Responses of Different Plant Taxonomic Groups to Complex Environmental Factors in Peri-Urban Wetlands
Abstract
:1. Introduction
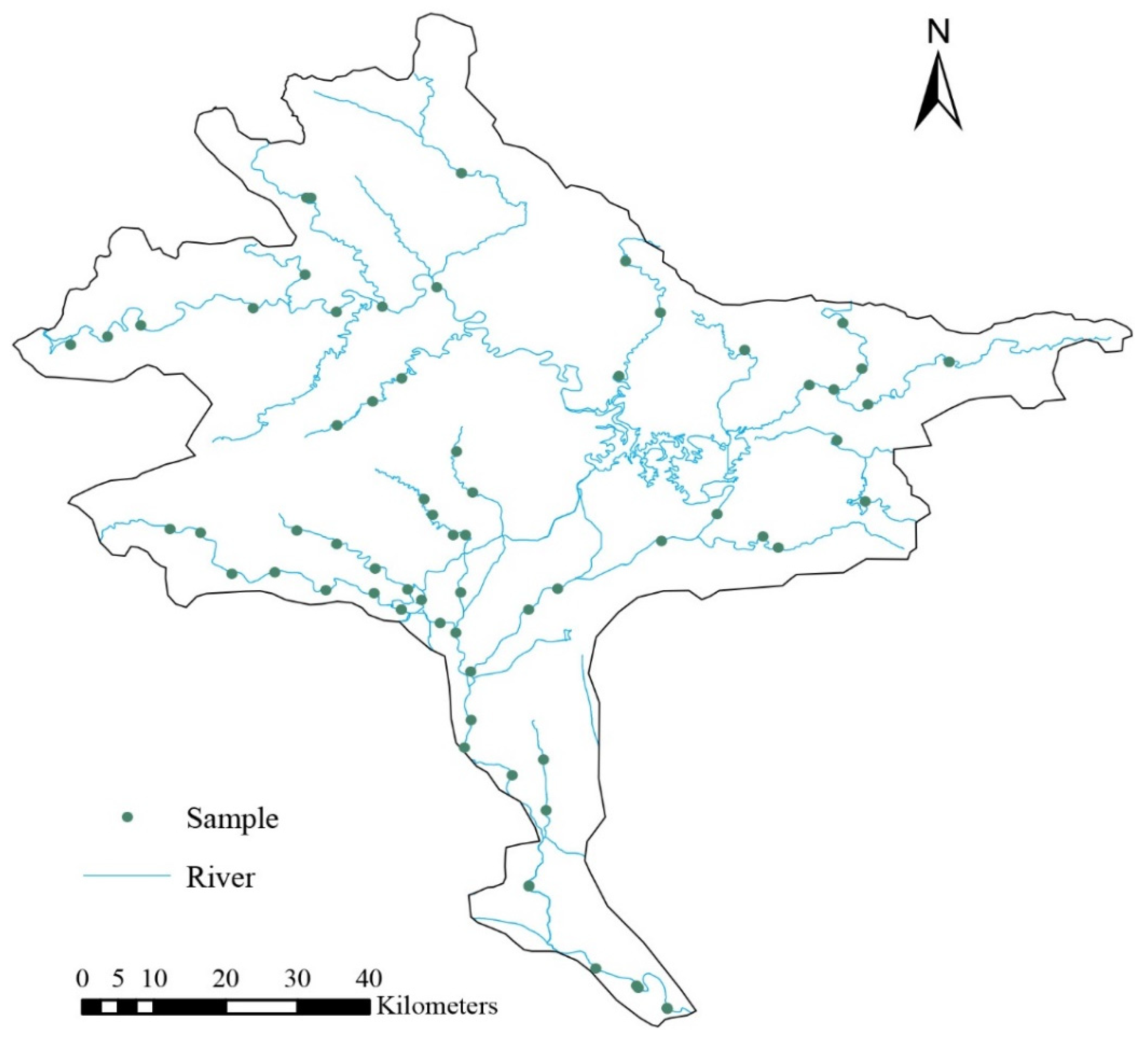
2. Materials and Methods
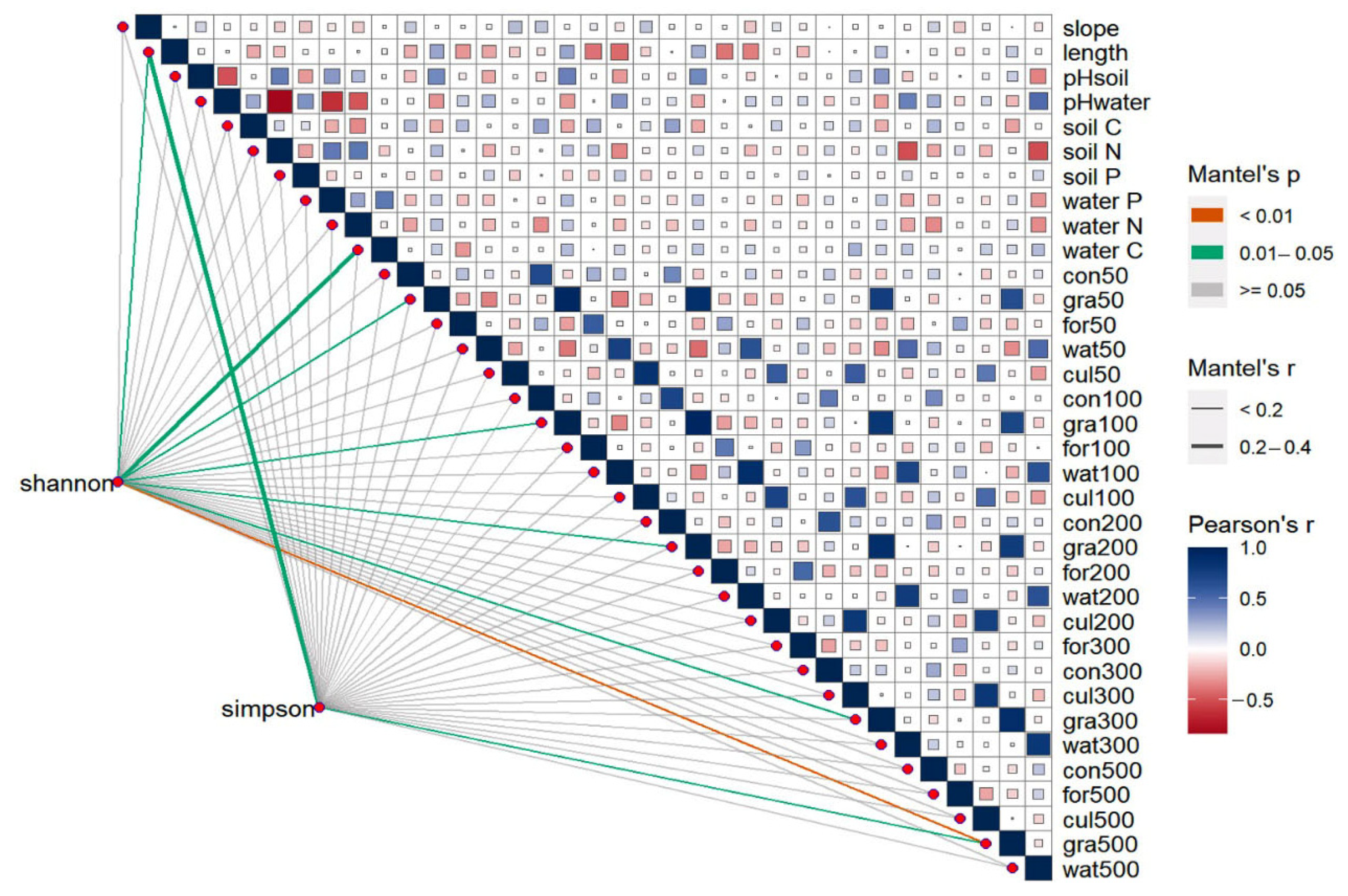
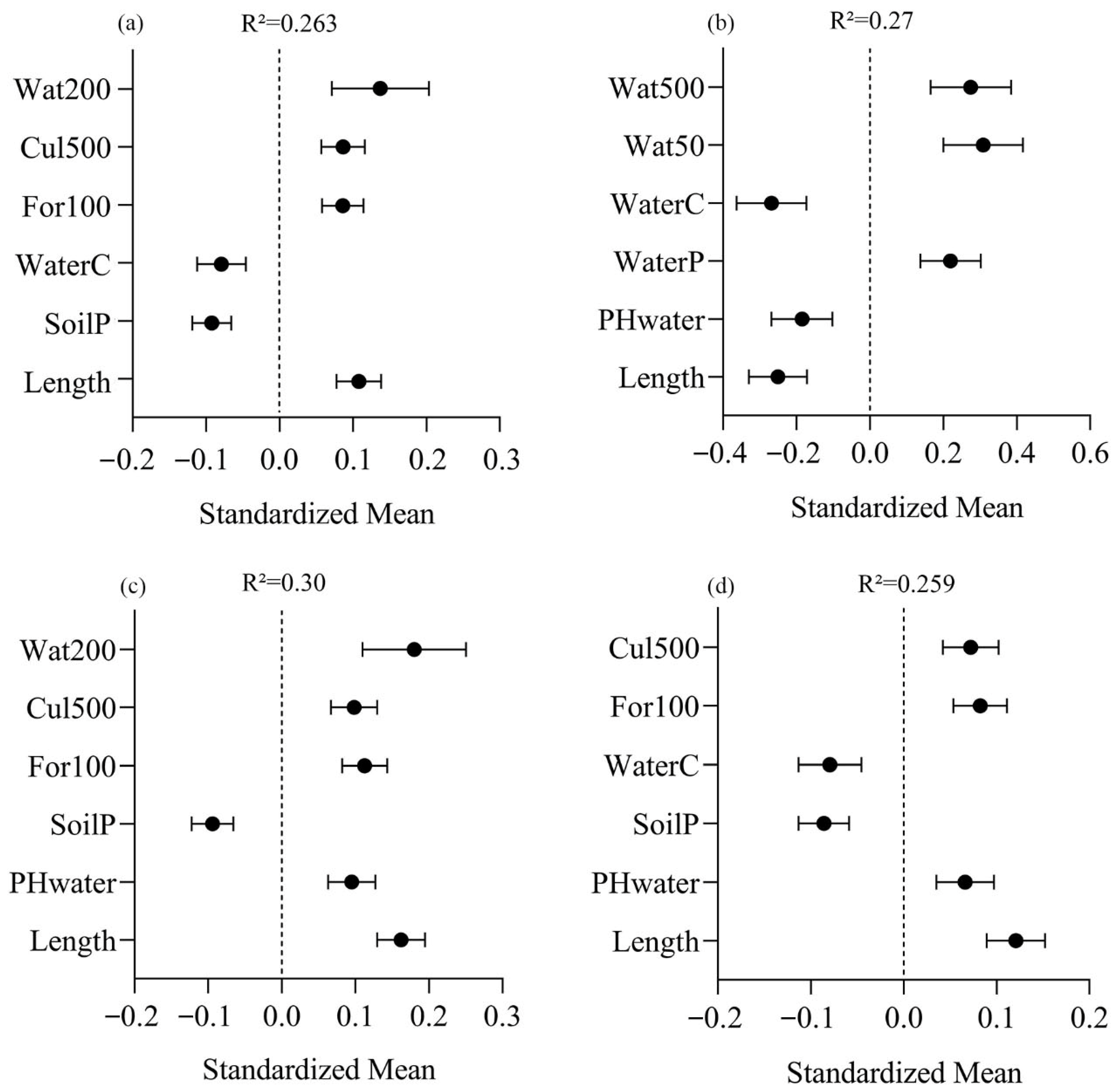
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Jiao, L.; Wu, X.; Du, D.; Wu, J.; Zhang, P. Ecosystem health assessment and comparison of natural and constructed wetlands in the arid zone of northwest China. Ecol. Indic. 2023, 154, 110576. [Google Scholar] [CrossRef]
- Cao, L.; Fox, A.D. Birds and people both depend on China’s wetlands. Nature 2009, 460, 173. [Google Scholar] [CrossRef] [PubMed]
- Fluet-Chouinard, E.; Stocker, B.D.; Zhang, Z.; Malhotra, A.; Melton, J.R.; Poulter, B.; Kaplan, J.O.; Goledwijck, K.K.; Siebert, S.; Minayeva, T.; et al. Extensive global wetland loss over the past three centuries. Nature 2023, 614, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Van Asselen, S.; Verburg, P.H.; Vermaat, J.E.; Janse, J.H. Drivers of Wetland Conversion: A Global Meta-Analysis. PLoS ONE 2013, 8, e81292. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ziegler, A.D.; Chen, D.; McNicol, G.; Ciais, P.; Jiang, X.; Zheng, K.; Wu, J.; Lin, X.; He, X.; et al. Rewetting global wetlands effectively reduces major greenhouse gas emissions. Nat. Geosci. 2022, 15, 627–632. [Google Scholar] [CrossRef]
- King, C.M.; Hovick, S.M. Wetland plant community variation across replicate urban to rural gradients: Non-native species as both drivers and passengers in systems impacted by anthropogenic land-use. Urban Ecosyst. 2020, 23, 1209–1226. [Google Scholar] [CrossRef]
- Andrew, S.M.; Totland, Ø.; Moe, S.R. Spatial variation in plant species richness and diversity along human disturbance and environmental gradients in a tropical wetland. Wetl. Ecol. Manag. 2015, 23, 395–404. [Google Scholar] [CrossRef]
- Bowman Cutway, H.; Ehrenfeld, J.G. The influence of urban land use on seed dispersal and wetland invasibility. Plant Ecol. 2010, 210, 153–167. [Google Scholar] [CrossRef]
