Removal of Emerging Contaminants by Degradation during Filtration: A Review of Experimental Procedures and Modeling
Abstract
:1. Introduction
2. Model Equations and Analysis: Removal of Emerging Contaminants by Degradation during Filtration
3. Removal of Taste and Odor Molecules from Water in Fish Ponds
4. Removal of Cyanotoxins from Water
4.1. Removal of Cyanobacteria and Cyanotoxins by Filtration
4.2. Removal of Cyanotoxins by Degradation during Filtration
- A.
- Laboratory experiments on removal of microcystins [40]
- B.
- Simulation of results in (A) and a proposed treatment for larger toxin concentrations
- C.
- Addition of a micelle–clay filter
5. Removal of ECs Using Enzymes
5.1. Use of Enzymes in Filtration Processes
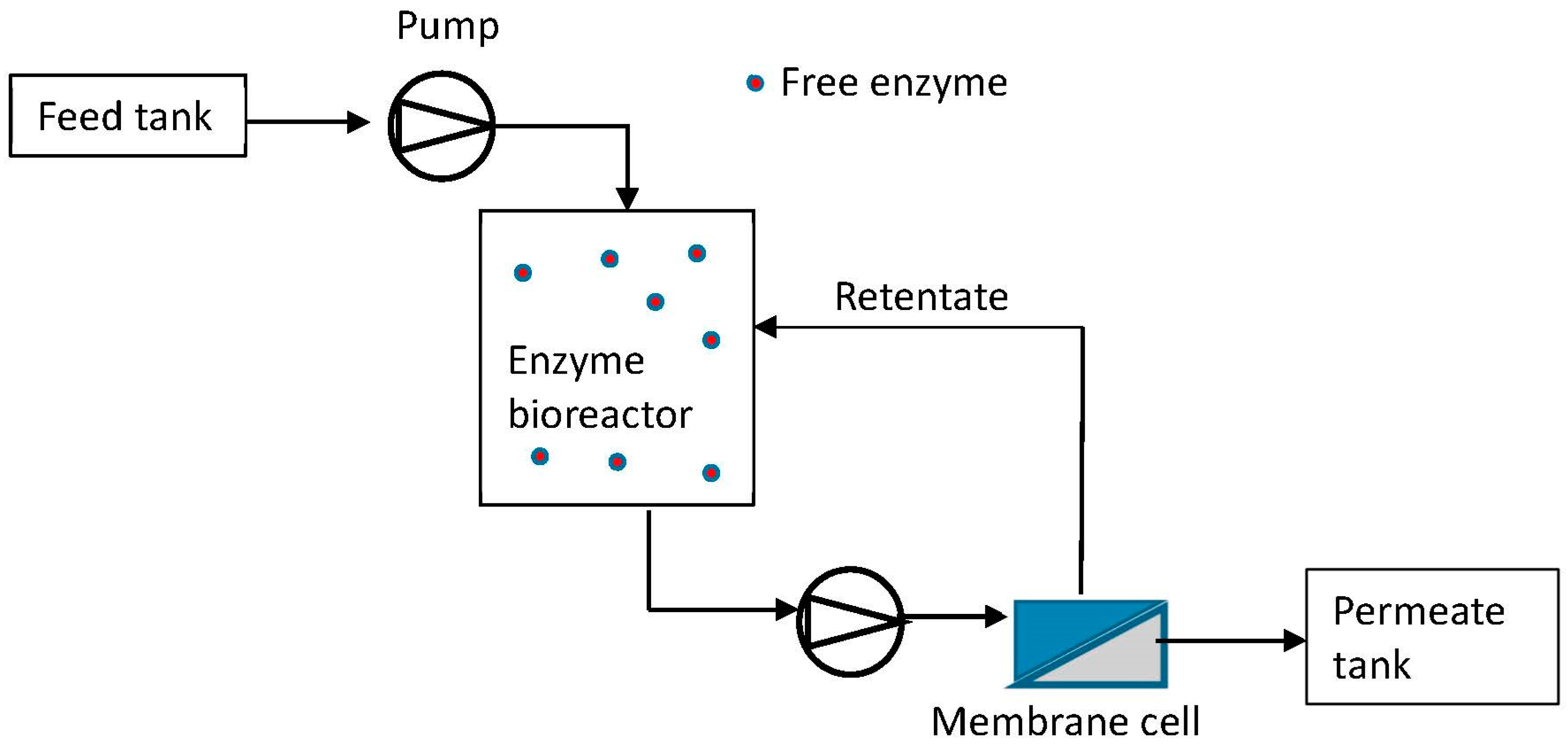
5.1.1. Membrane Technologies
5.1.2. Bed Reactors
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Sengupta, A.; Jebur, M.; Kamaz, M.; Wickramasinghe, R. Removal of emerging contaminants from wastewater streams using membrane bioreactors: A review. Membranes 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Thuptimdang, P.; Siripattanakul-Ratpukdi, S.; Ratpukdi, T.; Youngwilai, A.; Khan, E. Biofiltration for treatment of recent emerging contaminants in water: Current and future perspectives. Water Environ. Res. 2021, 93, 972–992. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, L.; Hong, Y.; Duan, X.; Ai, C.; Zhang, L.; Zhang, T.; Chen, Y.; Lin, X.; Shi, W.; et al. Iron phthalocyanine nanodots decorated ultra-thin porous carbon nitride: A combination of photocatalysis and Fenton reaction to achieve two-channel efficient tetracycline degradation J. Alloys Compd. 2023, 966, 171580. [Google Scholar] [CrossRef]
- Nir, S.; Zadaka-Amir, D.; Kartaginer, A.; Gonen, Y. Simulation of adsorption and flow of pollutants in a column filter: Application to Micelle-clay mixtures with sand. Appl. Clay Sci. 2012, 67–68, 134–140. [Google Scholar] [CrossRef]
- Lelario, F.; Gardi, I.; Mishael, Y.; Dolev, N.; Undabeytia, T.; Nir, S.; Scarano, L.; Bufo, S.A. Pairing micropollutants and clay-composites for efficient water treatment: Filtration and modeling at a pilot scale. Appl. Clay Sci. 2017, 137, 225–232. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Tavassi, M.; Nir, S. Removal of cyanobacteria and cyanotoxins from lake water by composites of bentonite with micelles of the cation octadecyltrimethylammonium (ODTMA). Water Res. 2017, 120, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711. [Google Scholar] [CrossRef]
- Undabeytia, T.; Posada, R.; Nir, S.; Golindo, I.; Laiz, L.; Saiz-Jimenez, C.; Morillo, E. Removal of waterborne microorganisms by filtration using clay polymer complexes. J. Hazard. Mater. 2014, 279, 190–196. [Google Scholar] [CrossRef]
- Brienza, M.; Nir, S.; Plantard, G.; Goetz, V.; Chiron, S. Combining micelle-clay sorption to solar photo- fenton processes for domestic wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 18971–18978. [Google Scholar] [CrossRef]
- Benitez, A.R.; Ryskin, M.; Dor, M.; Shuali, U.; Nir, S.; Polubesova, T.; Ben-Ari, J.; Kerstnus, J.; Undabeytia, T. Modified compositions of micelle-clay and liposome-clay composites for optimal removal from water of hydrophobic neutral chemicals and bacteria. Appl. Sci. 2022, 12, 3044. [Google Scholar] [CrossRef]
- Nir, S.; Brook, I.; Anavi, Y.; Ben-Ari, J.; Sheveky-Huterer, R.; Etkin, H.; Zadaka-Amir, D.; Shuali, U. Water purification from perchlorate micelle-clay complex. Laboratory and pilot experiments. Appl. Clay Sci. 2015, 114, 151–156. [Google Scholar] [CrossRef]
- Azaria, S.; Nussinovitch, A.; Nir, S.; van Rijn, J. Removal of geosmin and 2-methylisoborneolfrom aquaculture water by novel, alginate-based carriers: Performance and metagenomic analysis. J. Water Process Eng. 2021, 42, 102125. [Google Scholar] [CrossRef]
