SWRO Brine Characterisation and Critical Analysis of Its Industrial Valorisation: A Case Study in the Canary Islands (Spain)
Abstract
:1. Introduction
1.1. Desalination Brine Valorisation Technologies
2. Materials and Methods
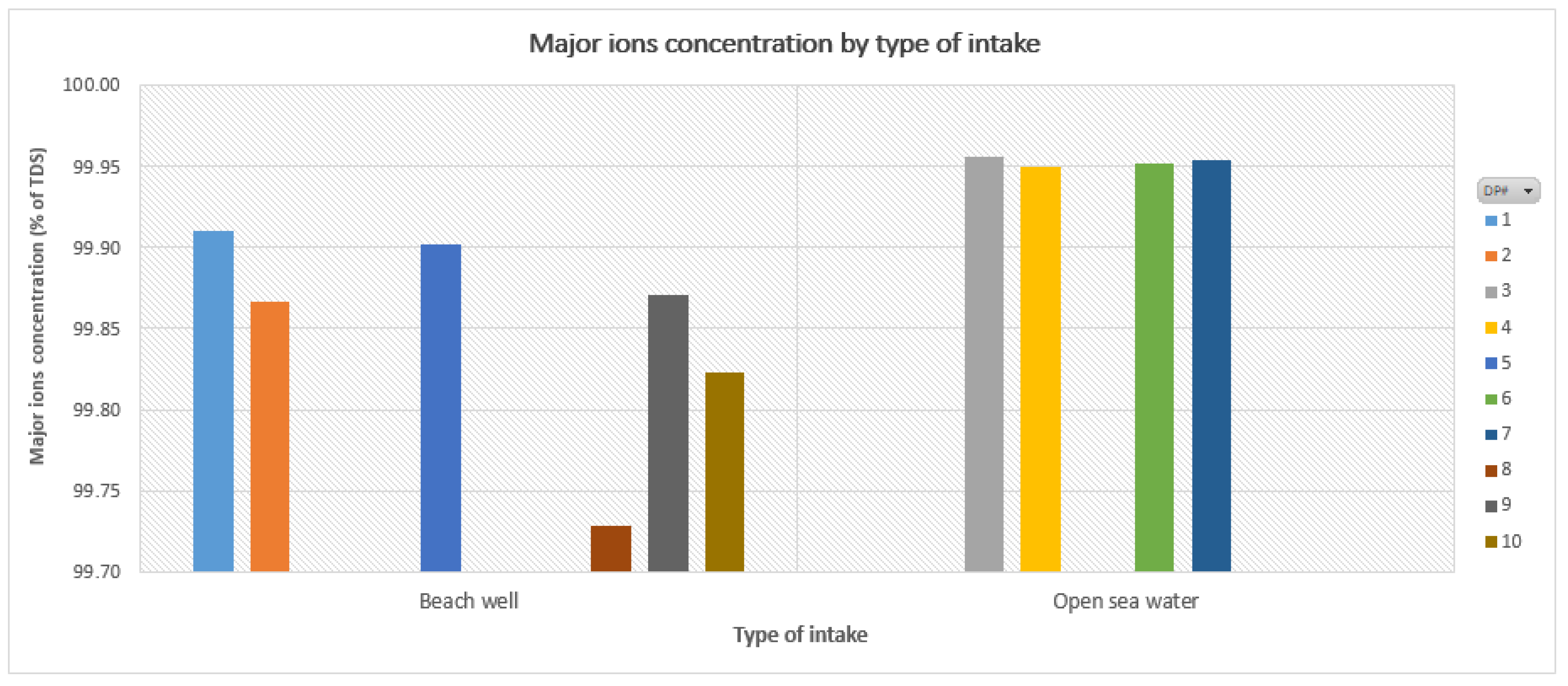
2.1. Brine Characterisation Analysis
2.2. Identification of Industrial DBV Factors
3. Results and Discussion
3.1. Brine Characterisation and Categorisation of SWRO Brine in the Canary Islands
3.2. Strengths of Industrial DBV
3.2.1. Environmental Impact Mitigation of Brine Discharge
3.2.2. Resource or Energy Recovery
3.2.3. Desalinated Water Production Increase
3.2.4. Hybrid Solutions—Zero Liquid Discharge (ZLD)
3.2.5. Potential Integration with Renewable Energies and Waste Heat
3.2.6. New Employment/Business Opportunities
3.3. Impacts and Barriers of Industrial DBV
3.3.1. Pre-Treatment
3.3.2. Technology Readiness Level of Emerging Technologies
3.3.3. Environmental Impact
3.3.4. High Capex/Opex
3.3.5. Legal Restrictions
3.3.6. Limited Available Research Data
3.3.7. Commercialisation of By-Products
3.3.8. Lack of Specific Materials/Components
3.3.9. Lack of Specific Simulation Software Tools
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Brine concentrator |
| BCr | Brine crystallizer |
| BMED | Bipolar membrane electrodialysis |
| BMSED | Selectrodialysis with bipolar membranes |
| CCU | Carbon capture and utilisation |
| CP | Chemical precipitation |
| DB | Desalination brine |
| DBV | Desalination brine valorisation |
| DP | Desalination plant |
| EC | Electrical conductivity |
| EDM | Electrodialysis metathesis |
| FO | Forward osmosis |
| FTIR | Fourier-transform infrared spectroscopy |
| HPLC | High-performance liquid chromatography |
| HPRO | High pressure reverse osmosis |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| IEx | Ion exchange |
| MCr | Membrane crystallisation |
| MD | Membrane distillation |
| MED | Multi-effect distillation |
| MLD | Minimal liquid discharge |
| MSF | Multistage flash distillation |
| NF | Nanofiltration |
| OARO | Osmotically assisted reverse osmosis |
| PRO | Pressure retarded osmosis |
| RED | Reverse electrodialysis |
| SWRO | Seawater reverse osmosis |
| RO | Reverse osmosis |
| SD | Spray drying |
| SEC | Specific energy consumption |
| SED | Selective electrodialysis |
| TDS | Total dissolved solids |
| TOC | Total organic carbon |
| TRL | Technology readiness level |
| TSSE | Temperature Swing Solvent Extraction |
| VCD | Vapour compression evaporation |
| ZLD | Zero liquid discharge |
References
- GWI DesalData. IDA Desalination & Reuse Handbook, 2021–2022. Available online: https://www.globalwaterintel.com/products-and-services/market-research-reports/ida-desalination-reuse-handbook (accessed on 30 March 2021).
