Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles
Abstract
:1. Introduction
2. Material and Methods
2.1. Anaerobic Inoculum and Feedstock
2.2. Microparticles as Additives
2.3. Anaerobic Digestion Tests
2.4. Analytical Procedure
2.5. Modelling
2.6. Data Analysis
3. Results and Discussion
3.1. The Effect of Microparticles on the Production of Biogas
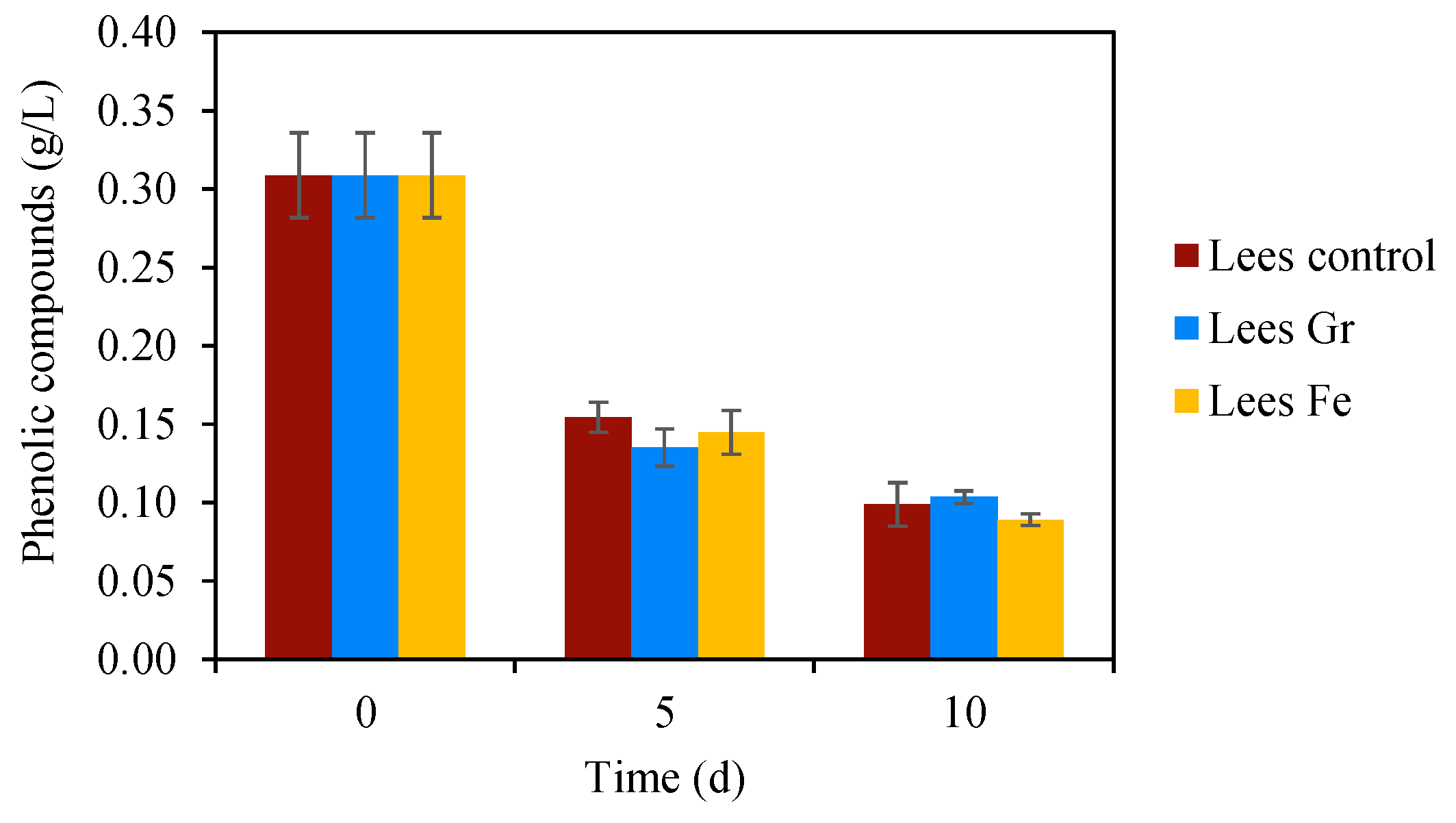
3.2. Potential Inhibitors along the Anaerobic Digestion Process
3.3. Modelling
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginni, G.; Kavitha, S.; Kannah, Y.; Bhatia, S.K.; Kumar, A.; Rajkumar, M.; Gopalakrishnan, K.; Arivalagan, P.; Nguyen, T.L.C.; Rajesh, B.J.; et al. Valorization of agricultural residues: Different biorefinery routes. J. Environ. Chem. Eng. 2021, 9, 105435. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Waiho, K.; Fazhan, H.; Necibi, M.C.; Hafsa, J.; Ben Mrid, R.; Fal, S.; El Arroussi, H.; Peng, W.; Tabatabaei, M.; et al. Progress in valorisation of agriculture, aquaculture and shellfish biomass into biochemicals and biomaterials towards sustainable bioeconomy. Chemosphere 2021, 291, 133036. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Summary Progress Update 2021: SDG 15-Life on Land. 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=SDG_15_-_Life_on_land (accessed on 1 June 2023).