- Kaya, A.U.; Güner, S.; Ryskin, M.; Lameck, A.S.; Benitez, A.R.; Shuali, U.; Nir, S. Effect of microwave radiation on regeneration of a granulated micelle–clay complex after adsorption of bacteria. Appl. Sci. 2020, 10, 2530. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018. [Google Scholar]
- van Rijn, J. Waste treatment in recirculating aquaculture systems. Aquac. Eng. 2013, 53, 49–56. [Google Scholar] [CrossRef]
- Azaria, S.; van Rijn, J. Off-flavor in recirculating aquaculture systems (RAS): Production and removal processes. Aquac. Eng. 2018, 83, 57–64. [Google Scholar] [CrossRef]
- Howgate, P. Tainting of farmed fish by geosmin and 2-methylisoborneol: A review of sensory aspect and uptake/depuration. Aquaculture 2004, 234, 155–181. [Google Scholar] [CrossRef]
- Tucker, C.S. Off-flavor problems in aquaculture. Rev. Fish. Sci. 2000, 8, 45–88. [Google Scholar] [CrossRef]
- Guttman, L.; van Rijn, J. Isolation of bacteria capable of growth with 2-methylisoborneol and geosmin as the sole carbon and energy sources. Appl. Environ. Microbiol. 2012, 78, 363–370. [Google Scholar] [CrossRef]
- Kawaguchi, O.; Tanaka, M.; Yoshii, M.; Iwamoto, Y.; Midooka, A.; Toutani, F.; Nagao, N.; Matsumoto, T.; Mabuchi, R.; Tanimoto, S. Off-flavor of red sea bream Pagrus major reared in recirculating aquaculture systems with low salinity is caused by 2-methylisoborneol. Fish. Sci. 2019, 85, 553–560. [Google Scholar] [CrossRef]
- Lukassen, M.B.; Podduturi, R.; Rohaan, B.; Jørgensen, N.O.; Nielsen, J.L. Dynamics of geosmin-producing bacteria in a full-scale saltwater recirculated aquaculture system. Aquaculture 2019, 500, 170–177. [Google Scholar] [CrossRef]
- Chen, G.; Dussert, B.W.; Suffet, I.H. Evaluation of granular activated carbons for removal of methylisoborneol to below odor threshold concentration in drinking water. Water Res. 1997, 31, 1155–1163. [Google Scholar] [CrossRef]
- Burr, G.S.; Wolters, W.R.; Schrader, K.K.; Summerfelt, S.T. Impact of depuration of earthy-musty off-flavors on fillet quality of Atlantic salmon, Salmo salar, cultured in a recirculating aquaculture system. Aquac. Eng. 2012, 50, 28–36. [Google Scholar] [CrossRef]
- Davidson, J.; Schrader, K.K.; Ruan, E.; Swift, B.; Aalhus, J.; Juarez, M.; Wolters, W.; Burr, G.; Good, C.; Summerfelt, S.T. Evaluation of depuration procedures to mitigate the off-flavor compounds geosmin and 2-methylisoborneol from Atlantic salmon, Salmo salar, raised to market-size in recirculating aquaculture systems. Aquac. Eng. 2014, 61, 27–34. [Google Scholar] [CrossRef]
- Azaria, S.; Nir, S.; van Rijn, J. Combined adsorption and degradation of the off-flavor compound 2-methylisoboneol in sludge derived from a recirculating aquaculture system. Chemosphere 2017, 169, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Azaria, S.; Post, A.F.; van Rijn, J. Changes in the bacterial community structure of denitrifying sludge from a recirculating aquaculture system (RAS) after geosmin and 2-methylisoborneol enrichment. Curr. Microbiol. 2020, 77, 353–360. [Google Scholar] [CrossRef]
- Szlag, D.; Sinclair, J.; Southwell, B.; Westrick, J. Cyanobacteria and cyanotoxins occurrence and removal/inactivation from five high- risk conventional treatment drinking water plants. Toxins 2015, 7, 2198–2220. [Google Scholar] [CrossRef]
- Markensten, H.; Moore, K.; Persson, I. Simulated lake phytoplankton composition shifts toward cyanobacteria dominance in a future warmer climate. Ecol. Appl. 2010, 20, 752–767. [Google Scholar] [CrossRef]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef]