- Eke, J.; Yusuf, A.; Giwa, A.; Sodiq, A. The global status of desalination: An assessment of current desalination technologies, plants and capacity. Desalination 2020, 495, 114633. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine management in desalination industry: From waste to resources generation. Desalination 2019, 472, 114187. [Google Scholar]
- Ihsanullah, I.; Atieh, M.A.; Sajid, M.; Nazal, M.K. Desalination and environment: A critical analysis of impacts, mitigation strategies, and greener desalination technologies. Sci. Total Environ. 2021, 780, 146585. [Google Scholar]
- Panagopoulos, A.; Haralambous, K.J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [PubMed]
- Sola, I.; Zarzo, D.; Carratalá, A.; Fernández-Torquemada, Y.; de-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Sánchez-Lizaso, J.L. Review of the management of brine discharges in Spain. Ocean. Coast. Manag. 2020, 196, 105301. [Google Scholar]
- Portillo, E.; Louzara, G.; Ruiz de la Rosa, M.; Quesada, J.; Gonzalez, J.C.; Roque, F.; Antequera, M.; Mendoza, H. Venturi diffusers as enhancing devices for the dilution process in desalination plant brine discharges. Desalination Water Treat. 2013, 51, 525–542. [Google Scholar]
- Instituto Español de Oceanografía. Estrategia Marina—Demarcación Marina Canaria; Catálogo de Publicaciones de la Administración General del Estado: Madrid, Spain, 2012. [Google Scholar]
- Veza, J.M. Desalination in the Canary Islands: An update. Desalination 2001, 133, 259–270. [Google Scholar]
- Gómez-Gotor, A.; Del Río-Gamero, B.; Prado, I.P.; Casañas, A. The history of desalination in the Canary Islands. Desalination 2018, 428, 86–107. [Google Scholar]
- Ministerio Para la Transición Ecológica y el Reto Demográfico (Gobierno de España). Censo Nacional de Vertidos. 2020. Available online: https://www.miteco.gob.es/es/agua/publicaciones/cnv-resumen-ejecutivo-2020_tcm30-536914.pdf (accessed on 5 April 2021).
- European Parliamentary Research Service. Circular Economy. 2018. Available online: http://www.europarl.europa.eu/thinktank/infographics/circulareconomy/public/index.html (accessed on 12 April 2022).
- European Commission. Circular Economy Action Plan. March 2020. Available online: http://ec.europa.eu/environment/strategy/circular-economy-action-plan_en (accessed on 12 April 2022).
- Ministerio Para la Transición Ecológica y el Reto Demográfico—Gobierno de España. Estrategia Española de Economía Circular. Available online: https://www.miteco.gob.es/es/calidad-y-evaluacion-ambiental/temas/economia-circular/estrategia/ (accessed on 10 May 2021).
- Gobierno de Canarias. Estrategias Canarias Economía Azul y Circular. 13 Octubre 2020. Available online: http://www.gobiernodecanarias.org/ece/economia-azul-y-circular/index.html (accessed on 13 April 2022).
