Monitoring Adenosine Triphosphate Concentrations in a Chloraminated Drinking Water Distribution System for Risk and Asset Management
Abstract
:1. Introduction
- Characterize ATP concentrations both at the DWTP and in the DWDS, as well as the factors influencing those concentrations.
- Compare ATP and HPC results collected in 2019 to assess each parameters value to decision making.
- Define ATP concentration thresholds that should result in preventative or corrective action to manage risk and water quality changes.
- Evaluate the use of ATP monitoring for management of treated water storage facilities using two outlying reservoir case studies.
2. Materials and Methods
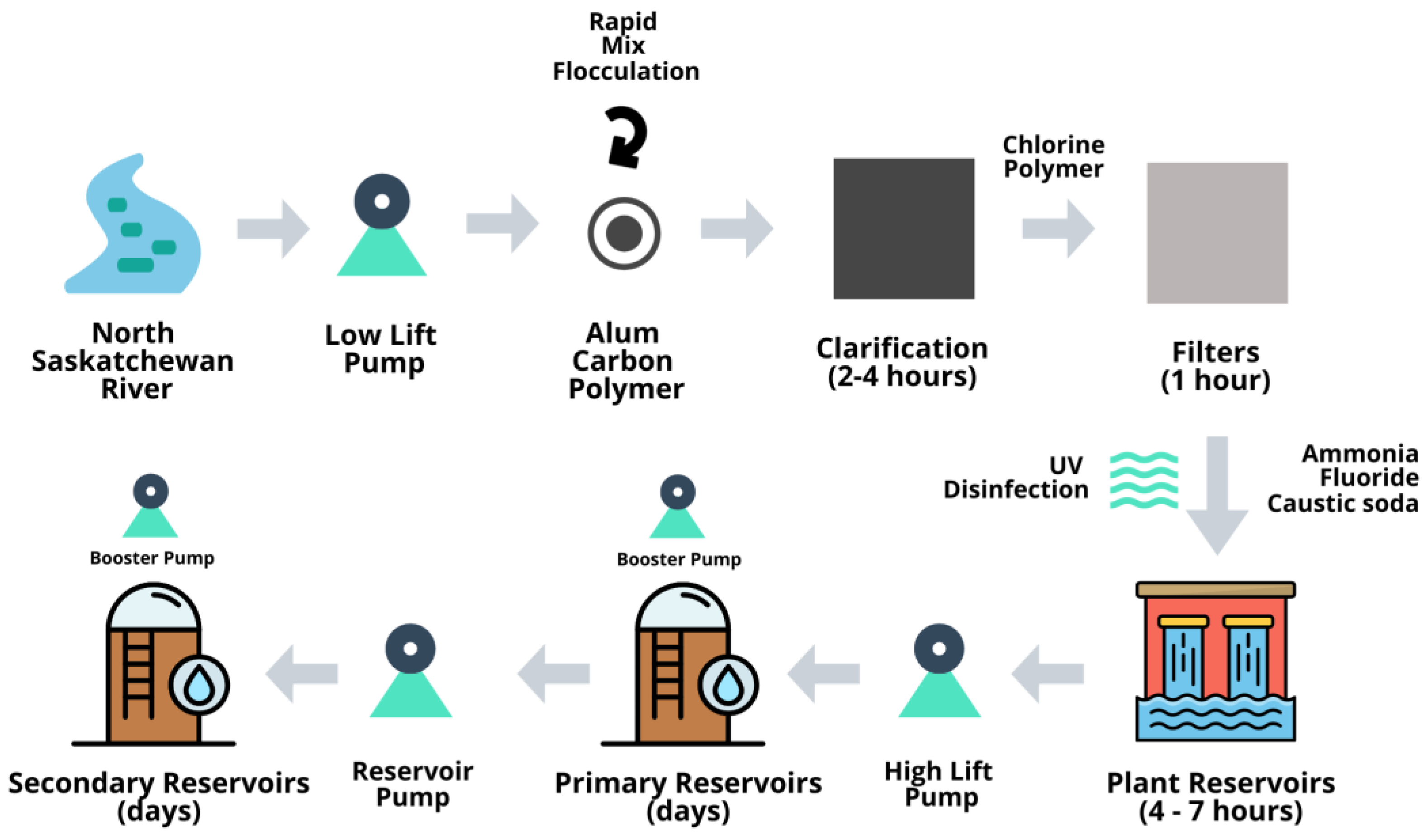
2.1. Drinking Water Treatment, Storage and Distribution
2.2. Sampling Protocol for Water Samples
2.3. Analytical Testing
2.4. Data Processing and Statistical Analyses