- Visser, P.M.; Verspagen, J.M.; Sandrini, G.; Stal, L.J.; Matthijs, H.C.; Davis, T.W.; Paerl, H.W.; Huisman, J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae 2016, 54, 145–159. [Google Scholar] [CrossRef]
- Scholz, S.N.; Esterhuizen-Londt, M.; Pflugmacher, S. Rise of toxic cyanobacterial blooms in temperate freshwater lakes: Causes, correlations and possible countermeasures. Toxicol. Environ. Chem. 2017, 99, 543–577. [Google Scholar] [CrossRef]
- Mishael, Y.G.; Undabeytia, T.; Rytwo, G.; Papahadjpoulos-Steinberg, B.; Rubin, B.; Nir, S. Sulfometuron adsorption via alkylammonium cations adsorption as monomers and micelles on montmorillonite. J. Agric. Food Chem. 2002, 50, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Nir, S.; Shuali, U. Water Purification by Micelle-Clay Nano-Particles; Nova Science Publishers: New York, NY, USA, 2019. [Google Scholar]
- Rytwo, G. The use of clay-polymer nanocomposites in wastewater pretreatment. Sci. World J. 2012, 2012, e498503. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G.; Lavi, R.; Rytwo, Y.; Monchase, H.; Dultz, S.; König, T.N. Clarification of olive mill and winery wastewater by means of clay-polymer nanocomposites. Sci. Total Environ. 2013, 442, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rytwo, G. Hybrid Clay-Polymer Nanocomposites for the Clarification of Water and Effluents. Recent Pat. Nanotechnol. 2017, 11, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Sukenik, A.; Kaplan, A. Cyanobacterial Harmful Algal Blooms in Aquatic Ecosystems: A Comprehensive Outlook on Current and Emerging Mitigation and Control Approaches. Microorganisms 2021, 9, 1472. [Google Scholar] [CrossRef] [PubMed]
- Keliri, E.; Paraskeva, C.; Sofokleous, A.; Sukenik, A.; Dziga, D.; Chernova, E.; Brient, L.; Antoniou, M.G. Occurrence of a single-species cyanobacterial bloom in a lake in Cyprus: Monitoring and treatment with hydrogen peroxide-releasing granules. Environ. Sci. Eur. 2021, 33, 31–44. [Google Scholar] [CrossRef]
- Cook, D.; Newcombe, G. Comparison and modeling of the adsorption of two microcystin analogues onto powdered activated carbon. Environ. Technol. 2008, 29, 525–534. [Google Scholar] [CrossRef]
- Westrick, J.A.; Szlag, D.C.; Southwell, B.J.; Sinclair, J. A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal. Bioanal. Chem. 2010, 397, 1705–1714. [Google Scholar] [CrossRef]
- Sukenik, A.; Viner-Mozzini, Y.; Mizrahi, D.; Tamam, I.; Benitez, A.R.; Nir, S. Removal of Cyanotoxins–Microcystins from Water by Filtration through Granulated Composites of Bentonite with Micelles of the Cation Octadecyltrimethyl Ammonium (ODTMA). Appl. Nano 2021, 2, 67–81. [Google Scholar] [CrossRef]
- Smith, M.J.; Shaw, G.R.; Eaglesham, G.K.; Ho, L.; Brookes, J.D. Elucidating the Factors Influencing the Biodegradation of Cylindrospermopsin in Drinking Water Sources. Environ. Toxicol. 2008, 23, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ho, L.; Lewis, D.M.; Brookes, J.D.; Newcombe, G. Discriminating and assessing adsorption and biodegradation removal mechanisms during granular activated carbon filtration of microcystin toxins. Water Res. 2007, 41, 4262–4270. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Meyn, T.; Keegan, A.; Hoefel, D.; Brookes, J.; Saint, C.P.; Newcombe, G. Bacterial degradation of microcystin toxins within a biologically active sand filter. Water Res. 2006, 40, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Rakovitsky, N.; Brook, I.; Van Rijn, J.; Ryskin, M.; Mkhweli, Z.; Etkin, H.; Nir, S. Purification of greywater by a moving bed reactor followed by a filter including a granulated micelle-clay composite. Appl. Clay Sci. 2016, 132, 267–272. [Google Scholar] [CrossRef]