- Campion, B.B.; Venzke, J.-F. Spatial patterns and determinants of wetland vegetation distribution in the Kumasi Metropolis, Ghana. Wetl. Ecol. Manag. 2011, 19, 423–431. [Google Scholar] [CrossRef]
- Perron, M.A.C.; Pick, F.R. Stormwater ponds as habitat for Odonata in urban areas: The importance of obligate wetland plant species. Biodivers. Conserv. 2020, 29, 913–931. [Google Scholar] [CrossRef]
- Wondie, A. Ecological conditions and ecosystem services of wetlands in the Lake Tana Area, Ethiopia. Ecohydrol. Hydrobiol. 2018, 18, 231–244. [Google Scholar] [CrossRef]
- Larson, M.A.; Heintzman, R.L.; Titus, J.E.; Zhu, W. Urban Wetland Characterization in South-Central New York State. Wetlands 2016, 36, 821–829. [Google Scholar] [CrossRef]
- van Wyk, E.; Cilliers, S.S.; Bredenkamp, G.J. Vegetation analysis of wetlands in the Klerksdorp Municipal Area, North West Province, South Africa. S. Afr. J. Bot. 2000, 66, 52–62. [Google Scholar] [CrossRef]
- Asongwe, G.A.; Bame, I.B.; Ndam, L.M.; Orock, A.E.; Tellen, V.A.; Bumtu, K.P.; Tening, A.S. Influence of urbanisation on phytodiversity and some soil properties in riverine wetlands of Bamenda municipality, Cameroon. Sci. Rep. 2022, 12, 19766. [Google Scholar] [CrossRef] [PubMed]
- Magee, T.K.; Ernst, T.L.; Kentula, M.E.; Dwire, K.A. Floristic comparison of freshwater wetlands in an urbanizing environment. Wetlands 1999, 19, 517–534. [Google Scholar] [CrossRef]
- Du Toit, M.J.; Du Preez, C.; Cilliers, S.S. Plant diversity and conservation value of wetlands along a rural–urban gradient. Bothalia ABC 2021, 51, 1–18. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Li, G.; Qi, W. Negative effects of urbanization on plants: A global meta-analysis. Ecol. Evol. 2023, 13, e9894. [Google Scholar] [CrossRef]
- Wandl, A.; Magoni, M. Sustainable Planning of Peri-Urban Areas: Introduction to the Special Issue. Plan. Pract. Res. 2017, 32, 1–3. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban biodiversity: Patterns and mechanisms: Urban biodiversity. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; Rodríguez-Correa, H.A.; MacGregor-Fors, I. Trees and the City: Diversity and Composition along a Neotropical Gradient of Urbanization. Int. J. Ecol. 2011, 2011, 704084. [Google Scholar] [CrossRef]