3. Results
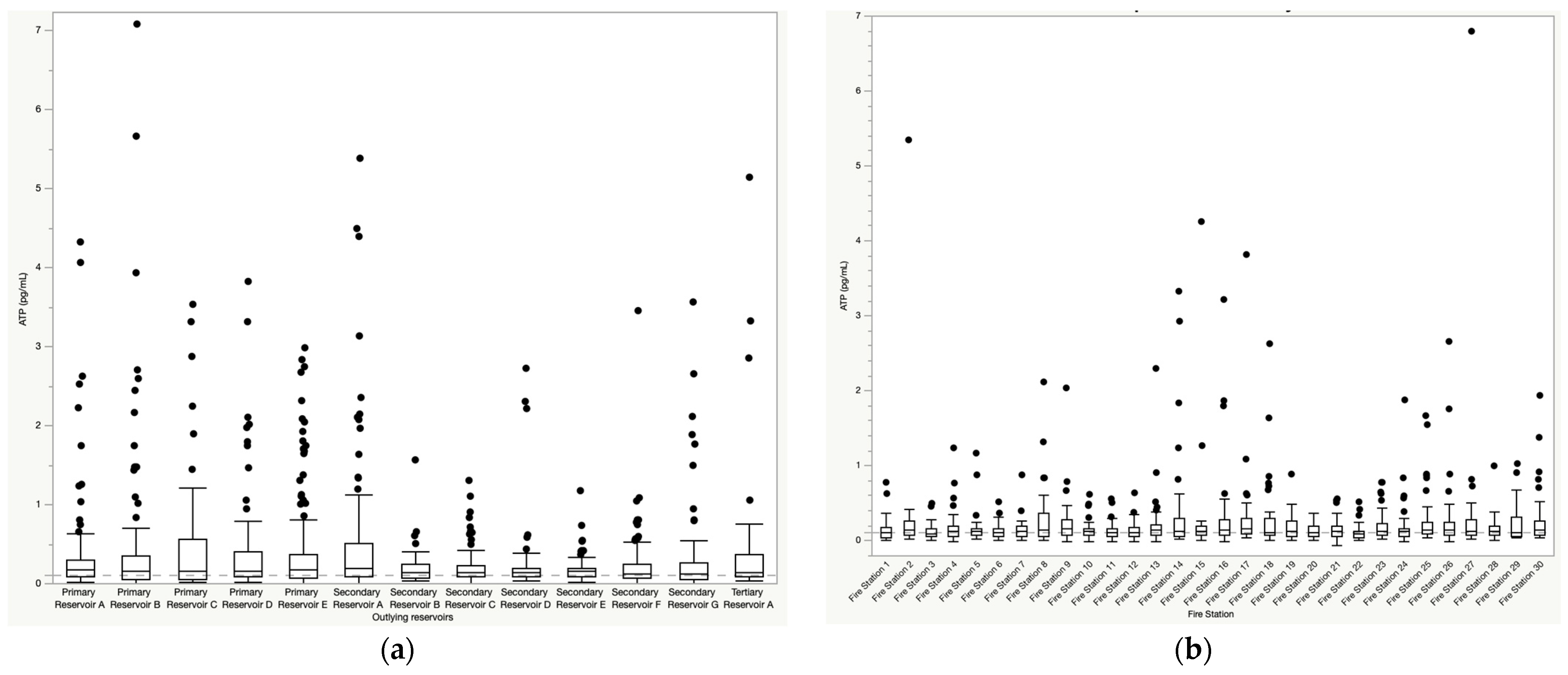
3.1. Mean ATP Concentrations and Water Quality Parameters by Sampling Location
3.2. Variability in ATP Concentrations and Water Quality by Season and Sampling Location
3.2.1. Temporal and Spatial Variability in ATP Concentrations
3.2.2. Relationships between ATP Concentrations and Water Quality Variables by Sampling Location
3.3. Comparing ATP Concentrations and HPC Counts
3.4. Proposed Operational Thresholds for Utilities
3.5. Outlying Reservoir Case Studies
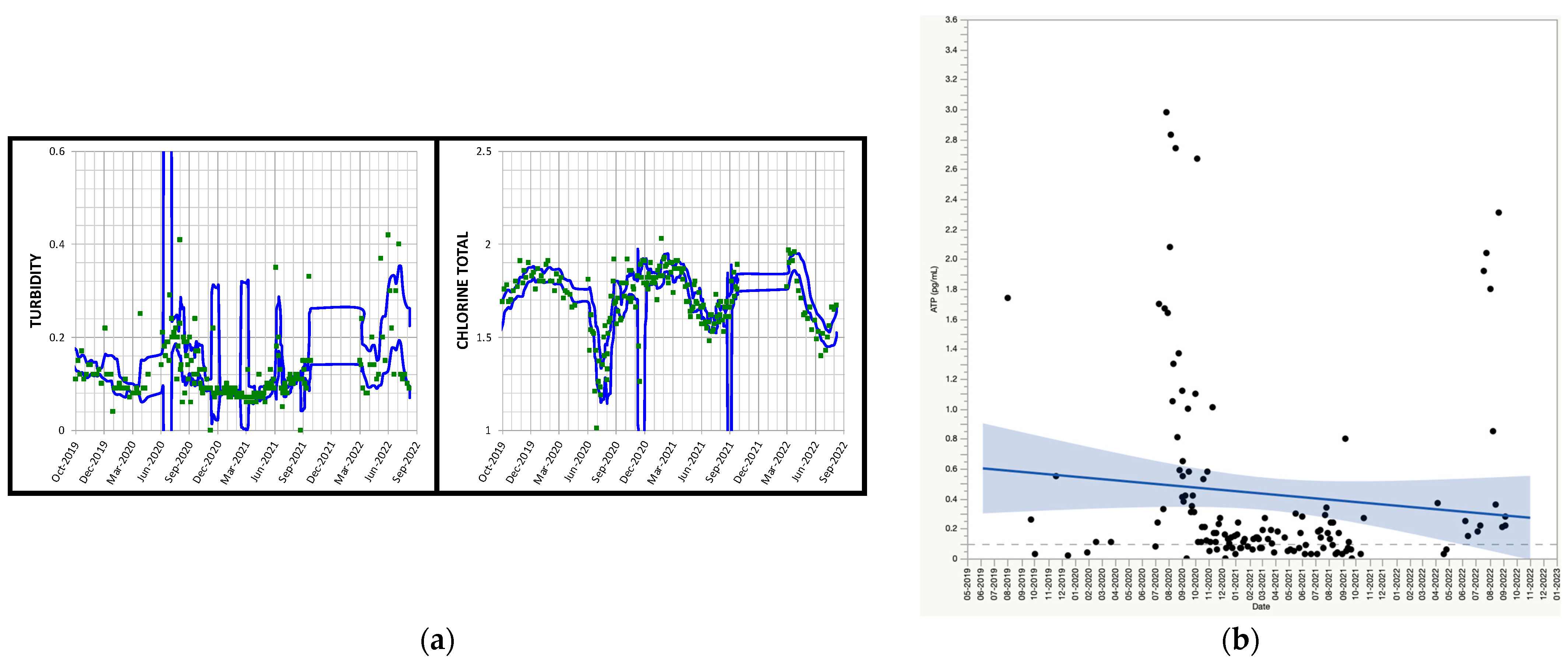
3.5.1. Case Study 1: Outlying Primary Reservoir Maintenance
3.5.2. Case Study 2: Outlying Primary Reservoir Draining
4. Discussion
5. Conclusions
- Using large, long-term ATP concentration datasets by sampling multiple locations in the DWDS starting with the treatment plant allows utilities and researchers to draw reliable and scientifically sound conclusions around the factors that may affect drinking water quality and microbial regrowth in the real world.
- ATP concentrations exhibited an increase between the DWTP and the outlying reservoirs and a decrease after leaving the outlying reservoirs potentially signaling the importance of TOC removal in controlling microbial regrowth in this chloraminated DWDS.
- The higher ATP values in outlying reservoirs compared to other sampling locations indicate the importance of reservoir management practices and the usefulness of a robust and accurate monitoring parameter.
- ATP concentrations were highest in the summer and exhibited significant spatial heterogeneity within sampling locations (e.g., outlying reservoirs, fire stations).
- ATP values provide better operational decision support than HPCs, which were negative (<1 CFU/mL) more than 90% of the time.