- Kummel, M.L.; Shabtai, I.A.; Nir, S.; Mishael, Y.G. DOM removal from surface water by activated carbon vs. a nanocomposite: An experimental and modeling approach to optimize treatment. Environ. Sci. Water Res. Technol. 2023, 9, 1531–1544. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A. Removal of pharmaceutical compounds in water and wastewater using fungal oxidoreductase enzymes. Environ. Pollut. 2018, 234, 190–213. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, e136572. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.; Sanromán, M.A.; Moldes, D. Recent developments and applications of immobilized laccase. Biotechnol. Adv. 2013, 31, 1808–1825. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B 2002, 16, 231–240. [Google Scholar] [CrossRef]
- Astolfi, P.; Brandi, P.; Galli, C.; Gentili, P.; Gerini, M.F.; Greci, L.; Lanzalunga, O. New mediators for the enzyme laccase: Mechanistic features and selectivity in the oxidation of non-phenolic substrates. New J. Chem. 2005, 29, 1308–1317. [Google Scholar] [CrossRef]
- Hata, T.; Shintate, H.; Kawai, S.; Okamura, H.; Nishida, T. Elimination of carbamazepine by repeated treatment with laccase in the presence of 1-hydroxybenzotriazole. J. Hazard. Mater. 2010, 181, 1175–1178. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wu, Y.; Zou, B.; Lou, Q.; Zhang, W.; Zhong, J.; Lu, L.; Dai, G. Simultaneous removal and degradation characteristics of sulfonamide, tetracycline, and quinolone antibiotics by laccase-mediated oxidation coupled with soil adsorption. J. Hazard. Mater. 2016, 307, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–200. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-S.; Ku, K.-L.; Lai, H.-T. The implication of mediators for enhancement of laccase oxidation of sulfonamide antibiotics. Bioresour. Technol. 2012, 113, 259–264. [Google Scholar] [CrossRef]
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor. J. Biotechnol. 2009, 143, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Urase, T.; Kusakabe, O. Biodegradation characteristics of pharmaceutical substances by whole fungal culture Trametes versicolor and its laccase. J. Water Environ. Technol. 2010, 8, 125–140. [Google Scholar] [CrossRef]
- Li, X.; Alves de Toledo, R.; Wang, S.; Shim, H. Removal of carbamazepine and naproxen by immobilized Phanerochaete chrysosporium under non-sterile condition. New Biotechnol. 2015, 32, 282–289. [Google Scholar] [CrossRef]
- Jelic, A.; Cruz-Morato, C.; Marco-Urrea, E.; Sarrà, M.; Pérez, S.; Vicent, T.; Petrović, M.; Barceló, D. Degradation of carbamazepine by Trametes versicolor in an air pulsed fluidized bed bioreactor and identification of intermediates. Water Res. 2012, 46, 955–964. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of pharmaceuticals, steroid hormones, phytoestrogens, UV-filters, industrial chemicals and pesticide by Trametes versicolor: Role of biosorption and biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175. [Google Scholar] [CrossRef]
- Arca-Ramos, A.; Kumar, V.V.; Eibes, G.; Moreira, M.T.; Cabana, H. Recyclable cross-linked laccase aggregates coupled to magnetic silica microbeads for elimination of pharmaceuticals from municipal wastewater. Environ. Sci. Pollut. Res. 2016, 23, 8929–8939. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Hou, J.; Price, W.E.; Ghen, V.; Hai, F.I. Removal of trace organic contaminants by enzymatic membrane bioreactors: Role of membrane retention and biodegradation. J. Membr. Sci. 2020, 611, e118345. [Google Scholar] [CrossRef]
- Margot, J.; Copin, P.-J.; von Gunten, U.; Barry, D.A.; Holliger, C. Sulfamethoxazole and isoproturon degradation and detoxification by a laccase-mediator system: Influence of treatment conditions and mechanistic aspects. Biochem. Eng. J. 2015, 103, 47–59. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Magram, S.F.; Price, W.E.; Nghiem, L.D. Enhancement of trace organic contaminant degradation by crude enzyme extract from Trametes versicolor culture: Effect of mediator type and concentration. J. Taiwan Inst. Chem. Eng. 2014, 45, 1855–1862. [Google Scholar] [CrossRef]