- Tian, Z.; Song, K.; Da, L. Distribution patterns and traits of weed communities along an urban-rural gradient under rapid urbanization in Shanghai, China: Weed communities on urban-rural gradient. Weed Biol. Manag. 2015, 15, 27–41. [Google Scholar] [CrossRef]
- Wanek, A.; Hargiss, C.L.M.; Norland, J. Hydric vegetation communities across rural, peri-urban, and urban zones within the Prairie Pothole Region. Urban For. Urban Green. 2022, 70, 127539. [Google Scholar] [CrossRef]
- Mao, D.; Wang, Z.; Wu, J.; Wu, B.; Zeng, Y.; Song, K.; Luo, L. China’s wetlands loss to urban expansion. Land Degrad. Dev. 2018, 29, 2644–2657. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Q.; You, Y.; Zhang, K.; Gao, C.; Peng, Y. Compositional and structural characteristics of dissolved organic matter in overlying water of the Chaobai River and its environment significance. Environ. Sci. Pollut. Res. 2021, 28, 59673–59686. [Google Scholar] [CrossRef]
- Geng, B.; Cao, Y.; Su, R.; Liu, S.; Feng, Z. Influence of land-use change on ecosystem services in the Chaobai River region of Beijing-Tianjin-Hebei. J. Agric. Resour. Environ. 2020, 37, 583–593. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Huang, H.; Zhou, L.; Chen, J.; Wei, T. ggcor: Extended Tools for Correlation Analysis and Visualization; R Package Version 0.9.7; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Morandeira, N.S.; Kandus, P. Do taxonomic, phylogenetic and functional plant α- and β-diversity reflect environmental patterns in the Lower Paraná River floodplain? Plant Ecol. Divers. 2017, 10, 153–165. [Google Scholar] [CrossRef]
- Schouten, M.A.; Verweij, P.A.; Barendregt, A.; Kleukers, R.M.J.C.; Kalkman, V.J.; De Ruiter, P.C. Determinants of species richness patterns in the Netherlands across multiple taxonomic groups. Biodivers. Conserv. 2009, 18, 203–217. [Google Scholar] [CrossRef]
- Ding, Y.; Zang, R. Determinants of aboveground biomass in forests across three climatic zones in China. For. Ecol. Manag. 2021, 482, 118805. [Google Scholar] [CrossRef]