- Reza Moradi, M.; Pihlajamäki, A.; Hesampour, M.; Ahlgren, J.; Mänttäri, M. End-of-life RO membranes recycling: Reuse as NF membranes by polyelectrolyte layer-by-layer deposition. J. Membr. Sci. 2019, 584, 300–308. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular economy in membrane technology: Using end-of-life reverse osmosis modules for preparation of recycled anion exchange membranes and validation in electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar]
- Pervov, A.; Andrianov, A.; Rudakova, G.; Popov, K. A comparative study of some novel “green” and traditional antiscalants efficiency for the reverse osmotic Black Sea water desalination. Desalination Water Treat. 2017, 73, 11–21. [Google Scholar]
- Kurihara, M.; Ito, Y. Sustainable seawater reverse Osmosis desalination as green desalination in the 21st century. J. Membr. Sci. Res. 2020, 6, 20–29. [Google Scholar]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [PubMed]
- Bello, A.S. An overview of brine management: Emerging desalination technologies, life cycle assessment, and metal recovery methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar]
- ITC; ULPGC; ULL. DESAL+ Living Lab. Available online: http://www.desalinationlab.com/ (accessed on 22 August 2022).
- Pérez-González, A.; Ibáñez, R.; Gómez, P.; Urtiaga, A.; Ortiz, I.; Irabien, J. Recovery of desalination brines: Separation of calcium, magnesium and sulfate as a pre-treatment step. Desalination Water Treat. 2015, 56, 3617–3625. [Google Scholar]
- Mohammad, A.F.; El-Naas, M.H.; Al-Marzouqi, A.H.; Suleiman, M.I.; Al Musharfy, M. Optimization of magnesium recovery from reject brine for reuse in desalination post-treatment. J. Water Process Eng. 2019, 31, 100810. [Google Scholar]
- Ramasamy, B. Short Review of Salt Recovery from Reverse Osmosis Reject; IntechOpen: London, UK, 2019. [Google Scholar]
- Ali, M.E. Nanofiltration process for enhanced treatment of RO brine discharge. Membranes 2021, 11, 212. [Google Scholar]
- Park, K.; Kim, J.; Hong, S. Brine management systems using membrane concentrators: Future directions for membrane development in desalination. Desalination 2022, 535, 115839. [Google Scholar]
- García-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A. Environmental challenges of the chlor-alkali production: Seeking answers from a life cycle approach. Sci. Total Environ. 2017, 580, 147–157. [Google Scholar] [PubMed] [Green Version]
- Kumar, A.; Phillips, K.R.; Thiel, G.; Schröder, U.; Lienhard, J.H.V. Direct electrosynthesis of sodium hydroxide and hydrochloric acid from brine streams. Nat. Catal. 2019, 2, 106–113. [Google Scholar]
- Malholtra, V. Methods and Systems for Making Hypochlorite Solution from Reverse Osmosis Brine. U.S. Patent 10,934,627, 2 March 2021. [Google Scholar]
- Luo, X.; Li, X.; Wei, C.; Deng, Z.; Liu, Y.; Li, M.; Zheng, S.; Huang, X. Recovery of NaCl and Na2SO4 from high salinity brine by purification and evaporation. Desalination 2022, 530, 115631. [Google Scholar]
- Sakai, H.; Ueyama, T.; Irie, M.; Matsuyama, K.; Tanioka, A.; Saito, K.; Kumano, A. Energy recovery by PRO in sea water desalination plant. Desalination 2016, 389, 52–57. [Google Scholar]
- Schunke, A.J.; Hernández-Herrera, G.A.; Padhye, L.; Berry, T.-A. Energy recovery in SWRO desalination: Current status and new possibilities. Front. Sustain. Cities 2020, 2, 9. [Google Scholar]
- Tawalbeh, M.; Al-Othman, A.; Abdelwahab, N.; Alami, A.H.; Olabi, A.G. Recent developments in pressure retarded osmosis for desalination and power generation. Renew. Sustain. Energy Rev. 2021, 138, 110492. [Google Scholar]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar]
- Ihsanullah, I.; Mustafa, J.; Mannan Zafar, A.; Obaid, M.; Atieh, M.A.; Ghaffour, N. Waste to wealth: A critical analysis of resource recovery from desalination brine. Desalination 2022, 543, 116093. [Google Scholar]
- Mustafa, J.; Mourad, A.A.-H.; Al-Marzouqi, A.H.; El-Naas, M.H. Simultaneous treatment of reject brine and capture of carbon dioxide: A comprehensive review. Desalination 2020, 483, 114386. [Google Scholar]
- Das, P.; Dutta, S.; Kumar Singh, K. Insights into membrane crystallization: A sustainable tool for value added product recovery from effluent streams. Sep. Purif. Technol. 2020, 257, 117666. [Google Scholar]
- Yang, G.; Ng, D.; Huang, Z.; Zhang, J.; Gray, S.; Xie, Z. Janus hollow fibre membranes with intrusion anchored structure for robust desalination and leachate treatment in direct contact membrane distillation. Desalination 2023, 551, 116423. [Google Scholar]