- Barber, E.A.; Liu, Z.; Smith, S.R. Organic contaminant biodegradation by oxidoreductase enzymes in wastewater treatment. Microorganisms 2020, 8, 122. [Google Scholar] [CrossRef]
- Golan-Rozen, N.; Chefetz, B.; Ben-Ari, J.; Geva, J.; Hadar, Y. Transformation of the recalcitrant pharmaceutical compound carbamazepine by Pleurotus ostreatus: Role of cytochrome P450 monooxygenase and manganese peroxidase. Environ. Sci. Technol. 2011, 45, 6800–6805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geissen, S.-U. In vitro degradation of carbamazepine and diclofenac by crude lignin peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of carbamazepine by laccase-mediator system: Kinetics, by-products and toxicity assessment. Process Biochem. 2018, 67, 147–154. [Google Scholar] [CrossRef]
- Auriol, M.; Filali-Meknassi, U.; Adams, C.D.; Tyagi, R.J.; Noguerol, T.-N.; Piña, B. Removal of estrogenic activity of natural and synthetic hormones from a municipal wastewater: Efficiency of horseradish peroxidase and laccase from Trametes versicolor. Chemosphere 2008, 70, 445–452. [Google Scholar] [CrossRef]
- Montanez-Hurtado, E.; Pramparo, L. Degradation of a nonsteroidal anti-inflammatory drug using horseradish peroxidase enzyme. Res. J. Appl. Sci. 2018, 13, 425–430. [Google Scholar] [CrossRef]
- Camarillo-Ravelo, D.; Loera Corral, O.; González-Martínez, I.; Cupul, W.C.; Rodríguez Nava, C.O. Evaluation of bezafibrate, gemfibrozil, indomethacin, sulfamethoxazole, and diclofenac removal by ligninolytic enzymes. Prep. Biochem. Biotechnol. 2020, 50, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Jia, Y.; Li, J. Enzymatic degradation of tetracycline and oxytetracycline by curde manganese peroxidase prepared from Phanerochaete chrysosporium. J. Hazard. Mater. 2010, 177, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Demarche, P.; Enaud, E.; García-Morales, R.; Agathos, S.N.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Dey, P. Optimization of growth conditions for enhancing the production of microbial laccase and its application in treating antibiotic contamination in wastewater. 3 Biotech. 2021, 11, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.; Godjevargova, T.; Portaccio, M.; Lepore, M.; Mita, D.G. Advantages in using non-isothermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera. J. Mol. Catal. B 2008, 55, 177–184. [Google Scholar] [CrossRef]
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Matijošyte, I.; Arends, I.W.C.E.; de Vries, S.; Sheldon, R.A. Preparation and use of cross-linked enzyme aggregates (CLEAs) of laccases. J. Mol. Catal. B 2010, 62, 142–148. [Google Scholar] [CrossRef]
- Catapane, M.; Nicolucci, C.; Menale, C.; Mita, L.; Rossi, S.; Mita, D.G.; Diano, N. Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J. Hazard. Mater. 2013, 248–249, 337–346. [Google Scholar] [CrossRef]
- Cabana, H.; Alexandre, C.; Agathos, S.N.; Jones, J.P. Immobilization of laccase from the white rot fungus Coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresour. Technol. 2009, 100, 3447–3458. [Google Scholar] [CrossRef]
- de Cazes, M.; Belleville, M.-P.; Petit, E.; Llorca, M.; Rodríguez-Mozaz, S.; de Gunzburg, J.; Barceló, D.; Sánchez-Marcano, J. Design and optimization of an enzymatic membrane reactor for tetracycline degradation. Catal. Today 2014, 236, 146–152. [Google Scholar] [CrossRef]