- ATP concentrations were used effectively for outlying reservoir management decisions and detected both ingress and suction pipe condition deterioration when chlorine and turbidity did not signal significant water quality changes.
- While a significant number of ATP measurements fell below the detection limit (<0.1 pg/mL), values in the 1–3 pg/mL range were also common providing higher granularity and potential ability to detect early changes and microbial regrowth throughout the system.
- The results suggest that an ATP operational threshold concentration of 10 pg/mL could be used to trigger utility intervention (e.g., flushing, maintenance, disinfection). It also suggests that an internal utility action limit of 5 pg/mL could be a useful metric to trigger additional testing and resampling.
- Given its granularity and speed of testing, researchers should continue to explore potential sensors and real-time monitoring tools that enable continuous monitoring of ATP at sensitive locations at the DWTP and in the DWDS.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpitella, S.; Del Olmo, G.; Izquierdo, J.; Husband, S.; Boxall, J.; Douterelo, I. Decision-Making Tools to Manage the Microbiology of Drinking Water Distribution Systems. Water 2020, 12, 1247. [Google Scholar] [CrossRef]
- Chen, L.; Ling, F.; Bakker, G.; Liu, W.-T.; Medema, G.; van der Meer, W.; Liu, G. Assessing the transition effects in a drinking water distribution system caused by changing supply water quality: An indirect approach by characterizing suspended solids. Water Res. 2020, 168, 115159. [Google Scholar] [CrossRef]
- Douterelo, I.; Boxall, J.B.; Deines, P.; Sekar, R.; Fish, K.E.; Biggs, C.A. Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res. 2014, 65, 134–156. [Google Scholar] [CrossRef]
- Douterelo, I.; Husband, S.; Loza, V.; Boxall, J. Dynamics of Biofilm Regrowth in Drinking Water Distribution Systems. Appl. Environ. Microbiol. 2016, 82, 4155–4168. [Google Scholar] [CrossRef]
- Douterelo, I.; Sharpe, R.L.; Husband, S.; Fish, K.E.; Boxall, J.B. Understanding microbial ecology to improve management of drinking water distribution systems. Wiley Interdiscip. Rev. Water 2019, 6, e01325. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; van der Mark, E.; Magic-Knezev, A.; Pinto, A.; Bogert, B.V.D.; Liu, W.; van der Meer, W.; Medema, G. Assessing the origin of bacteria in tap water and distribution system in an unchlorinated drinking water system by SourceTracker using microbial community fingerprints. Water Res. 2018, 138, 86–96. [Google Scholar] [CrossRef]
- Tscheikner-Gratl, F.; Sitzenfrei, R.; Hammerer, M.; Rauch, W.; Kleidorfer, M. Prioritization of Rehabilitation Areas for Urban Water Infrastructure. A Case Study. Procedia Eng. 2014, 89, 811–816. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological Stability of Drinking Water: Controlling Factors, Methods, and Challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- de Vera, G.A.; Wert, E.C. Using discrete and online ATP measurements to evaluate regrowth potential following ozonation and (non)biological drinking water treatment. Water Res. 2019, 154, 377–386. [Google Scholar] [CrossRef]
- van der Kooij, D.; van der Wielen, P.W. Problems, Causes, Control and Research Needs. In Microbial Growth in Drinking-Water Supplies; IWA Publishing: London, UK, 2013. [Google Scholar] [CrossRef]
- US EPA. Finished Water Storage Facilities; US EPA: Washington, DC, USA, 2002.
- Martel, K.D.; Kirmeyer, G.J.; Murphy, B.M.; Noran, P.F.; Kirby, L.; Lund, T.W.; Anderson, J.L.; Medhurst, R.; Caprara, M. Preventing Water Quality deterioration in Finished Water Storage Facilities. J. AWWA 2002, 94, 139–148. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Nescerecka, A.; Juhna, T.; Hammes, F. Behavior and stability of adenosine triphosphate (ATP) during chlorine disinfection. Water Res. 2016, 101, 490–497. [Google Scholar] [CrossRef]
- Nescerecka, A.; Rubulis, J.; Vital, M.; Juhna, T.; Hammes, F. Biological Instability in a Chlorinated Drinking Water Distribution Network. PLoS ONE 2014, 9, e96354. [Google Scholar] [CrossRef]
- Van der Wielen, P.W.; van der Kooij, D. Effect of water composition, distance and season on the adenosine triphosphate concentration in unchlorinated drinking water in the Netherlands. Water Res. 2010, 44, 4860–4867. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Drinking Water Guidelines 6; NHMRC: Canberra, Australia, 2011.