- United Nations. Summary Progress Update 2021: SDG 13-Climate Action. 2021. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=SDG_13_-_Climate_action (accessed on 1 June 2023).
- Wei, M.; Ma, T.; Ge, Q.; Li, C.; Zhang, K.; Fang, Y.; Sun, X. Challenges and opportunities of winter vine pruning for global grape and wine industries. J. Clean. Prod. 2022, 380, 135086. [Google Scholar] [CrossRef]
- Mastoras, P.; Zkeri, E.; Panara, A.; Dasenaki, M.E.; Maragou, N.C.; Vakalis, S.; Fountoulakis, M.S.; Thomaidis, N.S.; Stasinakis, A.S. Application of a pilot-scale solar still for wine lees management: Characterization of by-products and valorization potential. J. Environ. Chem. Eng. 2023, 11, 111227. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero-García, J.M.; López-Linares, J.C.; Romero, I.; Castro, E. Residues from grapevine and wine production as feedstock for a biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Summo, C.; Caponio, F. Bioactive compounds from vine shoots, grape stalks, and wine lees: Their potential use in agro-food chains. Foods 2021, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, C.B.A.; Chiappero, M.; Gomez, X.; Fiore, S.; Martínez, E.J. Improving the anaerobic digestion of wine-industry liquid wastes: Treatment by electro-oxidation and use of biochar as an additive. Energies 2020, 13, 5971. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Palomar, C.R.; Torre, R.M.; Redondo, D.H.; Crespo, I.d.G. Hybridization of anaerobic digestion with solar energy: A solution for isolated livestock farms. Energy Convers. Manag. X 2023, 20, 100488. [Google Scholar] [CrossRef]
- Holm-Nielsen, J.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Poirier, S.; Chapleur, O. Inhibition of anaerobic digestion by phenol and ammonia: Effect on degradation performances and microbial dynamics. Data Brief 2018, 19, 2235–2239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.; Arjmand, M.; Eskicioglu, C. Nanomaterial-amended anaerobic sludge digestion: Effect of pH as a game changer. Environ. Res. 2024, 240, 117463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, D.; Zhang, K.; Ma, Y.; Liu, F.; Li, Z.; Gao, X.; Gao, W.; Du, L. Effects of initial volatile fatty acid concentrations on process characteristics, microbial communities, and metabolic pathways on solid-state anaerobic digestion. Bioresour. Technol. 2023, 369, 128461. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Cavinato, C.; Pavan, P.; Bolzonella, D. Mesophilic and thermophilic anaerobic co-digestion of winery wastewater sludge and wine lees: An integrated approach for sustainable wine production. J. Environ. Manag. 2017, 203, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hungría, J.; Siles, J.A.; Chica, A.F.; Gil, A.; Martín, M.A. Anaerobic co-digestion of winery waste: Comparative assessment of grape marc waste and lees derived from organic crops. Environ. Technol. 2021, 42, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.J.; Burgess, J.E. Fungal and enzymatic remediation of a wine lees and five wine-related distillery wastewaters. Bioresour. Technol. 2008, 99, 6134–6142. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Li, Y.; Li, Q.; Li, P.; Luo, L.; Zhen, F.; Zheng, G.; Sun, Y. Low-Temperature Pretreatment of Biomass for Enhancing Biogas Production: A Review. Fermentation 2022, 8, 562. [Google Scholar] [CrossRef]
- François, M.; Lin, K.-S.; Rachmadona, N.; Khoo, K.S. Advancement of nanotechnologies in biogas production and contaminant removal: A review. Fuel 2023, 340, 127470. [Google Scholar] [CrossRef]