- Davenport, D.M.; Deshmukh, A.; Werber, J.R.; Elimelech, M. High-pressure reverse osmosis for energy-efficient hypersaline brine desalination: Current status, design considerations, and research needs. Environ. Sci. Technol. Lett. 2018, 5, 467–475. [Google Scholar]
- Chen, B.; Jiang, C.; Wang, Y.; Fu, R.; Liu, Z.; Xu, T. Selectrodialysis with bipolar membrane for the reclamation of concentrated brine from RO plant. Desalination 2018, 442, 8–15. [Google Scholar]
- Panagopoulos, A. Beneficiation of saline effluents from seawater desalination plants: Fostering the zero liquid discharge (ZLD) approach—A techno-economic evaluation. J. Environ. Chem. Eng. 2021, 9, 105338. [Google Scholar]
- Cipolleta, G.; Lancioni, N.; Akyol, Ç.; Eusebi, A.L.; Fatone, F. Brine treatment technologies towards minimum/zero liquid discharge and resource recovery: State of the art and techno-economic assessment. J. Environ. Manag. 2021, 300, 113681. [Google Scholar]
- Morgante, C.; Vassallo, F.; Xevgenos, D.; Cipollina, A.; Micari, M.; Tamburini, A.; Micale, G. Valorisation of SWRO brines in a remote island through a circular approach: Techno-economic analysis and perspectives. Desalination 2022, 542, 116005. [Google Scholar]
- Panagopoulos, A.; Haralambous, K.-J. Minimal liquid discharge (MLD) and zero liquid discharge (ZLD) strategies for wastewater management and resource recovery—Analysis, challenges and prospects. J. Environ. Chem. Eng. 2020, 8, 104418. [Google Scholar]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar]
- European Commission. Study on the Review of the List of Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020.
- Yang, S.; Zhang, F.; Ding, H.; He, P.; Zhou, H. Lithium metal extraction from seawater. Joule 2018, 2, 1648–1651. [Google Scholar]
- Wiechert, A.I.; Ladshaw, A.P.; Gill, G.A.; Wood, J.R.; Yiacoumi, S.; Tsouris, C. Uranium resource recovery from desalination plant feed and reject water using amidoxime functionalized adsorbent. Ind. Eng. Chem. Res. 2018, 57, 17237–17244. [Google Scholar]
- Chen, W.-S.; Lee, C.-H.; Chung, Y.-F.; Tien, K.-W.; Chen, Y.-J.; Chen, Y.-A. Recovery of rubidium and cesium resources from brine of desalination through t-BAMBP extraction. Metals 2020, 10, 607. [Google Scholar]
- Sea4value. Mining Value from Brines. 2020. Available online: http://sea4value.eu/ (accessed on 10 January 2022).
- Ministerio de la Presidencia—Gobierno de España. Real Decreto 140/2003, de 7 de Febrero, por el que se Establecen los Criterios Sanitarios de la Calidad del agua de Consumo Humano. 2003. Available online: https://www.boe.es/eli/es/rd/2003/02/07/140/con (accessed on 24 January 2022).
- Ministerio de la Presidencia—Gobierno de España. Real Decreto 1620/2007, de 7 de Diciembre, por el que se Establece el Régimen Jurídico de la Reutilización de las Aguas Depuradas. 2007. Available online: https://www.boe.es/eli/es/rd/2007/12/07/1620 (accessed on 24 January 2022).
- Shah, K.M.; Billinge, I.H.; Chen, X.; Fan, H.; Huang, Y.; Winton, R.K.; Yin Yip, N. Drivers, challenges, and emerging technologies for desalination of high-salinity brines: A critical review. Desalination 2022, 538, 115827. [Google Scholar] [CrossRef]
- Khan, M.; Al-Absi, R.S.; Khraisheh, M.; Al-Ghouti, M.A. A better understanding of seawater reverse osmosis brine: Characterizations, uses, and energy requirements. Case Stud. Chem. Environ. Eng. 2021, 4, 100165. [Google Scholar] [CrossRef]
- Amitouche, M.; Remini, B. Operation of cap djinet desalination plant and dilution of brine with power station cooling water. Desalination Water Treat. 2014, 57, 3514–3521. [Google Scholar] [CrossRef]
- Barros Rebello, L.R.; Siepman, T.; Drexler, S. Correlations between TDS and electrical conductivity for high-salinity formation brines characteristic of South Atlantic pre-salt basins. Water SA 2020, 46, 602–609. [Google Scholar] [CrossRef]
- Hernández Moreno, J.M. Suelos Volcánicos de Canarias—Propiedades Específicas e Implicaciones Agronómicas y Ambientales; Cabildo Insular de Tenerife: Tenerife, Spain, 2021. [Google Scholar]
- Dehwah, A.H.; AlMashharawi, S.K.; Kammourie, N.; Missimer, T.M. Impact of well intake systems on bacterial, algae, and organic carbon reduction in SWRO desalination systems, SAWACO, Jeddah, Saudi Arabia. Desalination Water Treat. 2014, 55, 2594–2600. [Google Scholar] [CrossRef]
- Royal Society of Chemistry. Chemistry of the oceans. In Education—Inspiring Your Teaching and Learning; Available online: https://edu.rsc.org/download?ac=11242 (accessed on 25 January 2022).