- Stoddart, A.K.; Serracin-Pitti, D.; Gagnon, G.A.; Evans, A.; Slabaugh, R.; Alspach, B. ATP & Coliform Analysis Comparison for Infrastructure Release for Service; Centre for Water Resources Studies: Halifax, NS, Canada, 2021. [Google Scholar]
- Health Canada. Guidance on Monitoring the Biological Stability of Drinking Water in Distribution Systems; Health Canada: Ottawa, ON, Canada, 2020.
- Bartram, J.; Cotruvo, J.; Exner, M.; Fricker, C.; Glasmacher, A. Heterotrophic Plate Counts and Drinking-Water Safety; IWA Publishing: London, UK, 2003. [Google Scholar]
- Shama, G.; Malik, D. The uses and abuses of rapid bioluminescence-based ATP assays. Int. J. Hyg. Environ. Health 2013, 216, 115–125. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Sapers, G.M.; Fett, W.F. ATP Bioluminescence Assay for Estimation of Microbial Populations of Fresh-Cut Melon. J. Food Prot. 2005, 68, 2427–2432. [Google Scholar] [CrossRef]
- Siragusa, G.R.; Cutter, C.N.; Dorsa, W.J.; Koohmaraie, M. Use of a Rapid Microbial ATP Bioluminescence Assay to Detect Contamination on Beef and Pork Carcasses. J. Food Prot. 1995, 58, 770–775. [Google Scholar] [CrossRef]
- Hossain, S.; Chow, C.W.K.; Cook, D.; Sawade, E.; Hewa, G.A. Review of Nitrification Monitoring and Control Strategies in Drinking Water System. Int. J. Environ. Res. Public Health 2022, 19, 4003. [Google Scholar] [CrossRef]
- Favere, J.; Waegenaar, F.; Boon, N.; De Gusseme, B. Online microbial monitoring of drinking water: How do different techniques respond to contaminations in practice? Water Res. 2021, 202, 117387. [Google Scholar] [CrossRef]
- Siebel, E.; Wang, Y.; Egli, T.; Hammes, F. Correlations between total cell concentration, total adenosine tri-phosphate concentration and heterotrophic plate counts during microbial monitoring of drinking water. Drink. Water Eng. Sci. 2008, 1, 1–6. [Google Scholar] [CrossRef]
- Hammes, F.; Goldschmidt, F.; Vital, M.; Wang, Y.; Egli, T. Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res. 2010, 44, 3915–3923. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Hwang, C.; Liu, W.-T.; Boon, N.; Köster, O.; Vrouwenvelder, H.; Egli, T.; Hammes, F. A microbiology-based multi-parametric approach towards assessing biological stability in drinking water distribution networks. Water Res. 2013, 47, 3015–3025. [Google Scholar] [CrossRef]
- Delahaye, E.; Welté, B.; Levi, Y.; Leblon, G.; Montiel, A. An ATP-based method for monitoring the microbiological drinking water quality in a distribution network. Water Res. 2003, 37, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Deininger, R.A.; Lee, J. Rapid determination of bacteria in drinking water using an ATP assay. Field Anal. Chem. Technol. 2001, 5, 185–189. [Google Scholar] [CrossRef]
- van der Wielen, P.W.; Brouwer-Hanzens, A.; Italiaander, R.; Hijnen, W.A. Initiating guidance values for novel biological stability parameters in drinking water to control regrowth in the distribution system. Sci. Total. Environ. 2023, 871, 161930. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Duong, T.H.; Noh, J.H.; Chung, S.-Y.; Son, H.; Prest, E.; Oh, S.; Maeng, S.K. Occurrences and changes in bacterial growth-promoting nutrients in drinking water from source to tap: A review. Environ. Sci. Water Res. Technol. 2021, 7, 2206–2222. [Google Scholar] [CrossRef]
- Prest, E.I.; Schaap, P.G.; Besmer, M.D.; Hammes, F. Dynamic Hydraulics in a Drinking Water Distribution System Influence Suspended Particles and Turbidity, But Not Microbiology. Water 2021, 13, 109. [Google Scholar] [CrossRef]