- Hassanein, A.; Kumar, A.N.; Lansing, S. Impact of electro-conductive nanoparticles additives on anaerobic digestion performance-A review. Bioresour. Technol. 2021, 342, 126023. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Panahi, H.K.S.; Nizami, A.-S. A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 nanoparticles facilitated anaerobic digestion of organic fraction of municipal solid waste for enhancement of methane production. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2018, 275, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Mayumi, M.; Syo, K.; Satoshi, A.; Seiichi, Y.; Takashima, S.; Ono, H.; Ap, Y.; Yamashiro, T.; Ahmed, M.M.; et al. Potential of biogas production from manure of dairy cattle fed on natural soil supplement rich in iron under batch and semi-continuous anaerobic digestion. Bioresour. Technol. 2020, 309, 123298. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, Q.; Ye, X.; Wang, C.; Jia, Z.; Du, J.; Kong, X.; Xi, Y. Effect of different charged Fe3O4 nanoparticles on methane production for anaerobic digestion of wheat straw. J. Clean. Prod. 2021, 328, 129655. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Perendeci, N.A. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Hassaneen, F.Y.; Faisal, Y.; Mansour, M.S.; Ibrahim, A.; Abo-Elfadl, S.; Salem, H.; Allam, N.K. Effect of Ni-Ferrite and Ni-Co-Ferrite nanostructures on biogas production from anaerobic digestion. Fuel 2019, 254, 115673. [Google Scholar] [CrossRef]
- Ma, H.; Hu, Y.; Kobayashi, T.; Xu, K.-Q. The role of rice husk biochar addition in anaerobic digestion for sweet sorghum under high loading condition. Biotechnol. Rep. 2020, 27, e00515. [Google Scholar] [CrossRef]
- Lee, J.T.; Lim, E.Y.; Zhang, L.; Tsui, T.-H.; Tian, H.; Yan, M.; Lim, S.; Majid, M.b.A.; Jong, M.-C.; Zhang, J.; et al. Methanosarcina thermophila bioaugmentation and its synergy with biochar growth support particles versus polypropylene microplastics in thermophilic food waste anaerobic digestion. Bioresour. Technol. 2022, 360, 127531. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, Y.; Ji, D.; Li, X.; Zhang, J.; Zang, L. Synergetic promotion of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with graphite felt in anaerobic digestion. Bioresour. Technol. 2019, 287, 121373. [Google Scholar] [CrossRef] [PubMed]
- Muratçobanoğlu, H.; Gökçek, B.; Mert, R.A.; Zan, R.; Demirel, S. Simultaneous synergistic effects of graphite addition and co-digestion of food waste and cow manure: Biogas production and microbial community. Bioresour. Technol. 2020, 309, 123365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Reyes, F.L.d.L.; Call, D.F. Call, Amending anaerobic bioreactors with pyrogenic carbonaceous materials: The influence of material properties on methane generation. Environ. Sci. Water Res. Technol. 2018, 4, 1794–1806. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K.; et al. Programmed Iron Oxide Nanoparticles Disintegration in Anaerobic Digesters Boosts Biogas Production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- Álvaro, A.G.; Palomar, C.R.; Redondo, D.H.; Torre, R.M.; Crespo, I.d.G. Simultaneous production of biogas and volatile fatty acids through anaerobic digestion using cereal straw as substrate. Environ. Technol. Innov. 2023, 31, 103215. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Kasama, T.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Effect of metal oxide based TiO2 nanoparticles on anaerobic digestion process of lignocellulosic substrate. Energy 2019, 191, 116580. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water & Wastewater, 22nd ed.; American Water Works Association: Denver, CO, USA, 2012. [Google Scholar]
- Box, J.D. Investigation of the Folin-Ciocalteau phenol reagent for the determination of polyphenolic substances in natural waters. Water Res. 1983, 17, 511–525. [Google Scholar] [CrossRef]
