Strain Screening and Conditions Optimization in Microalgae-Based Monosodium Glutamate Wastewater (MSGW) Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strains, Culture Conditions, and MSGW Preparation
2.2. Experimental Design
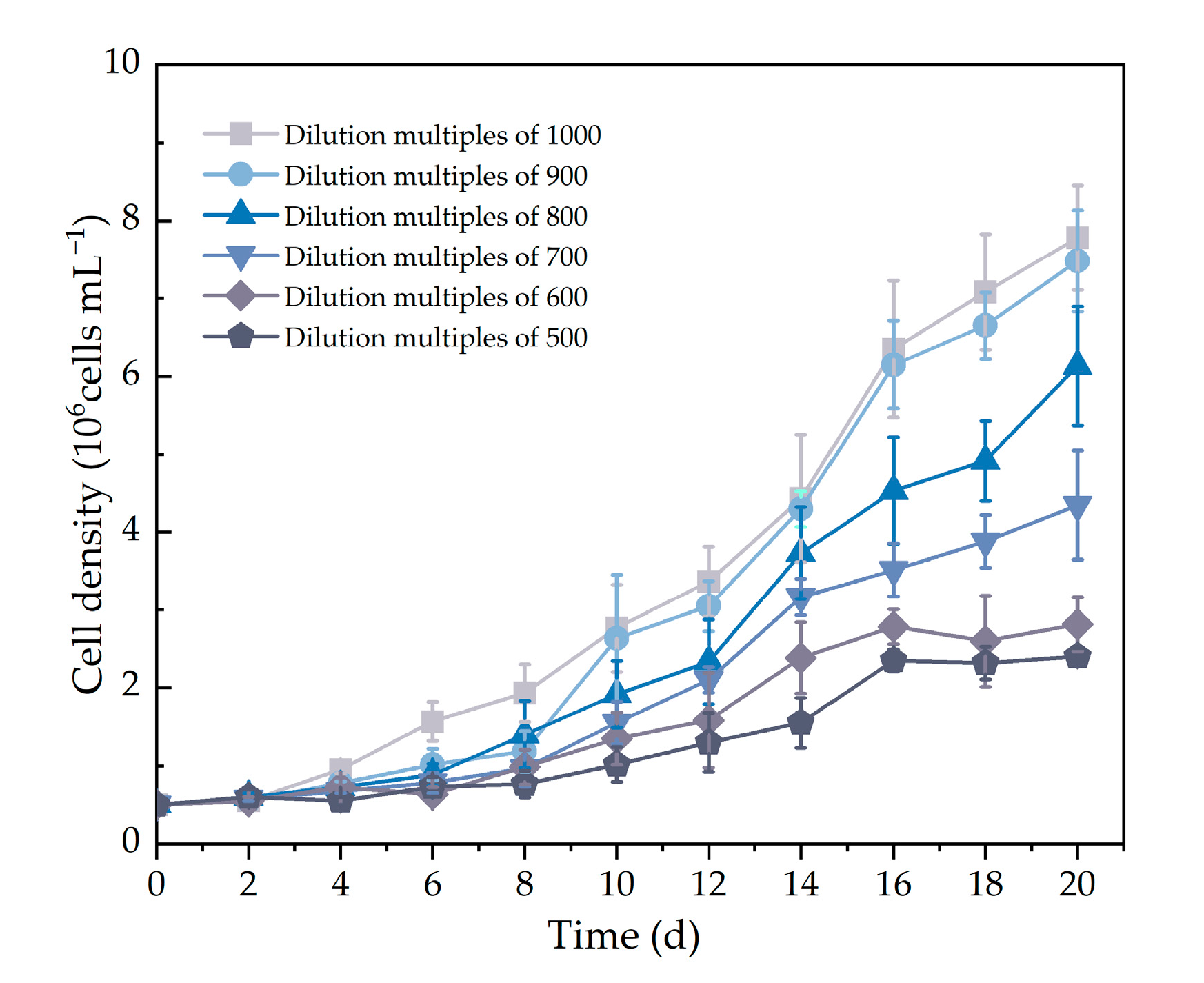
2.2.1. Screening of MSGW Dilution Factor for Promoting Microalgal Growth
2.2.2. Selection of Microalgae Species Suitable for Cultivation in MSGW
2.2.3. Batch Experiments for Optimization of Cultivation Condition
2.3. Analytical Methods
2.3.1. Determination of Cell Growth
2.3.2. Determination of COD, TN, TP, and -N
2.4. Statistical Analysis
3. Results
3.1. Microalgae Species Screening Based on Growth Performance and Removal of Nutrients
3.2. Growth of FACHB-30 under Different Light Intensities
3.3. Growth of FACHB-30 under Different Initial Inoculum Concentrations
3.4. Growth of FACHB-30 under Different Fe3+ Concentrations
3.5. Comparison of the Nutrient Removal Rate and Biomass Production before and after Optimization
4. Discussion
4.1. The Selecting of the Tolerant Strains Grown in the MSGW
4.2. Optimizing the Conditions for the Maximum Biomass Production and Nutrient Removal Rates
4.2.1. Light Intensity
4.2.2. Initial Inoculum Concentration
4.2.3. Fe3+ Concentrations
4.3. Comparison of Other Research for the MSGW Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, L.; Li, Y.; Wang, P.; Feng, Z.; Ding, N. Cleaner production of monosodium glutamate in China. J. Clean. Prod. 2018, 190, 452–461. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Y.; Liang, P.; Wang, C.; Hu, Y.; Xie, M.; Wang, Y.; Xiao, B.; Du, C.; Tian, H. Performance and microbial community variations of a upflow anaerobic sludge blanket (UASB) reactor for treating monosodium glutamate wastewater: Effects of organic loading rate. J. Environ. Manag. 2020, 253, 109691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, M.; Zhang, S.; Lv, W. Treatment of wastewater from a monosodium glutamate manufacturing plant using successive yeast and activated sludge systems. Process Biochem. 2004, 40, 2483–2488. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Z.; Jiang, L.; Hou, Q.; Xie, Z.; Ma, M.; Yu, S.; Pei, H. Monosodium glutamate wastewater assisted seawater to increase lipid productivity in single-celled algae. Renew. Energy 2021, 179, 1793–1802. [Google Scholar] [CrossRef]
- Singh, S.; Refeha, P.D.; Arun, A.B.; Huang, Y.M.; Shen, F.T.; Young, C.C. Wastewater from monosodium glutamate industry as a low cost fertilizer source for corn (Zea mays L.). Biomass Bioenergy 2011, 35, 4001–4007. [Google Scholar] [CrossRef]
- Chen, W.J.; Su, W.T.; Hsu, H.Y. Continuous flow electrocoagulation for MSG wastewater treatment using polymer coagulants via mixture-process design and response-surface methods. J. Taiwan Inst. Chem. Eng. 2012, 43, 246–255. [Google Scholar] [CrossRef]