- Learbuch, K.; Smidt, H.; van der Wielen, P. Water and biofilm in drinking water distribution systems in the Netherlands. Sci. Total. Environ. 2022, 831, 154940. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Huang, T.; Liu, X.; Miao, Y.; Liu, K.; Qian, X. Indoor heating triggers bacterial ecological links with tap water stagnation during winter: Novel insights into bacterial abundance, community metabolic activity and interactions. Environ. Pollut. 2021, 269, 116094. [Google Scholar] [CrossRef]
- Hallam, N.; West, J.; Forster, C.; Simms, J. The potential for biofilm growth in water distribution systems. Water Res. 2001, 35, 4063–4071. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, L.; Huang, T.; Yan, M.; Liu, K.; Miao, Y.; He, H.; Li, S.; Sekar, R. Combined effects of seasonality and stagnation on tap water quality: Changes in chemical parameters, metabolic activity and co-existence in bacterial community. J. Hazard. Mater. 2021, 403, 124018. [Google Scholar] [CrossRef]
- Ling, F.; Whitaker, R.; Lechevallier, M.W.; Liu, W.-T. Drinking water microbiome assembly induced by water stagnation. ISME J. 2018, 12, 1520–1531. [Google Scholar] [CrossRef]
- Sherchan, S.; Miles, S.; Ikner, L.; Yu, H.-W.; Snyder, S.A.; Pepper, I.L. Near Real-Time Detection of E. coli in Reclaimed Water. Sensors 2018, 18, 2303. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.; Kerrouche, A.; Tatari, K.; Rasmussen, A.; Ryan, T.; Summersgill, P.; Desmulliez, M.; Bridle, H.; Albrechtsen, H.-J. Monitoring of drinking water quality using automated ATP quantification. J. Microbiol. Methods 2019, 165, 105713. [Google Scholar] [CrossRef] [PubMed]
- EPA. Regional guidance on handling chemical concentration data near the detection limit in risk assessments. In Regional Technical Guidance Manual; US Environmental Protection Agency—Region 3: Philadelphia, PA, USA, 1991. [Google Scholar]
- Helsel, D. Less than obvious-statistical treatment of data below the detection limit. Environ. Sci. Technol. 1990, 24, 1766–1774. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2022.
- Axelsson-Olsson, D.; Svensson, L.; Olofsson, J.; Salomon, P.; Waldenström, J.; Ellström, P.; Olsen, B. Increase in Acid Tolerance of Campylobacter jejuni through Coincubation with Amoebae. Appl. Environ. Microbiol. 2010, 76, 4194–4200. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Laxander, M.; Miettinen, I.T.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Res. 2006, 40, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.A.; Stockman, L.J.; Hicks, L.A.; Garrison, L.E.; Zhou, F.J.; Beach, M.J. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol. Infect. 2012, 140, 2003–2013. [Google Scholar] [CrossRef]
- Health Canada. Guidance on the Use of Heterotrophic Plate Counts in Canadian Drinking Water Supplies; Health Canada: Ottawa, ON, Canada, 2013.
- Ghazali, M.; McBean, E.A.; Whalen, P. Kelvin Journal Supporting a drinking water contaminant warning system using the adenosine triphosphate test. Can. J. Civ. Eng. 2010, 37, 1423–1431. [Google Scholar] [CrossRef]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm structures (EPS and bacterial communities) in drinking water distribution systems are conditioned by hydraulics and influence discolouration. Sci. Total Environ. 2017, 593, 571–580. [Google Scholar] [CrossRef]
- Farhat, N.; Kim, L.; Mineta, K.; Alarawi, M.; Gojobori, T.; Saikaly, P.; Vrouwenvelder, J. Seawater desalination based drinking water: Microbial characterization during distribution with and without residual chlorine. Water Res. 2022, 210, 117975. [Google Scholar] [CrossRef]
- Norton, C.D.; LeChevallier, M.W. A Pilot Study of Bacteriological Population Changes through Potable Water Treatment and Distribution. Appl. Environ. Microbiol. 2000, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A. Finished Wter Storage and Quality Concerns. Available online: https://wcponline.com/2018/09/15/finished-water-storage-quality-concerns/ (accessed on 10 February 2022).