- LAY, J.J.; Li, Y.Y.; Noike, T. Student, Effect of moisture content and chemical nature on methane fermentation characteristics of municipal solid wastes. Doboku Gakkai Ronbunshu 1996, 552, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Nopharatana, A.; Pullammanappallil, P.C.; Clarke, W.P. Kinetics and dynamic modelling of batch anaerobic digestion of municipal solid waste in a stirred reactor. Waste Manag. 2007, 27, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Shamurad, B.; Gray, N.; Petropoulos, E.; Tabraiz, S.; Membere, E.; Sallis, P. Predicting the effects of integrating mineral wastes in anaerobic digestion of OFMSW using first-order and Gompertz models from biomethane potential assays. Renew. Energy 2020, 152, 308–319. [Google Scholar] [CrossRef]
- Cano, R.; Nielfa, A.; Fdz-Polanco, M. Thermal hydrolysis integration in the anaerobic digestion process of different solid wastes: Energy and economic feasibility study. Bioresour. Technol. 2014, 168, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, C.; Cavinato, C.; Bolzonella, D.; Pavan, P. Renewable energy from thermophilic anaerobic digestion of winery residue: Preliminary evidence from batch and continuous lab-scale trials. Biomass Bioenergy 2016, 91, 150–159. [Google Scholar] [CrossRef]
- Fabbri, A.; Bonifazi, G.; Serranti, S. Micro-scale energy valorization of grape marcs in winery production plants. Waste Manag. 2014, 36, 156–165. [Google Scholar] [CrossRef]
- Jasko, J.; Skripsts, E.; Dubrovskis, V. Biogas production of winemaking waste in anaerobic fermentation process. In Proceedings of the 11th International Scientific Conference Engineering for Rural Development, Jelgava, Latvia, 24–25 May 2012. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Kalia, V.; Kumar, A.; Jain, S.; Joshi, A. Biomethanation of plant materials. Bioresour. Technol. 1992, 41, 209–212. [Google Scholar] [CrossRef]
- Almeida, P.; Gando-Ferreira, L.; Quina, M. Biorefinery perspective for industrial potato peel management: Technology readiness level and economic assessment. J. Environ. Chem. Eng. 2023, 11, 110049. [Google Scholar] [CrossRef]
- Khoufi, S.; Louhichi, A.; Sayadi, S. Optimization of anaerobic co-digestion of olive mill wastewater and liquid poultry manure in batch condition and semi-continuous jet-loop reactor. Bioresour. Technol. 2015, 182, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. A detailed assessment of resource of biomethane from first, second and third generation substrates. Renew. Energy 2016, 87, 656–665. [Google Scholar] [CrossRef]
- Galí, A.; Benabdallah, T.; Astals, S.; Mata-Alvarez, J. Modified version of ADM1 model for agro-waste application. Bioresour. Technol. 2009, 100, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Vivekanand, V. Combined fungal and bacterial pretreatment of wheat and pearl millet straw for biogas production—A study from batch to continuous stirred tank reactors. Bioresour. Technol. 2020, 321, 124523. [Google Scholar] [CrossRef] [PubMed]
- Sravan, J.S.; Tharak, A.; Mohan, S.V. Chapter 1-Status of biogas production and biogas upgrading: A global scenario. In Emerging Technologies and Biological Systems for Biogas Upgrading; Aryal, N., Mørck Ottosen, L.D., Wegener Kofoed, M.V., Pant, D., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–26. [Google Scholar] [CrossRef]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Dhar, B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 244, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.; Tyagi, V.K. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, H.-J.; Kim, Y.; Kim, N.-K.; Park, H.-D. Different contribution of exoelectrogens in methanogenesis via direct interspecies electron transfer (DIET) by the different substrate in continuous anaerobic bioreactor. Bioresour. Technol. 2022, 364, 128115. [Google Scholar] [CrossRef]