- Becker, D.; della Giustina, S.V.; Rodriguez-Mozaz, S.; Shoevart, R.; Barceló, D.; de Cazes, M.; Belleville, M.-P.; Sánchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of antibiotics in wastewater by enzymatic treatment with fungal laccase-Degradation of compounds does not always eliminate toxicity. Bioresour. Technol. 2016, 219, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Cabana, H.; Jones, J.P.; Agathos, S.N. Utilization of cross-linked laccase aggregates in a perfusion basket reactor for the continuous elimination of endocrine-disrupting chemicals. Biotechnol. Bioeng. 2009, 102, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous adsorption and biotransformation of micropollutants by granular activated carbon-bound laccase in a packed-bed enzyme reactor. Bioresour. Technol. 2016, 210, 108–116. [Google Scholar] [CrossRef]
- Asif, M.; Hai, F.I.; Dhar, B.R.; Ngo, H.H.; Guo, W.; Jegatheesan, V.; Price, W.E.; Nghiem, L.D.; Yamamoto, K. Impact of simultaneous retention of micropollutants and laccase on micropollutant degradation in enzymatic membrane bioreactor. Bioresour. Technol. 2018, 267, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Escalona, I.; de Grooth, J.; Font, J.; Nijmeijer, K. Removal of BPA by enzyme polymerization using NF membranes. J. Membr. Sci. 2014, 468, 192–201. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; Pathak, N.; Zdarta, J.; Jesionowski, T.; Semblante, G.U.; Hai, F.I.; Nguyen, H.K.D.; Nghiem, L.D. A novel approach in crude enzyme laccase production and application in emerging contamination bioremediation. Processes 2020, 8, 648. [Google Scholar] [CrossRef]
- Lante, A.; Crapisi, A.; Krastanov, A.; Spettoli, P. Biodegradation of phenols by laccase immobilized in a membrane reactor. Process Biochem. 2000, 36, 51–58. [Google Scholar] [CrossRef]
- Moeder, M.; Martin, C.; Koeller, G. Degradation of hydroxylated compounds using laccase and horseradish peroxidase immobilized on microporous polypropylene hollow fiber membranes. J. Membr. Sci. 2004, 245, 183–190. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; McAdam, E.J.; Magram, S.F.; Nghiem, L.D. Continuous biotransformation of bisphenol A and diclofenac by laccase in an enzymatic membrane reactor. Int. Biodeterior. Biodegrad. 2014, 95, 25–32. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Vladisavljević, G.T. Biocatalytic membrane reactors. Phys. Sci. Rev. 2016, 1, 20150015. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wang, X.-M.; Yang, H.-W.; Xie, Y.F. Adsorption of pharmaceuticals onto isolated polyamide active layer of NF/RO membranes. Chemosphere 2018, 200, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hachi, M.; Chergui, A.; Yeddou, A.R.; Selatnia, A.; Cabana, H. Removal of acetaminophen and carbamazepine in single and binary systems with immobilized laccase from Trametes hirsuta. Biocatal. Biotransform. 2017, 35, 51–62. [Google Scholar] [CrossRef]
- Pylpchuk, I.; Kessler, V.G.; Seisenbaeva, G.A. Simultaneous removal of acetaminophen, diclofenac, and Cd (II) by Trametes versicolor laccase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain. Chem. Eng. 2018, 6, 9979–9989. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Price, W.E.; Leusch, F.D.L.; Roddick, F.; Ngo, H.H.; Guo, W.; Magram, S.F.; Nghiem, L.D. The effects of mediator and granular activated carbon addition on degradation of trace organic contaminants by an enzymatic membrane reactor. Bioresour. Technol. 2014, 167, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cui, B.-K.; Wu, X.-J.; Meng, G.; Liu, H.-X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Kumar, V.V.; Cabana, H. Towards high potential magnetic biocatalysts for on-demand elimination of pharmaceuticals. Bioresour. Technol. 2016, 200, 81–89. [Google Scholar] [CrossRef]