- Loewenstein, N.J.; Loewenstein, E.F. Non-native plants in the understory of riparian forests across a land use gradient in the Southeast. Urban Ecosyst. 2005, 8, 79–91. [Google Scholar] [CrossRef]
- Houlahan, J.E.; Keddy, P.A.; Makkay, K.; Findlay, C.S. The effects of adjacent land use on wetland species richness and community composition. Wetlands 2006, 26, 79–96. [Google Scholar] [CrossRef]
- Burton, M.L.; Samuelson, L.J. Influence of urbanization on riparian forest diversity and structure in the Georgia Piedmont, US. Plant Ecol. 2008, 195, 99–115. [Google Scholar] [CrossRef]
- Moges, A.; Beyene, A.; Ambelu, A.; Mereta, S.T.; Triest, L.; Kelbessa, E. Plant species composition and diversity in wetlands under forest, agriculture and urban land uses. Aquat. Bot. 2017, 138, 9–15. [Google Scholar] [CrossRef]
- Yuan, Z.; Xiao, M.; Su, X.; Zhao, H.; Li, Y.; Zhang, H.; Wang, W. Effects of Environment and Human Activities on Plant Diversity in Wetlands along the Yellow River in Henan Province, China. Diversity 2022, 14, 470. [Google Scholar] [CrossRef]
- von Behren, C.; Dietrich, A.; Yeakley, J.A. Riparian vegetation assemblages and associated landscape factors across an urbanizing metropolitan area. Écoscience 2013, 20, 373–382. [Google Scholar] [CrossRef]
- Lyons, M.N. The riparian flora and plant communities of the Pilbara region of Western Australia. Rec. West Aust. Mus. Sup. 2015, 78, 485. [Google Scholar] [CrossRef]
- Kallimanis, A.S.; Mazaris, A.D.; Tzanopoulos, J.; Halley, J.M.; Pantis, J.D.; Sgardelis, S.P. How does habitat diversity affect the species–area relationship? Glob. Ecol. Biogeogr. 2008, 17, 532–538. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Exotic invasive species in urban wetlands: Environmental correlates and implications for wetland management. J. Appl. Ecol. 2008, 45, 1160–1169. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Alston, K.P.; Richardson, D.M. The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biol. Conserv. 2006, 132, 183–198. [Google Scholar] [CrossRef]