- Spencer, C. Water quality in the distribution system: A review. J. AWWA 2012, 104, 48–55. [Google Scholar] [CrossRef]
- Kirmeyer, G.J. Maintaining Water Quality in Finished Water Storage Facilities; American Water Works Association: Denver, CO, USA, 1999. [Google Scholar]
- Vang, Ó.K.; Corfitzen, C.B.; Smith, C.; Albrechtsen, H.-J. Evaluation of ATP measurements to detect microbial ingress by wastewater and surface water in drinking water. Water Res. 2014, 64, 309–320. [Google Scholar] [CrossRef]
- Gősi, P.; Rátkai, S.; Shetty, P.; Wirth, R.; Maróti, G.; Oszvald, F.; Knisz, J. Prediction of long-term localized corrosion rates in a carbon steel cooling water system is enhanced by metagenome analysis. Eng. Fail. Anal. 2022, 141, 106733. [Google Scholar] [CrossRef]





| ATP 1 | Total Chlorine | Turbidity | Conductivity | TOC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling Location | N | Mean | Std Dev | N | Mean | Std Dev | N | Mean | Std Dev | N | Mean | Std Dev | N | Mean | Std Dev |
| DWTP1 Raw | 94 | 80.96 | 77.45 | NT 2 | 1204 | 26.56 | 85.00 | 1204 | 14.14 | 17.67 | 40 | 8.2 | 0.1 | ||
| DWTP1 Treated | 598 | 0.14 | 0.23 | NT | 1383 | 0.05 | 0.02 | NT | NT | ||||||
| DWTP1 Reservoir | 570 | 0.13 | 0.12 | 1451 | 1.99 | 0.21 | 1430 | 0.05 | 0.12 | 1287 | 1.07 | 0.40 | 1451 | 7.8 | 0.8 |
| DWTP2 Raw | 101 | 93.47 | 85.78 | NT | 1203 | 29.64 | 126.22 | 1203 | 14.27 | 18.19 | 40 | 8.2 | 0.1 | ||
| DWTP2 Treated | 519 | <0.1 | <0.1 | NT | 1341 | 0.05 | 0.01 | NT | NT | ||||||
| DWTP2 Reservoir | 496 | 0.10 | 0.08 | 1445 | 2.04 | 0.23 | 1336 | 0.04 | 0.01 | 1285 | 1.04 | 0.36 | 1445 | 7.8 | 0.8 |
| Primary Reservoirs | 481 | 0.44 | 0.79 | 1071 | 1.62 | 0.22 | 1066 | 0.10 | 0.14 | 272 | 379.1 | 53.3 | 134 | 1.63 | 0.81 |
| Secondary Reservoirs | 622 | 0.28 | 0.52 | 1469 | 1.79 | 0.19 | 1458 | 0.09 | 0.24 | 180 | 394.6 | 37.0 | 180 | 1.67 | 0.58 |
| Tertiary Reservoirs | 62 | 0.39 | 0.83 | 92 | 1.21 | 0.18 | 91 | 0.10 | 0.04 | 14 | 383.4 | 34.6 | 14 | 1.24 | 0.57 |
| Fire Stations | 1335 | 0.24 | 1.04 | 2833 | 1.70 | 0.25 | 2817 | 0.17 | 0.33 | NT | NT | ||||
| Random DWDS | 85 | 0.22 | 0.36 | 4179 | 1.73 | 0.26 | 4181 | 0.15 | 0.58 | NT | 13 | 1.75 | 0.56 | ||
| Renewals | 4 | <0.1 | <0.1 | 589 | 1.76 | 0.26 | 589 | 0.43 | 0.58 | NT | NT | ||||
| Complaints | 190 | 0.24 | 0.30 | 459 | 1.67 | 0.27 | 458 | 0.44 | 0.69 | NT | NT | ||||
| WHO Parameter Limit | N/A | Minimum 0.2 mg/L Maximum 5 mg/L 3 | Water entering the distribution system ≤ 1 NTU 4 | N/A | N/A | ||||||||||
| Y | X | Count | p-Value | FDR p-Value | FDR LogWorth | Effect Size | RSquare |
|---|---|---|---|---|---|---|---|
| DWTP1 Raw | |||||||
| ATP | Turbidity | 94 | 1.20 × 10−34 | 5.95 × 10−34 | 33.23 | 0.8937 | 0.807 |
| ATP | Colour | 94 | 2.30 × 10−16 | 5.77 × 10−16 | 15.24 | 0.7178 | 0.521 |