- de Cazes, M.; Belleville, M.P.; Mougel, M.; Kellner, H.; Sánchez-Marcano, J. Characterization of laccase-grafted ceramic membranes for pharmaceuticals degradation. J. Membrane Sci. 2015, 476, 384–393. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N.; Barceló, D. Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues—A review. Sci. Total Environ. 2019, 689, 160–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, J.; Liu, Z. Enhanced activity of immobilized or chemically modified enzymes. ACS Catal. 2015, 5, 4503–4513. [Google Scholar] [CrossRef]
- Carballares, D.; Rocha-Martín, J.; Fernández-Lafuente, R. Preparation of a six-enzyme multilayer combi-biocatalyst: Reuse of the most stable enzymes after inactivation of the least stable one. ACS Sustain. Chem. Eng. 2022, 10, 3920–3934. [Google Scholar] [CrossRef]
- Santiago-Arcos, J.; Velasco-Lozano, S.; Lopez-Gallego, F. Multienzyme Coimmobilization on Triheterofunctional Supports. Biomacromolecules 2023, 24, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.C.; Nidhi, C.; Tripathi, B.P. Nature inspired multienzyme immobilization: Strategies and concepts. ACS Appl. Bio Mater. 2021, 4, 1077–1114. [Google Scholar] [CrossRef]
- Mehandia, S.; Ahmad, S.; Sharma, S.C.; Arya, S.K. Decolorization and detoxification of textile effluent by immobilized laccase-ACS into chitosan-clay composite beads using a packed bed reactor system: An ecofriendly approach. J. Water Process Eng. 2022, 47, 102662. [Google Scholar] [CrossRef]

| kd = 0 | kd = 0.01 min−1 | kd = 0.001 min−1 | ||||
|---|---|---|---|---|---|---|
| Time (h) | Volume (L) | C/C0 | C/C0 | % degrad. | C/C0 | % degrad. |
| 1 | 1.2 | 0.025 | 0.025 | 18 | 0.025 | 2.1 |
| 5 | 6 | 0.075 | 0.039 | 63 | 0.068 | 12.2 |
| 10 | 12 | 0.16 | 0.041 | 78.7 | 0.123 | 22.5 |
| 24 | 28.8 | 0.45 | 0.041 | 88.7 | 0.232 | 41 |
| 48 | 57.6 | 0.83 | 0.041 | 92.3 | 0.284 | 54.6 |
| 96 | 115 | 0.993 | 0.041 | 93 | 0.289 | 62.8 |
| 120 | 144 | 1 | 0.041 | 94.5 | 0.289 | 64.5 |
| Time (h) | Volume (L) | Input Mole | Sorbed (Mole) | Sorbed (%) | Degraded (%) | C/C0 |
|---|---|---|---|---|---|---|
| 1 | 1.2 | 6 × 10−6 | 3.46 × 10−6 | 57.7 | 18.7 | 0.228 |
| 5 | 6 | 3 × 10−5 | 7.17 × 10−6 | 23.9 | 52 | 0.249 |
| 10 | 12 | 6 × 10−5 | 7.48 × 10−6 | 12.4 | 63 | 0.251 |
| 24 | 28.8 | 1.44 × 10−4 | 7.49 × 10−6 | 5.2 | 69.9 | 0.251 |
| 48 | 57.6 | 2.88 × 10−4 | 7.49 × 10−6 | 2.6 | 72.4 | 0.251 |
| 96 | 115.2 | 5.76 × 10−4 | 7.49 × 10−6 | 1.3 | 73.7 | 0.251 |
| % MIB Emerging from Filter | |||||
|---|---|---|---|---|---|
| Experimental | Calculated | MIB Degraded | |||
| Time | +Azide | −Azide | +Azide | −Azide | Calc. (%) |
| 1 h | 33.1 ± 1.7 | 30.6 | 18.7 | 18.2 | 27.7 |
| 2 h | NA b | NA | 22.3 | 18.9 | 48.0 |
| 10 h | NA | NA | 48.2 | 19.0 | 73.0 |
| 24 h | 77.8 ± 1.2 | 19.5 ± 0.9 | 77.8 | 19.0 | 78.0 |
| 48 h | 96.5 ± 4.0 | 20.3 ± 1.0 | 96.8 | 19.0 | 79.4 |
| 96 h | 100 ± 0.1 | 18.9 ± 0.9 | 99.9 | 19.0 | 80.1 |
| 168 h | 100 ± 0.1 | 19.5 ± 0.7 | 100 | 19.0 | 80.4 |
| 2 w | - | 17.4 ± 0.6 | - | 19.0 | 80.7 |
| 3 w | - | 18.0 ± 1.1 | - | 19.0 | 80.8 |
| 4 w | - | 15.1 ± 1.9 | - | 19.0 | 80.8 |
| 5 w | - | 17.8 ± 0.5 | - | 19.0 | |
| 6 w | - | 19.7 ± 0.3 | - | 19.0 | |
| Time (d) | C/C0 | Emerging Concentration, µg L−1 |
|---|---|---|
| 2 | 0 | 0 |
| 3 | 0.045 | 0.03 |
| 5 | 0.109 | 0.5 |
| 6 | 0.209 | 1.04 |
| 8 | 0.476 | 2.4 |
| 10 | 0.729 | 3.6 |
| 16 | 0.988 | 4.9 |
| Time (d) | C/C0 | Emerging Concentration (µg L−1) | Percent Degradation (Cumulative) |
|---|---|---|---|
| 2 | 0.00084 | 0.0047 | 37 |
| 4 | 0.008 | 0.04 | 56.9 |
| 5 | 0.014 | 0.07 | 63.4 |
| 6 | 0.02 | 0.1 | 68.3 |
| 10 | 0.03 | 0.15 | 79.4 |
| 20 | 0.031 | 0.155 | 88.1 |
| 34 | 0.031 | 0.155 | 91.4 |
| Time (d) | C/C0 | Emerging Concentration (µg L−1) | Percent Degradation (Cumulative) |
|---|---|---|---|
| 1 | 0 | 0 | 21.4 |
| 14 | 8.3 × 10−4 | 0.041 | 85.8 |