- Jin, R.; Zhang, Q.; Liu, J.; Yang, B.; Wu, K.; Zheng, P. Performance and stability of the partial nitrification process for nitrogen removal from monosodium glutamate wastewater. Sep. Purif. Technol. 2013, 103, 195–202. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Gong, Y.; Lin, N.; Yang, Q.; Zhang, X.; Wang, Y. Perovskite catalysts with different dimensionalities for environmental and energy applications: A review. Sep. Purif. Technol. 2023, 307, 122716. [Google Scholar] [CrossRef]
- Liu, J.; Yue, Q.; Gao, B.; Ma, Z.; Zhang, P. Microbial treatment of the monosodium glutamate wastewater by Lipomyces starkeyi to produce microbial lipid. Bioresour. Technol. 2011, 106, 69–73. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Prinsen, P.; Vuppaladadiyam, A.K.; Zhao, M.; Luque, R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J. Clean. Prod. 2018, 181, 42–59. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, J.; Nie, C.; Li, Y.; Jenkins, J.; Pei, H. Filamentous cyanobacteria triples oil production in seawater-based medium supplemented with industrial waste: Monosodium glutamate residue. Biotechnol. Biofuels 2019, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Hu, W.; Li, X.; Ma, G.; Song, M.; Pei, H. Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with diluted monosodium glutamate wastewater. Bioresour. Technol. 2014, 152, 471–476. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Miao, J.; Tian, Y. Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresour. Technol. 2018, 253, 79–84. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Liu, T.; Yuan, M.; Liu, G.; Fang, J.; Yang, B. Exploration of microalgal species for nutrient removal from anaerobically digested swine wastewater and potential lipids production. Microorganisms 2021, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Franchino, M.; Comino, E.; Bona, F.; Riggio, V.A. Growth of three microalgae strains and nutrient removal from an agro-zootechnical digestate. Chemosphere 2013, 92, 738–744. [Google Scholar] [CrossRef]
- Li, F.W.; Pei, C.C.; Ai, P.H.; Chi, M.L. The feasibility of biodiesel production by microalgae using industrial wastewater. Bioresour. Technol. 2012, 113, 14–18. [Google Scholar]
- Yu, S.; Yu, Z.; Hou, Q.; Pei, H. Seawater with added monosodium glutamate residue (MSGR) is a promising medium for the cultivation of two commercial marine microalgae. Water 2022, 14, 975. [Google Scholar] [CrossRef]
- Jiang, L.; Ji, Y.; Hu, W.; Pei, H.; Nie, C.; Ma, G.; Song, M. Adjusting irradiance to enhance growth and lipid production of Chlorella vulgaris cultivated with monosodium glutamate wastewater. J. Photochem. Photobiol. B Biol. 2016, 162, 619–624. [Google Scholar] [CrossRef]
- Jiang, L.; Pei, H.; Hu, W.; Ji, Y.; Han, L.; Ma, G. The feasibility of using complex wastewater from a monosodium glutamate factory to cultivate Spirulina subsalsa and accumulate biochemical composition. Bioresour. Technol. 2015, 180, 304–310. [Google Scholar] [CrossRef]
- Aron, N.; Khoo, K.S.; Chew, K.W.; Veeramuthu, A.; Show, P.L. Microalgae cultivation in wastewater and potential processing strategies using solvent and membrane separation technologies. J. Water Process Eng. 2020, 39, 101701. [Google Scholar] [CrossRef]