| ATP | Ambient Temp | 91 | 1.80 × 10−4 | 3.07 × 10−4 | 3.51 | 0.3859 | 0.146 |
| DWTP2 Raw | |||||||
| ATP | Colour | 101 | 2.90 × 10−21 | 1.45 × 10−20 | 19.84 | 1.501 | 0.597 |
| ATP | Turbidity | 101 | 6.44 × 10−9 | 1.61 × 10−8 | 7.79 | 1.045 | 0.290 |
| ATP | Ambient Temp | 97 | 8.73 × 10−7 | 1.46 × 10−6 | 5.84 | 0.939 | 0.226 |
| DWTP1 Treated | |||||||
| ATP | Turbidity | 558 | 1.80 × 10−4 | 3.55 × 10−4 | 3.45 | 0.192 | 0.025 |
| DWTP2 Treated | |||||||
| ATP | Turbidity | 481 | 2.00 × 10−4 | 4.08 × 10−4 | 3.39 | 0.214 | 0.028 |
| ATP | Ambient Temp | 472 | 1.34 × 10−3 | 1.34 × 10−3 | 2.87 | 0.183 | 0.022 |
| DWTP1 Reservoir | |||||||
| ATP | Turbidity | 560 | 4.53 × 10−7 | 2.72 × 10−6 | 5.57 | 0.266 | 0.045 |
| ATP | Colour | 493 | 2.30 × 10−4 | 6.93 × 10−4 | 3.16 | 0.215 | 0.027 |
| DWTP2 Reservoir | |||||||
| ATP | Colour | 439 | 3.06 × 10−6 | 1.84 × 10−5 | 4.74 | 0.244 | 0.049 |
| ATP | Ambient Temp | 455 | 2.00 × 10−5 | 5.83 × 10−5 | 4.24 | 0.225 | 0.040 |
| ATP | Turbidity | 463 | 5.14 × 10−5 | 1.03 × 10−4 | 3.99 | 0.212 | 0.035 |
| ATP | Chlorine | 495 | 5.00 × 10−4 | 7.53 × 10−4 | 3.12 | 0.175 | 0.024 |
| Outlying Reservoirs | |||||||
| ATP | Chlorine | 1169 | 7.90 × 10−40 | 4.76 × 10−39 | 38.32 | 0.373 | 0.139 |
| ATP | Ambient Temp | 1013 | 1.50 × 10−26 | 4.37 × 10−26 | 25.36 | 0.344 | 0.107 |
| ATP | Conductivity | 248 | 5.00 × 10−5 | 1.01 × 10−4 | 4.00 | 0.272 | 0.065 |
| ATP | TOC | 147 | 1.60 × 10−4 | 1.97 × 10−4 | 3.71 | 0.347 | 0.094 |
| ATP | Turbidity | 1168 | 1.50 × 10−4 | 1.97 × 10−4 | 3.71 | 0.110 | 0.012 |
| DWDS Samples (Fire stations, Randoms, Complaints) | |||||||
| ATP | Chlorine | 1608 | 7.00 × 10−19 | 2.79 × 10−18 | 17.56 | 0.088 | 0.048 |
| ATP | Ambient Temp | 1414 | 3.02 × 10−9 | 6.04 × 10−9 | 8.22 | 0.066 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maal-Bared, R.; McCracken, M.; Busawon, B.; Simpson, D. Monitoring Adenosine Triphosphate Concentrations in a Chloraminated Drinking Water Distribution System for Risk and Asset Management. Water 2023, 15, 1636. https://doi.org/10.3390/w15091636
Maal-Bared R, McCracken M, Busawon B, Simpson D. Monitoring Adenosine Triphosphate Concentrations in a Chloraminated Drinking Water Distribution System for Risk and Asset Management. Water. 2023; 15(9):1636. https://doi.org/10.3390/w15091636
Chicago/Turabian StyleMaal-Bared, Rasha, Michael McCracken, Bharatee Busawon, and Darlyce Simpson. 2023. "Monitoring Adenosine Triphosphate Concentrations in a Chloraminated Drinking Water Distribution System for Risk and Asset Management" Water 15, no. 9: 1636. https://doi.org/10.3390/w15091636
APA StyleMaal-Bared, R., McCracken, M., Busawon, B., & Simpson, D. (2023). Monitoring Adenosine Triphosphate Concentrations in a Chloraminated Drinking Water Distribution System for Risk and Asset Management. Water, 15(9), 1636. https://doi.org/10.3390/w15091636