| 18 | 9.6 × 10−4 | 0.048 | 88.9 |
| 24 | 9.8 × 10−4 | 0.049 | 91.7 |
| 30 | 9.8 × 10−4 | 0.049 | 93.3 |
| 34 | 9.8 × 10−4 | 0.049 | 94.1 |
| Enzyme (Producer) | EC(s) 1 | EC Concentration | Enzyme Concentration | Degradation (%) | Reference |
|---|---|---|---|---|---|
| Crude MnP (Pleurotus ostreatus) | CBZ | 10 mg/L | 7.7 U/L | 99.7 | [68] |
| Crude LiP (Phanerochaete chrysosporium) | CBZ, DC | 5 mg/L 5 mg/L | 180 U/L | 100 (pH 3.0–4.5) | [69] |
| Laccase (Trametes versicolor) | CBZ | 1 mg/L | 60 U/L | 95 | [70] |
| HRP laccase (Trametes versicolor) | Steroid estrogen mixture (EE, E2, E3) | 100 ng/L of each | 8 × 103–10 × 103 U/L 20 × 103 U/L | 100 100 | [71] |
| HRP | DC | 25 mg/L | 6.4 × 103 U/L | 47 | [72] |
| Crude MnP (Trametes maxima) | DC SMX IND GFB BZF | 2 mg/L of each | 387.6 ± 67.4 U/mg | 90.2 72.6 60.8 43.4 32.6 | [73] |
| Crude MnP (Phanerochaete chrysosporium) | TC OTC | 50 mg/L | 40 U/L | 72.5 84.3 | [74] |
| Laccase (Pycnoporus sanguineus CS43) | NP TCS | 10 mg/L | 100 U/L | 94 92 | [75] |
| Laccase (Pleurotus eryngii) | LV | 5 mg/L | 2 × 103 U/L | 97 | [76] |
| Enzymatic System (Source) | Type of Filtration (Mode) | EC(s) a | Enzyme Immobilization Method (Support) | Reference |
|---|---|---|---|---|
| Laccase (Trametes versicolor) | Fluid bed (continuous recirculation) | OP, NP | Covalent (polyacrylonitrile beads) | [80] |
| Laccase (Coriolopsis polizona) | Packed bed bioreactor (dead-end) | TCS, BPA, NP | Covalent (diatomaceous earth) | [81] |
| Laccase (Trametes versicolor) | EMR (cross-flow) | TC | Covalent (ceramic membrane) | [82] |
| Laccase (Trametes versicolor) | EMR (cross-flow) | Antibiotics: 12 SFAs, 6 PN, 10 FQ, 4 QN, 4 TCs | Covalent (ceramic membrane) | [83] |
| Laccase (Coriolopsis polizona) | Fluid bed (perfusion basket reactor, dead-end) | BPA, TCS, NP | CLEAs | [84] |
| Laccase (genetically modified Aspergillus oryzae) | Packed bed bioreactor (dead-end) | SMX, CBZ, DC, BPA | Adsorption (GAC) | [85] |
| Whole fungal cells and extracellular enzymes (P. chrysosporium) | Packed bed bioreactor (dead-end) | NPX, CBZ | Adsorption (wood chips) | [60] |
| Laccase (genetically modified Aspergillus oryzae) | EMR (cross-flow) | CBZ, DC, SMX, AT | - | [62] |
| Laccase (genetically modified Aspergillus oryzae) | EMR (cross flow) | 17 non-phenolic and 12 phenolic TrOCs | - | [64] |
| Laccase (genetically modified Aspergillus oryzae) | EMR (cross flow) | CBZ, SMX, DC, AT, OXY | - | [86] |
| Laccase (T. versicolor), HRP | EMR (cross flow) | BPA | - | [87] |
| Crude enzyme laccase (Pleurotus ostreatus) | EMR (cross flow) | 30 ECs of which 11 were PhAcs | - | [88] |
| Laccase (P. oryzae) | EMR (cross flow) | 17 phenols | Physical entrapment | [89] |
| HRP, laccase (Rhus vernificera) | EMR (cross flow) | 5 hydroxylated compounds | Physical entrapment | [90] |
| Laccase (genetically modified A. oryzae) | EMR, (cross flow) | BPA, DC | - | [91] |
| Mycelial pellets (Trametes versicolor) | Fluid bed bioreactor (dead-end) | CBZ | - | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Undabeytia, T.; Jiménez-Barrera, J.M.; Nir, S. Removal of Emerging Contaminants by Degradation during Filtration: A Review of Experimental Procedures and Modeling. Water 2024, 16, 110. https://doi.org/10.3390/w16010110
Undabeytia T, Jiménez-Barrera JM, Nir S. Removal of Emerging Contaminants by Degradation during Filtration: A Review of Experimental Procedures and Modeling. Water. 2024; 16(1):110. https://doi.org/10.3390/w16010110
Chicago/Turabian StyleUndabeytia, Tomás, José Manuel Jiménez-Barrera, and Shlomo Nir. 2024. "Removal of Emerging Contaminants by Degradation during Filtration: A Review of Experimental Procedures and Modeling" Water 16, no. 1: 110. https://doi.org/10.3390/w16010110