- Abdallah, A.; Samir, A.S.; Hassan, R.; Ibrahim, E.-A.E.; Reham, E.; Shih-Hsin, H.; Tamer, E.; Shengnan, L.; El-Sheekh, M.M.; Schagerl, M. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar]
- Do, C.V.T.; Pham, M.H.T.; Pham, T.Y.T.; Dinh, C.T.; Bui, T.U.T.; Tran, T.D. Microalgae and bioremediation of domestic wastewater. Curr. Opin. Green Sustain. Chem. 2022, 34, 100595. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, G.; Zhang, X. Isolation and characterization of Chlorella sorokiniana GXNN01 (chlorophyta) with the properties of heterotrephic and microaerobic growth. J. Phycol. 2009, 45, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Graham, J.E.S.; Hutchinson, T.C. The role of temperature and heterotrophism in determining crude oil toxicity. Water Qual. Res. J. 1975, 10, 73–83. [Google Scholar] [CrossRef]
- Singh, S.; Rekha, P.D.; Arun, A.B.; Hameed, A.; Singh, S.; Shen, F.-T.; Young, C.-C. Glutamate wastewater as a culture medium for Azospirillum rugosum production and its impact on plant growth. Biol. Fertil. Soils 2011, 47, 419–426. [Google Scholar] [CrossRef]
- Jia, C.; Kang, R.; Zhang, Y.; Zhang, Y.; Cong, W. Degradation and decolorization of monosodium glutamate wastewater with Coriolus versicolor. Biodegradation 2007, 18, 551–557. [Google Scholar] [CrossRef]
- You, K.; Ge, F.; Wu, X.; Song, K.; Yang, Z.; Zhang, Q.; Liu, Y.; Roger, R.; Zheng, H. Nutrients recovery from piggery wastewater and starch wastewater via microalgae-bacteria consortia. Algal Res. 2021, 60, 102551. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. The promising way to treat wastewater by microalgae: Approaches, mechanisms, applications and challenges. J. Water Process Eng. 2022, 49, 103012. [Google Scholar] [CrossRef]
- Liu, X.; Hong, Y.; Zhao, G.; Zhang, H.; Zhai, Q.; Wang, Q. Microalgae-based swine wastewater treatment: Strain screening, conditions optimization, physiological activity and biomass potential. Sci. Total Environ. 2021, 807, 151008. [Google Scholar] [CrossRef] [PubMed]
- Huss, V.A.R.; Frank, C.; Hartmann, E.C.; Hirmer, M.; Kloboucek, A.; Seidel, B.M.; Wenzeler, P.; Kessler, E. Biochemical taxonomy and molecular phylogeny of the fenus Chlorella sensu lato (chlorophyta). J. Phycol. 1999, 35, 587–598. [Google Scholar] [CrossRef]
- Cheng, S.; Cut, Y.; Liu, Y.; Xia, F.; Ding, S. The effect of heavy metal or metal elements pollution on biological treatment and comprehensive utilization for monosodium glutamate wastewater. Environ. Chem. 1995, 05, 454–459. [Google Scholar]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.S.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2010, 102, 1649–1655. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y. Effect of trace elements on biomass, lipid productivity and fatty acid composition in Chlorella sorokiniana. Braz. J. Bot. 2017, 40, 871–881. [Google Scholar] [CrossRef]