- Skultety, D.; Matthews, J.W. Human land use as a driver of plant community composition in wetlands of the Chicago metropolitan region. Urban Ecosyst. 2018, 21, 447–458. [Google Scholar] [CrossRef]
- Chu, S.; Molano-Flores, B. Impacts of agricultural to urban land-use change on floristic quality assessment indicators in Northeastern Illinois wetlands. Urban Ecosyst. 2013, 16, 235–246. [Google Scholar] [CrossRef]
- Stefanidis, K.; Oikonomou, A.; Papastergiadou, E. Responses of different facets of aquatic plant diversity along environmental gradients in Mediterranean streams: Results from rivers of Greece. J. Environ. Manag. 2021, 296, 113307. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of aquatic plants to abiotic factors: A review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Franklin, H.M.; Robinson, B.H.; Dickinson, N.M. Plants for nitrogen management in riparian zones: A proposed trait-based framework to select effective species. Ecol. Manag. Restor. 2019, 20, 202–213. [Google Scholar] [CrossRef]
- Panuccio, M.; Foschi, F.; Audinet, J.-P.; Calò, C.; Bologna, M. Urban wetlands: Wastelands or hotspots for conservation? Two case studies from Rome, Italy. Avocetta 2017, 41, 13–18. [Google Scholar] [CrossRef]
- Gleason, H.A. The Individualistic Concept of the Plant Association. Bull. Torrey Bot. Club 1926, 53, 7–26. [Google Scholar] [CrossRef]
- Uddin, M.N.; Robinson, R.W. Can nutrient enrichment influence the invasion of Phragmites australis? Sci. Total Environ. 2018, 613–614, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.M.; Walbridge, M.R. Urbanization And Nutrient Retention In Freshwater Riparian Wetlands. Ecol. Appl. 2007, 17, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Nsor, C.A.; Acolatse, R.; Mensah, J.N.; Oppong, S.K.; Dompreh, D.; Addai-Wireko, L. Structural assemblages of plant species in the Owabi Ramsar Wetland in the Ashanti Region of Ghana. Afr. J. Aquat. Sci. 2022, 47, 100–114. [Google Scholar] [CrossRef]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Colbert, M.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Haase, D.; Hamman, M.; Hillel, O.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]