- Kassab, G.; Khater, D.; Odeh, F.; Shatanawi, K.; Halalsheh, M.; Arafah, M.; van Lier, J.B. Impact of Nanoscale Magnetite and Zero Valent Iron on the Batch-Wise Anaerobic Co-Digestion of Food Waste and Waste-Activated Sludge. Water 2020, 12, 1283. [Google Scholar] [CrossRef]
- Başar, I.A.; Eskicioglu, C.; Perendeci, N.A. Biochar and wood ash amended anaerobic digestion of hydrothermally pretreated lignocellulosic biomass for biorefinery applications. Waste Manag. 2022, 154, 350–360. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Z.; Zhao, Z.; Quan, X.; Zhao, Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017, 149, 1101–1108. [Google Scholar] [CrossRef]
- Powell, C.D.; Atkinson, A.J.; Ma, Y.; Marcos-Hernandez, M.; Villagran, D.; Westerhoff, P.; Wong, M.S. Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J. Nanoparticle Res. 2020, 22, 48. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Sarathy, V.; Tratnyek, P.G.; Baer, D.R.; Amonette, J.E.; Karkamkar, A. Recovery of iron/iron oxide nanoparticles from solution: Comparison of methods and their effects. J. Nanoparticle Res. 2010, 13, 1937–1952. [Google Scholar] [CrossRef]
- Chiappero, M.; Berruti, F.; Fiore, S. Biomethane potential of wine lees from mesophilic anaerobic digestion. Biochem. Eng. J. 2023, 196, 108954. [Google Scholar] [CrossRef]
- Yeole, T.; Gokhale, S.; Hajarnis, S.; Ranade, D. Effect of brackish water on biogas production from cattle dung and methanogens. Bioresour. Technol. 1996, 58, 323–325. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Gavala, H.N.; Angelidaki, I.; Ahring, B.K. Kinetics and Modeling of Anaerobic Digestion Process. In Biomethanation; Ahring, B.K., Angelidaki, I., de Macario, E.C., Gavala, H.N., Hofman-Bang, J., Macario, A.J.L., Elferink, S.J.W.H.O., Raskin, L., Stams, A.J.M., Westermann, P., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 57–93. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Jakubíková, M.; Sádecká, J.; Hroboňová, K. Determination of total phenolic content and selected phenolic compounds in sweet wines by fluorescence spectroscopy and multivariate calibration. Microchem. J. 2022, 181, 107834. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 34. [Google Scholar] [CrossRef]
- Freitas, P.V.; da Silva, D.R.; Beluomini, M.A.; da Silva, J.L.; Stradiotto, N.R. Determination of Phenolic Acids in Sugarcane Vinasse by HPLC with Pulse Amperometry. J. Anal. Methods Chem. 2018, 2018, 4869487. [Google Scholar] [CrossRef]
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Steyer, J.-P.; Sadowski, A.G.; Béline, F. A waste characterisation procedure for ADM1 implementation based on degradation kinetics. Water Res. 2012, 46, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Pavlostathis, S.G.; Giraldo-Gomez, E. Kinetics of Anaerobic Treatment. Water Sci. Technol. 1991, 24, 35–59. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.A.; Vavilin, V.A. The IWA Anaerobic digestion model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources. In Focus on Catalysts, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2011; p. 8. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Singh, R.N.; Tripathi, A.K.; Rauniyar, S.; Saxena, P.; Thakur, P.; Sani, R.K. Chapter 11-Biochemical and molecular mechanisms of sulfate-reducing bacterial biofilms. In Understanding Microbial Biofilms; Das, S., Kungwani, N.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 165–172. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Pandey, A. Chapter 6-Landfill Gas as an Energy Source. In Current Developments in Biotechnology and Bioengineering; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 93–117. [Google Scholar] [CrossRef]
- Gunaseelan, V. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]