- Tuantet, K.; Janssen, M.; Temmink, H.; Zeeman, G.; Wijffels, R.H.; Buisman, C.J.N. Microalgae growth on concentrated human urine. J. Appl. Phycol. 2014, 26, 287–297. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Cui, D.; Liu, Y.; Yang, L.; Chen, H.; Qiu, G.; Geng, Y.; Xiong, Z.; Shao, P.; et al. A pilot scale study on the treatment of rare earth tailings (REEs) wastewater with low C/N ratio using microalgae photobioreactor. J. Environ. Manag. 2023, 328, 125593. [Google Scholar] [CrossRef]
- Kathirvel, B.; Thangavel, M.; Rene, R.E.; Sabarathinam, S.; Lan, C.N.T.; Arivalagan, P. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 119058. [Google Scholar]
- Boda, R.K.; Venkata Mohan, S. Photosynthetic transients in Chlorella sorokiniana during phycoremediation of dairy wastewater under distinct light intensities. Bioresour. Technol. 2021, 340, 125593. [Google Scholar]
- Qu, W.; Zhang, C.; Zhang, Y.; Ho, S.-H. Optimizing real swine wastewater treatment with maximum carbohydrate production by a newly isolated indigenous microalga Parachlorella kessleri QWY28. Bioresour. Technol. 2019, 289, 121702. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Raza, M.A.; Li, Z.; Chen, Y.; Khalid, M.H.B.; Du, J.; Liu, W.; Wu, X.; Song, C.; Yu, L.; et al. The influence of light intensity and leaf movement on photosynthesis characteristics and carbon balance of soybean. Front. Plant Sci. 2019, 9, 1952. [Google Scholar] [CrossRef]
- Serena, S.; Fernie, A.R.; Peter, G.; Dario, L.; Torsten, M.; Belen, N.; Ekkehard, N.H. Chloroplasts are key players to cope with light and temperature stress. Trends Plant Sci. 2022, 27, 577–587. [Google Scholar]
- Li, S.F.; Fanesi, A.; Martin, T.; Lopes, F. Biomass production and physiology of Chlorella vulgaris during the early stages of immobilized state are affected by light intensity and inoculum cell density. Algal Res. 2021, 59, 102453. [Google Scholar] [CrossRef]
- Wang, P.; Shao, Y.; Geng, Y.; Rubina, M.; Yang, W.; Li, M.; Sun, X.; Wang, H.; Chen, G. Advanced treatment of secondary effluent from wastewater treatment plant by a newly isolated microalga Desmodesmus sp. SNN1-13. Front. Microbiol. 2023, 14, 1111468. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.S.; Tam, N.F.Y.; Wong, Y.S. Effect of algal density on nutrient removal from primary settled wastewater. Environ. Pollut. 1995, 89, 59–66. [Google Scholar] [CrossRef]
- Park, S.; Ahn, Y.; Park, Y.-T.; Ji, M.-K.; Choi, J. The effect of mixed wastewaters on the biomass production and biochemical content of microalgae. Energies 2019, 12, 12183431. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van Montagu, M.; Inze, D. Effects of iron excess on nicotiana plumbaginifolia plants (implications to oxidative stress). Plant Physiol. 1995, 107, 725–735. [Google Scholar] [CrossRef]
- Gallego, S.M.; Benavídes, M.P.; Tomaro, M.L. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 1996, 121, 151–159. [Google Scholar] [CrossRef]
