 205 | 94 |
| bream1 | 170 | 225 | 120 |
| bream3 | 205 | 255 | 186 |
| bream6 | 240 | 295 | 304 |
| bream2 | 290 | 355 | 518 |
| bream4 | 310 | 375 | 628 |
| bream7 | 325 | 400 | 622 |
| Ramping | Model M1 Component | edf | F | p |
|---|---|---|---|---|
| fast | bi | 5 | 2441 | <2 × 10−16 |
| ssize | 1 | 87 | <2 × 10−16 | |
| s50 | 14 | 33,001 | <2 × 10−16 | |
| s60 | 14 | 40,008 | <2 × 10−16 | |
| s70 | 14 | 49,688 | <2 × 10−16 | |
| s80 | 14 | 44,410 | <2 × 10−16 | |
| s90 | 14 | 40,766 | <2 × 10−16 | |
| s100 | 14 | 47,679 | <2 × 10−16 | |
| s110 | 14 | 41,846 | <2 × 10−16 | |
| s120 | 14 | 40,478 | <2 × 10−16 | |
| s130 | 14 | 44,044 | <2 × 10−16 | |
| slow | bi | 4 | 4662 | <2 × 10−16 |
| ssize | 1 | 18 | <2 × 10−16 | |
| s50 | 14 | 25,782 | <2 × 10−16 | |
| s60 | 14 | 26,706 | <2 × 10−16 | |
| s70 | 14 | 24,904 | <2 × 10−16 | |
| s80 | 14 | 28,241 | <2 × 10−16 | |
| s90 | 14 | 29,702 | <2 × 10−16 | |
| s100 | 14 | 25,088 | <2 × 10−16 | |
| s110 | 14 | 27,635 | <2 × 10−16 | |
| s120 | 14 | 29,312 | <2 × 10−16 | |
| s130 | 14 | 24,902 | <2 × 10−16 |
| Deviance Explained [%] | F | ||||
|---|---|---|---|---|---|
| Ramping | Explanatory | GCV | bi | sangle | |
| fast | AMAX | 61 | 6 | 91 | 7580 |
| EL3dB | 17 | 28 | 51 | 128 | |
| EL6dB | 20 | 71 | 39 | 170 | |
| EL9dB | 35 | 93 | 108 | 311 | |
| EL12dB | 52 | 87 | 124 | 556 | |
| EL15dB | 71 | 61 | 164 | 1308 | |
| EL18dB | 81 | 40 | 127 | 2342 | |
| EL24dB | 61 | 104 | 127 | 875 | |
| slow | AMAX | 46 | 9 | 92 | 193 |
| EL3dB | 24 | 39 | 76 | 66 | |
| EL6dB | 31 | 46 | 44 | 178 | |
| EL9dB | 62 | 38 | 103 | 738 | |
| EL12dB | 68 | 52 | 186 | 1003 | |
| EL15dB | 71 | 63 | 175 | 1031 | |
| EL18dB | 69 | 77 | 124 | 852 | |
| EL24dB | 51 | 187 | 57 | 464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tušer, M.; Brabec, M.; Balk, H.; Draštík, V.; Kubečka, J.; Frouzová, J. Feasibility of Time-Dependent Amplitude in Pulse-Compressed Broadband Acoustic Signals for Determining the Dorsal Orientation of Fish. Water 2023, 15, 1596. https://doi.org/10.3390/w15081596
Tušer M, Brabec M, Balk H, Draštík V, Kubečka J, Frouzová J. Feasibility of Time-Dependent Amplitude in Pulse-Compressed Broadband Acoustic Signals for Determining the Dorsal Orientation of Fish. Water. 2023; 15(8):1596. https://doi.org/10.3390/w15081596
Chicago/Turabian StyleTušer, Michal, Marek Brabec, Helge Balk, Vladislav Draštík, Jan Kubečka, and Jaroslava Frouzová. 2023. "Feasibility of Time-Dependent Amplitude in Pulse-Compressed Broadband Acoustic Signals for Determining the Dorsal Orientation of Fish" Water 15, no. 8: 1596. https://doi.org/10.3390/w15081596
APA StyleTušer, M., Brabec, M., Balk, H., Draštík, V., Kubečka, J., & Frouzová, J. (2023). Feasibility of Time-Dependent Amplitude in Pulse-Compressed Broadband Acoustic Signals for Determining the Dorsal Orientation of Fish. Water, 15(8), 1596. https://doi.org/10.3390/w15081596