| Type of Particle | Type of Biomass | Particle Content | Biogas Production Increase | Reference |
|---|---|---|---|---|
| Metal-based particles | ||||
| Fe3O4 | Granular sludge | 500 mg∙L−1 | 24% | [25] |
| Fe3O4 | Municipal solid waste | 50–125 mg∙L−1 | 43–72% | [26] |
| Fe3O4 | Poultry litter | 15–100 mg∙L−1 | 26–28% | [27] |
| Fe2O3 | Cattle manure | 20–100 mg∙L−1 | 10–19% | [28] |
| Fe3O4 | Wheat straw | 100 mg∙L−1 | 51% | [29] |
| Al2O3 | Waste activated sludge | 50–500 mg∙L−1 | 8–15% | [30] |
| Nickel ferrite | Livestock manure | 20–130 mg∙L−1 | 18–31% | [31] |
| Carbon-based particles | ||||
| Biochar | Sweet sorghum | 5–20 g∙L−1 | 20–25% | [32] |
| Biochar | Wine lees | 10 g∙L−1 | 18% | [9] |
| Biochar | Food waste | 1 g∙L−1 | 32% | [33] |
| Graphene | Sewage sludge | 30 mg∙L−1 | 14% | [34] |
| Graphite | Synthetic wastewater | 6.5 g∙L−1 | 19% | [35] |
| Graphite | Food waste and cow manure | 1 g∙L−1 | 49% | [36] |
| Graphite | Swine sludge | 1–5 g∙L−1 | 2–23% | [37] |
| Parameter | WWTP Sludge | Wine Lees |
|---|---|---|
| COD (g∙L−1) | 19.21 ± 2.06 | 372.28 ± 3.42 |
| TS (g∙kg−1) | 16.20 ± 0.12 | 163.30 ± 0.15 |
| VS (g∙kg−1) | 11.18 ± 0.12 | 154.21 ± 0.85 |
| TOC (g∙L−1) | - | 139.45 ± 0.86 |
| TN (g∙L−1) | - | 6.37 ± 0.07 |
| C:N | - | 21.89 ± 0.26 |
| Phenolic compounds (g∙L−1) | 0.02 ± 0.00 | 1.59 ± 0.14 |
| pH | 7.2 ± 0.0 | 3.6 ± 0.1 |
| Parameter | Value |
|---|---|
| Reactor volume (mL) | 120 |
| Working volume (mL) | 70 |
| Substrate (type) | Wine lees |
| Inoculum (type) | WWTP sludge |
| Inoculum: Substrate ratio | 1.5 |
| Temperature (°C) | 35 |
| Agitation (rpm) | 100 |
| Type of added microparticles | Fe3O4, Graphite |
| Particle concentration (mg∙L−1) | 200 |
| Substrate | Experimental Conditions | TS (%) | VS (% of TS) | T (°C) | Methane Production (mL CH4 g−1 VS) | Reference |
|---|---|---|---|---|---|---|
| Tomato Pomace | Batch, 0.5 L | 30.1 | 96.1 | 40 | 180 | [52] |
| Apple pomace | Batch, 1 L | 50.2 | 95.6 | 40 | 157 | [53] |
| Potato peels | Batch, 0.075 L | 17.7 | 94.0 | 35 | 267 | [54] |
| Olive mill wastewater | Batch, 0.06 L | 12.0 | 87.5 | 37 | 183 | [55] |
| Bovine manure | Batch, 0.4 L | 9.8 | 76.0 | 37 | 36 | [56] |
| Pig slurry | Batch, 0.25 L | 3.7 | 78.5 | 35 | 150 | [57] |
| Wheat straw | Batch, 0.4 L | 93.5 | 95.8 | 37 | 226 | [58] |
| Rice straw | Batch, 0.5 L | 88.7 | 91.9 | 40 | 195 | [52] |
| Wine lees | Batch, 0.7 L | 16.3 | 94.4 | 35 | 192 | This study |
| Gompertz | First Order | |||||||
|---|---|---|---|---|---|---|---|---|
| P∞ (mL CH4∙gVS−1) | Rm (mL CH4∙gVS−1∙d−1) | λ (d) | R2 | P∞ (mL CH4∙gVS−1) | K (d−1) | λ (d) | R2 | |
| Lees Control | 136.00 | 33.46 | 0.38 | 98.6% | 136.00 | 0.350 | 0.17 | 96.5% |
| Lees Gr | 166.00 | 45.38 | 0.49 | 98.1% | 166.00 | 0.398 | 0.18 | 94.9% |
| Lees Fe | 192.00 | 46.54 | 0.53 | 98.4% | 192.00 | 0.401 | 0.21 | 93.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Álvaro, A.; Ruiz Palomar, C.; Hermosilla, D.; Gascó, A.; Muñoz, R.; de Godos, I. Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles. Water 2024, 16, 101. https://doi.org/10.3390/w16010101
García Álvaro A, Ruiz Palomar C, Hermosilla D, Gascó A, Muñoz R, de Godos I. Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles. Water. 2024; 16(1):101. https://doi.org/10.3390/w16010101
Chicago/Turabian StyleGarcía Álvaro, Alfonso, César Ruiz Palomar, Daphne Hermosilla, Antonio Gascó, Raúl Muñoz, and Ignacio de Godos. 2024. "Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles" Water 16, no. 1: 101. https://doi.org/10.3390/w16010101
APA StyleGarcía Álvaro, A., Ruiz Palomar, C., Hermosilla, D., Gascó, A., Muñoz, R., & de Godos, I. (2024). Improving the Anaerobic Digestion Process of Wine Lees by the Addition of Microparticles. Water, 16(1), 101. https://doi.org/10.3390/w16010101