- Sharkh, B.A.; Al-Amoudi, A.A.; Farooque, M.; Fellows, C.M.; Ihm, S.; Lee, S.; Li, S.; Voutchkov, N. Seawater desalination concentrate—A new frontier for sustainable mining of valuable minerals. Nat. Partn. J.—Clean Water 2022, 5, 9. [Google Scholar] [CrossRef]
- Lun Ang, W.; Mohammad, A.W.; Johnson, D.; Hilal, N. Unlocking the application potential of forward osmosis through integrated/hybrid process. Sci. Total Environ. 2020, 706, 136047. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Hu, S.; Zhang, X.; Song, L.; Wang, Y.; Tan, M.; Kong, L.; Zhang, Y. Integrated membrane system without adding chemicals for produced water desalination towards zero liquid discharge. Desalination 2020, 496, 114693. [Google Scholar] [CrossRef]
- Enviro Water Minerals Company. Full Recovery Desalination. In Innovative Ideas at Work Today: Texas Desal; 2017; Available online: https://www.texasdesal.com/wp-content/uploads/2017/09/SnyderSue.pdf (accessed on 21 February 2022).
- Tawalbeh, M.; Qalyoubi, L.; Al-Othman, A.; Qasim, M.; Shirazi, M. Insights on the development of enhanced antifouling reverse osmosis membranes: Industrial applications and challenges. Desalination 2023, 553, 116460. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, W.; Zhang, X.; Li, J.; Ma, J.; Zhang, T. Engineering a protective surface layer to resist membrane scaling and scale-induced wetting in membrane distillation for the treatment of hypersaline wastewater. Chem. Eng. J. 2023, 452, 139167. [Google Scholar] [CrossRef]
- Tedesco, M.; Cipollina, A.; Tamburini, A.; Micale, G. Towards 1 kW power production in a reverse electrodialysis pilot plant with saline waters and concentrated brines. J. Membr. Sci. 2017, 522, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Instituto Tecnológico de Canarias—Departamento de Agua. Valorización de Salmueras de Plantas Desaladoras de Agua de Mar; E5DES Project—FEDER—MAC 2014/2020 (MAC2/1.1a/309); Santa Lucía, Spain, 2022. [Google Scholar]
- Liu, Y.; Chen, H.; Zhu, N.; Zhang, J.; Li, Y.; Xu, D.; Gao, Y.; Zaho, J. Detection and remediation of mercury contaminated environment by nanotechnology: Progress and challenges. Environ. Pollut. 2022, 293, 118557. [Google Scholar] [CrossRef]
- Panagopoulos, A. A comparative study on minimum and actual energy consumption for the treatment of desalination brine. Energy 2020, 212, 118733. [Google Scholar] [CrossRef]
- Thiel, G.P.; Kumar, A.; Gómez-González, A.; Lienhard, J.H. Utilization of desalination brine for sodium hydroxide production: Technologies, engineering principles, recovery limits, and future directions. ACS Sustain. Chem. Eng. 2017, 5, 11147–11162. [Google Scholar] [CrossRef]
- Huertas, F.; Ordóñez, A.; Gutiérrez, B. Proyecto FOWE: Recuperación de energía de las salmueras procedentes de plantas desaladoras de agua de mar. TecnoAqua 2020, 46, 34–41. [Google Scholar]
- World Health Organization. Potable Reuse: Guidance for Producing Safe Drinking-Water. 2017. Available online: http://apps.who.int/iris/bitstream/handle/10665/258715/9789241512770-eng.pdf?sequence=1&isAllowed=y,Geneva (accessed on 22 August 2022).