| Abbreviation | Variable |
|---|---|
| Slope | Slope |
| Length | Length of the riparian zone |
| pHsoil | Soil pH |
| pHwater | Water pH |
| soil C | Soil organic matter |
| soil N | Total soil nitrogen |
| soil P | Total soil phosphorus |
| water C | Water organic matter |
| water N | Total water nitrogen |
| water P | Total water phosphorus |
| con50 | 0–50 m construction land area |
| con100 | 0–100 m construction land area |
| con200 | 0–200 m construction land area |
| con300 | 0–300 m construction land area |
| con500 | 0–500 m construction land area |
| gra50 | 0–50 m green land area |
| gra100 | 0–100 m green land area |
| gra200 | 0–200 m green land area |
| gra300 | 0–300 m green land area |
| gra500 | 0–500 m green land area |
| for50 | 0–50 m forestland area |
| for100 | 0–100 m forestland area |
| for200 | 0–200 m forestland area |
| for300 | 0–300 m forestland area |
| for500 | 0–500 m forestland area |
| cul50 | 0–50 m cultivated land area |
| cul100 | 0–100 m cultivated land area |
| cul200 | 0–200 m cultivated land area |
| cul300 | 0–300 m cultivated land area |
| cul500 | 0–500 m cultivated land area |
| wat50 | 0–50 m wetland area |
| wat100 | 0–100 m wetland area |
| wat200 | 0–200 m wetland area |
| wat300 | 0–300 m wetland area |
| wat500 | 0–500 m wetland area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Li, J.; Li, G.; Qi, W.; Jin, T.; Wang, Y. Responses of Different Plant Taxonomic Groups to Complex Environmental Factors in Peri-Urban Wetlands. Water 2024, 16, 46. https://doi.org/10.3390/w16010046
Hou Y, Li J, Li G, Qi W, Jin T, Wang Y. Responses of Different Plant Taxonomic Groups to Complex Environmental Factors in Peri-Urban Wetlands. Water. 2024; 16(1):46. https://doi.org/10.3390/w16010046
Chicago/Turabian StyleHou, Yuchen, Junsheng Li, Guo Li, Wei Qi, Tao Jin, and Ying Wang. 2024. "Responses of Different Plant Taxonomic Groups to Complex Environmental Factors in Peri-Urban Wetlands" Water 16, no. 1: 46. https://doi.org/10.3390/w16010046