- Marsh, H.V.; Evans, H.J.; Matrone, G. Investigations of the role of iron in chlorophyll metabolism. II. effect of iron deficiency on chlorophyll synthesis. Plant Physiol. 1963, 38, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Jin, W.; Ding, W.; Lu, S.; Song, K.; Chen, C.; Qin, C.; Chen, Y.; Tu, R.; Zhou, X. Effects of nutrient composition, lighting conditions, and metal ions on the growth and lipid yield of the high-lipid-yielding microalgae (Chlorella pyrenoidosa) cultivated in municipal wastewater. J. Environ. Chem. Eng. 2021, 9, 106491. [Google Scholar] [CrossRef]
- Atefeh, K.; Vida, T.; Ali, Z.M.; Eghrari, G.S.; Abdolhamid, A.S. Augmenting the expression of accD and rbcL genes using optimized iron concentration to achieve higher biomass and biodiesel in Chlorella vulgaris. Biotechnol. Lett. 2020, 42, 2631–2641. [Google Scholar]
- Vaishali, R.; Gergely, M. Light-dependent nitrate removal capacity of green microalgae. Int. J. Mol. Sci. 2023, 24, 77. [Google Scholar]








| Specific Growth Rate (d−1) | Microalgae Species | |||||
|---|---|---|---|---|---|---|
| Chlorella sorokiniana GXNN-01 | Chlorella sorokiniana FACHB-275 | Chlorella sp. FACHB-30 | Chlorella sp. FACHB-9 | Chlamydomonas reinhardtii FACHB-479 | Tetradesmus obliquus FACHB-416 | |
| BBM | 0.1756 ± 0.0129 bc | 0.1852 ± 0.0102 ab | 0.1728 ± 0.0099 bcd | 0.2031 ± 0.0083 a | 0.1572 ± 0.0057 d | 0.1604 ± 0.0054 cd |
| 1000MSGW | 0.1956 ± 0.0068 d | 0.2245 ± 0.0039 c | 0.2564 ± 0.0005 b | 0.2685 ± 0.0007 a | 0.1625 ± 0.0045 e | 0.1297 ± 0.0023 f |
| 1000MSGW + TMs | 0.1899 ± 0.0071 c | 0.2155 ± 0.0019 b | 0.2660 ± 0.0046 a | 0.2823 ± 0.0035 a | 0.1609 ± 0.0138 d | 0.1857 ± 0.0028 c |
| Parameters | Before Optimization | After Optimization | Increasing Rate |
|---|---|---|---|
| The removal rate of TP (%) | 69.10 ± 2.57 ** | 87.60 ± 1.50 ** | 26.77% |
| The removal rate of TN (%) | 26.93 ± 0.82 ** | 68.05 ± 3.89 ** | 152.69% |
| -N (%) | 51.91 ± 1.28 ** | 75.89 ± 1.82 ** | 46.19% |
| The removal rate of COD (%) | 61.50 ± 3.75 ** | 77.96 ± 2.95 ** | 26.76% |
| Biomass concentration (g L−1) | 1.196 ± 0.087 * | 1.423 ± 0.018 * | 18.98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Su, Q.; Wang, H.; Wu, C.; Tong, S.; Zhang, J.; Qiao, H. Strain Screening and Conditions Optimization in Microalgae-Based Monosodium Glutamate Wastewater (MSGW) Treatment. Water 2023, 15, 1663. https://doi.org/10.3390/w15091663
Zhuang Y, Su Q, Wang H, Wu C, Tong S, Zhang J, Qiao H. Strain Screening and Conditions Optimization in Microalgae-Based Monosodium Glutamate Wastewater (MSGW) Treatment. Water. 2023; 15(9):1663. https://doi.org/10.3390/w15091663
Chicago/Turabian StyleZhuang, Yanmin, Qingling Su, Haowen Wang, Chengzong Wu, Shanying Tong, Jumei Zhang, and Hongjin Qiao. 2023. "Strain Screening and Conditions Optimization in Microalgae-Based Monosodium Glutamate Wastewater (MSGW) Treatment" Water 15, no. 9: 1663. https://doi.org/10.3390/w15091663
APA StyleZhuang, Y., Su, Q., Wang, H., Wu, C., Tong, S., Zhang, J., & Qiao, H. (2023). Strain Screening and Conditions Optimization in Microalgae-Based Monosodium Glutamate Wastewater (MSGW) Treatment. Water, 15(9), 1663. https://doi.org/10.3390/w15091663