- Gálvez-Martos, J.-L.; Elhoweris, A.; Morrison, J.; Al-horr, Y. Conceptual design of a CO2 capture and utilisation process based on calcium and magnesium rich brines. J. CO2 Util. 2018, 27, 161–169. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Boo, C.; Winton, R.K.; Conway, K.M.; Yin Yip, N. Membrane-less and non-evaporative desalination of hypersaline brines by temperature swing solvent extraction. Environ. Sci. Technol. Lett. 2019, 6, 359–364. [Google Scholar] [CrossRef]




| Pre-Treatment | Concentration | Conversion |
|---|---|---|
| Chemical precipitation (CP) | Forward Osmosis (FO) | Bipolar Membrane Electrodialysis (BMED) |
| Nanofiltration (NF) | Osmotically Assisted Reverse Osmosis (OARO) | Membrane Crystallisation (MCr) |
| Carbon Capture and Utilisation (CCU) | Membrane Distillation (MD) | Pressure Retarded Osmosis (PRO) |
| Selective Electrodialysis (SED) | Electrodialysis Metathesis (EDM) | Reverse Electrodialysis (RED) |
| Ion Exchange resins (IEx resins) | Temperature Swing Solvent Extraction (TSSE) | Electrolysis (chlor-alkali) |
| SWRO Desalination Plant | Intake | Physical Pre-Treatment | Chemical Pre-Treatment | Fresh Water Production (m3/d) | Average Feed Conductivity @ 25 °C (µS/cm) | Number of Stages | Average Recovery Rate (%) | Average Brine Conductivity @ 25 °C (µS/cm) |
|---|---|---|---|---|---|---|---|---|
| DP#1 | Beach well | Sand and cartridge filters | No | 15,000 | 52,000 | 1 | 45.0 | 84,500 |
| DP#2 | Beach well | Sand and cartridge filters | Antiscalant | 100 | 55,500 | 1 | 36.5 | 80,000 |
| DP#3 | Open sea water | Sand and cartridge filters | NaHSO3 | 5000 | 55,000 | 1 | 41.0 | 82,500 |
| DP#4 | Open sea water | Sand and cartridge filters | Antiscalant | 79,000 | 55,000 | 2 | 50.0 | 96,000 |
| DP#5 | Beach well | Only cartridge filter | No | 3000 | 53,500 | 1 | 40.0 | 82,500 |
| DP#6 | Open sea water | Ultrafiltration and cartridge filter | HCl + NaClO + Antiscalant | 14,750 | 55,500 | 1 | 45.5 | 89,000 |
| DP#7 | Beach well | Sand and cartridge filters | NaClO + Na2S2O5 + Antiscalant | 1800 | 55,000 | 1 | 42.0 | 84,500 |
| DP#8 | Beach well | Sand and cartridge filters | No | 5200 | 52,500 | 1 | 42.5 | 84,000 |
| DP#9 | Beach well | Only cartridge filter | Antiscalant | 33,000 | 54,000 | 2 | 54.0 | 97,500 |
| DP#10 | Beach well | Sand and cartridge filters | Antiscalant | 16,500 | 48,500 | 1 | 45.0 | 81,000 |
| Environmental | Economic | Operational | Energy-Based | R&D | Commercial | Social | ||
|---|---|---|---|---|---|---|---|---|
| Strengths | Environmental impact mitigation of brine discharge | X | X | |||||
| Resource/energy recovery | X | X | ||||||
| Desalinated water production increase | X | |||||||
| Hybrid solutions/ZLD | X | X | ||||||
| Integration with renewable energies and waste heat | X | X | ||||||
| New employment opportunities | X | |||||||
| Barriers/limitations | Pre-treatment | X | X | |||||
| TRL of emerging technologies | X | |||||||
| Environmental impact | X | |||||||
| High Capex/Opex | X | |||||||
| Legal restrictions | X | |||||||
| Limited available research data | X | |||||||
| Commercialisation of by-products | X | X | ||||||
| Lack of specific materials/components | X | X | ||||||
| Lack of specific simulation software | X |
| Parameter | Unit | DP#1 | DP#2 | DP#3 | DP#4 | DP#5 | DP#6 | DP#7 | DP#8 | DP#9 | DP#10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EC@20 °C | µS/cm | 76,750 | 71,000 | 75,450 | 86,867 | 74,300 | 83,050 | 76,100 | 76,800 | 89,350 | 72,550 |
| pH | U. pH | 7.6 | 7.4 | 8.0 | 7.9 | 7.5 | 8.0 | 7.9 | 7.0 | 7.6 | 7.4 |
| TDS | mg/L | 63,375 | 58,756 | 62,972 | 70,444 | 62,080 | 66,120 | 63,595 | 63,743 | 71,356 | 59,333 |
| Cl− | mg/L | 34,730 | 32,510 | 35,000 | 39,120 | 33,890 | 36,420 | 34,875 | 34,645 | 39,645 | 32,975 |
| Na+ | mg/L | 19,207 | 17,761 | 18,330 | 20,507 | 18,740 | 19,987 | 18,975 | 19,217 | 21,070 | 16,869 |
| SO42− | mg/L | 5230 | 4630 | 5030 | 5423 | 4900 | 5150 | 5116 | 4970 | 5593 | 4765 |
| Mg2+ | mg/L | 2297 | 2105 | 2648 | 3036 | 2451 | 2480 | 2646 | 2396 | 2760 | 2453 |
| Ca2+ | mg/L | 704 | 703 | 800 | 969 | 798 | 801 | 736 | 1181 | 957 | 1085 |
| K+ | mg/L | 742 | 620 | 777 | 916 | 732 | 789 | 828 | 617 | 825 | 588 |
| HCO3− | mg/L | 287 | 203 | 235 | 296 | 388 | 276 | 252 | 398 | 261 | 324 |
| Br− | mg/L | 122 | 146 | 125 | 142 | 120 | 186 | 139 | 147 | 152 | 169 |
| Sr2+ | mg/L | 19.9 | 20.3 | 18.8 | 22.1 | 18.7 | 21.9 | 19.4 | 38.3 | 27.1 | 31.7 |
| SiO2 | mg/L | 15.4 | 48.2 | <2.4 | <2.4 | 29.7 | <2.4 | <2.4 | 78.9 | 49.1 | 43.1 |
| B | mg/L | 7.1 | 6.3 | 7.2 | 8.4 | 8.1 | 8.0 | 7.6 | 6.5 | 8.6 | 6.1 |
| TOC | mg/L | 1.0 | 0.7 | 1.4 | 1.8 | 0.7 | 1.4 | 1.1 | 1.1 | 0.9 | 1.0 |
| NO3− | mg/L | 13.5 | 2.9 | 0.2 | 3.3 | 4.0 | 0.4 | 1.2 | 48.2 | 6.7 | 23.0 |
| PO43− | mg/L | 0.15 | 0.27 | <0.10 | <0.10 | 0.44 | <0.10 | <0.10 | 0.59 | 0.15 | 0.37 |
| Total N | mg/L | 3 | <1 | <1 | <1 | <1 | <1 | <1 | 11 | 2 | 5 |
| Total P | mg/L | <0.10 | <0.10 | <0.10 | <0.10 | 0.13 | <0.10 | 0.10 | 0.20 | 0.48 | 0.13 |
| F− | mg/L | 1.4 | 0.6 | 1.2 | 1.3 | 1.2 | 1.4 | 1.2 | 0.4 | 0.9 | 0.8 |
| Li | µg/L | 305 | 198 | 306 | 367 | 305 | 278 | 331 | 226 | 330 | 260 |
| Mo | µg/L | 200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 | <200 |
| Ba | µg/L | 10 | 194 | 9 | 9 | 17 | 10 | 12 | 103 | 90 | 75 |
| Zn | µg/L | 21 | 25 | 2 | 9 | <2 | <2 | 3 | 3 | 3 | 23 |
| Al | µg/L | 15 | 3 | 4 | 12 | 3 | 3 | 8 | <2 | 4 | 3 |
| Fe | µg/L | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| V | µg/L | 8 | 8 | 3 | 4 | 15 | 4 | 3 | 9 | 8 | 16 |
| Ni | µg/L | <2 | 6 | <2 | <2 | 4 | <2 | <2 | 105 | <2 | 3 |
| As | µg/L | 4 | <2 | 3 | 4 | 3 | 3 | 3 | <2 | <2 | 201 |
| Co | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cu | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Mn | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cr | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Sn | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Pb | µg/L | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 | <2 |
| Cd | µg/L | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Hg | µg/L | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 | 0.23 |
| Parameter | Unit | DP#1 | DP#2 | DP#3 | DP#4 | DP#5 | DP#6 | DP#7 | DP#8 | DP#9 | DP#10 | Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl− | % of TDS | 54.80 | 55.33 | 55.58 | 55.53 | 54.59 | 55.08 | 54.84 | 54.35 | 55.56 | 55.58 | 54.0–56.0 |
| Na+ | % of TDS | 30.31 | 30.23 | 29.11 | 29.11 | 30.19 | 30.23 | 29.84 | 30.15 | 29.53 | 28.43 | 28.0–31.0 |
| SO42− | % of TDS | 8.25 | 7.88 | 7.99 | 7.70 | 7.89 | 7.79 | 8.04 | 7.80 | 7.84 | 8.03 | 7.0–9.0 |
| Mg2+ | % of TDS | 3.62 | 3.58 | 4.21 | 4.31 | 3.95 | 3.75 | 4.16 | 3.76 | 3.87 | 4.13 | 3.0–5.0 |
| Ca2+ | % of TDS | 1.11 | 1.20 | 1.27 | 1.38 | 1.29 | 1.21 | 1.16 | 1.85 | 1.34 | 1.83 | 1.0–2.0 |
| K+ | % of TDS | 1.17 | 1.05 | 1.23 | 1.30 | 1.18 | 1.19 | 1.30 | 0.97 | 1.16 | 0.99 | 0.9–1.5 |
| HCO3− | % of TDS | 0.45 | 0.34 | 0.37 | 0.42 | 0.62 | 0.42 | 0.40 | 0.62 | 0.37 | 0.55 | 0.3–0.7 |
| Br− | % of TDS | 0.19 | 0.25 | 0.20 | 0.20 | 0.19 | 0.28 | 0.22 | 0.23 | 0.21 | 0.28 | 0.1–0.3 |
| % Na+ | % Cl− | % Sum Na+ + Cl− | % Max NaCl | |
|---|---|---|---|---|
| DP#1 | 30.31 | 54.80 | 85.11 | 77.04 |
| DP#2 | 30.23 | 55.33 | 85.56 | 76.84 |
| DP#3 | 29.11 | 55.58 | 84.69 | 73.99 |
| DP#4 | 29.11 | 55.53 | 84.64 | 74.00 |
| DP#5 | 30.19 | 54.59 | 84.78 | 76.73 |
| DP#6 | 30.23 | 55.08 | 85.31 | 76.84 |
| DP#7 | 29.84 | 54.84 | 84.68 | 75.85 |
| DP#8 | 30.15 | 54.35 | 84.50 | 76.63 |
| DP#9 | 29.53 | 55.56 | 85.09 | 75.06 |
| DP#10 | 28.43 | 55.58 | 84.01 | 72.27 |
Overall Results | ||||
| Sum of Na+ and Cl− ≈ 85% TDS | % max. NaCl ≈ 75% TDS | |||
| Barrier | Likelihood-Value | Impact-Value | Total | ||
|---|---|---|---|---|---|
| Pre-treatment | Most technologies need an exhaustive pre-treatment | 4 | Lack of a proper pre-treatment will directly reduce performance in practically any DBV process | 4 | 16 |
| TRL of emerging technologies | Practically all DBV technologies have a low-medium TRL | 5 | Risks associated to non-commercially tested technologies will reduce their chance of success | 4 | 20 |
| Environmental impact | Emerging technologies are usually conceived with a so-called “green thinking” approach and harmful substances tend to disappear from all DBV processes | 2 | Processes with a high associated environmental impact will not be given the chance to progress | 5 | 10 |
| High Capex/Opex | Most DBV technologies will have high material and operating costs | 4 | The question of economic viability will always be a determining factor. It may be compensated by revenues from by-products or energy | 5 | 20 |
| Legal restrictions | Although it is a matter of concern, its likelihood is very low and limited to particular cases | 2 | These restrictions may stop/delay the development of a process | 4 | 8 |
| Limited available research data | Especially data related to pilot plants with real brines | 3 | The process of obtaining trustable data is a long one, thus delaying the commercialisation of any DBV solution in the short term | 3 | 9 |
| Commercialisation of by-products | Most DBV processes end up generating by-products | 4 | A process that generates non-marketable by- products will fail, as they will become more waste | 4 | 16 |
| Lack of specific materials/components | Emerging technologies usually need new materials/components in order to be optimised | 4 | Mostly needed to increase performance and to overcome common barriers | 3 | 12 |
| Lack of specific simulation software | No specific software has been developed as far the authors are aware | 5 | It would increase with the speed of the R&D process | 2 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivero-Falcón, Á.; Peñate Suárez, B.; Melián-Martel, N. SWRO Brine Characterisation and Critical Analysis of Its Industrial Valorisation: A Case Study in the Canary Islands (Spain). Water 2023, 15, 1600. https://doi.org/10.3390/w15081600
Rivero-Falcón Á, Peñate Suárez B, Melián-Martel N. SWRO Brine Characterisation and Critical Analysis of Its Industrial Valorisation: A Case Study in the Canary Islands (Spain). Water. 2023; 15(8):1600. https://doi.org/10.3390/w15081600
Chicago/Turabian StyleRivero-Falcón, Ángel, Baltasar Peñate Suárez, and Noemi Melián-Martel. 2023. "SWRO Brine Characterisation and Critical Analysis of Its Industrial Valorisation: A Case Study in the Canary Islands (Spain)" Water 15, no. 8: 1600. https://doi.org/10.3390/w15081600
APA StyleRivero-Falcón, Á., Peñate Suárez, B., & Melián-Martel, N. (2023). SWRO Brine Characterisation and Critical Analysis of Its Industrial Valorisation: A Case Study in the Canary Islands (Spain). Water, 15(8), 1600. https://doi.org/10.3390/w15081600








