Coupled In-Situ Fermentation for Enhanced Biological Phosphorus Removal from Digested Swine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setup of the Laboratory-Scale Reactors
2.2. Real Digested Swine Wastewater and Seed Sludge
2.3. Operational Procedure of the Sequencing Batch Reactors
2.4. Analysis of the Digested Swine Wastewater Quality
2.5. Characterization of Polyphosphate-Accumulating Organisms
2.6. Data Analysis and Reporting
3. Results and Discussion
3.1. Wastewater Treatment Performance of the Two Sequencing Batch Reactors
3.1.1. COD and Nitrogen Removal
3.1.2. Phosphorus Removal
3.1.3. Cycle Performance Study
3.2. Metabolic Activity and Kinetic Assessment for Enhanced Biological Phosphorus Removal
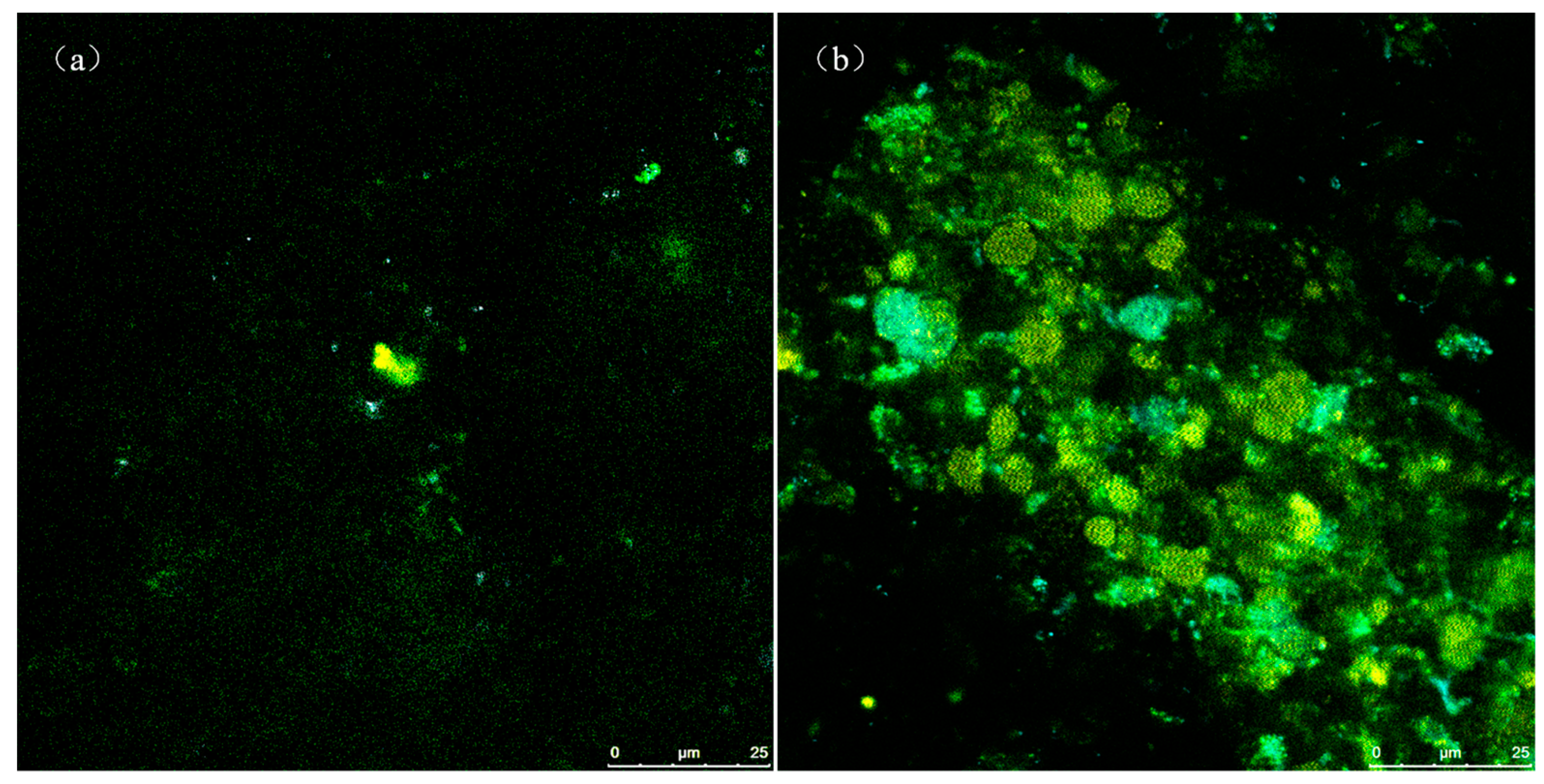
3.3. Microbial Community of Phosphate-Accumulating Organisms
3.4. Environmental Implications of This Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Yang, H.; Yang, H.; Wang, H.; Zheng, D.; Liu, Y.; Pu, X.; Deng, L. Improved biogas production of dry anaerobic digestion of swine manure. Bioresour. Technol. 2019, 294, 122188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.-S.; Zhao, J.-L.; Liu, W.-R.; Chen, J.; Zhang, Q.-Q.; He, L.-Y.; Ying, G.-G. Variations of antibiotic resistome in swine wastewater during full-scale anaerobic digestion treatment. Environ. Int. 2021, 155, 106694. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Ye, C.; Xiong, Y.; Xiang, J.; Wang, F. Simultaneous removal of ammonia, P and COD from anaerobically digested piggery wastewater using an integrated process of chemical precipitation and air stripping. J. Hazard. Mater. 2010, 178, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Choi, W.J.; Chae, A.N.; Park, J.; Kim, H.J.; Song, K.G. Evaluating integrated strategies for robust treatment of high saline piggery wastewater. Water Res. 2016, 89, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zheng, D.; Wang, S.; Wang, L.; Lei, Y.; Xu, Z.; Deng, L. Remedying acidification and deterioration of aerobic post-treatment of digested effluent by using zero-valent iron. Bioresour. Technol. 2018, 247, 477–485. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Qudah, Y.; Assirey, E. Combined biological wastewater treatment with electrocoagulation as a post-polishing process: A review. Sep. Sci. Technol. 2020, 55, 2334–2352. [Google Scholar] [CrossRef]
- Song, Y.-H.; Qiu, G.-L.; Yuan, P.; Cui, X.-Y.; Peng, J.-F.; Zeng, P.; Duan, L.; Xiang, L.-C.; Qian, F. Nutrients removal and recovery from anaerobically digested swine wastewater by struvite crystallization without chemical additions. J. Hazard. Mater. 2011, 190, 140–149. [Google Scholar] [CrossRef]
- Wang, T.; Ni, Z.; Kuang, B.; Zhou, L.; Chen, X.; Lin, Z.; Guo, B.; Zhu, G.; Jia, J. Two-stage hybrid microalgal electroactive wetland-coupled anaerobic digestion for swine wastewater treatment in South China: Full-scale verification. Sci. Total Environ. 2022, 820, 153312. [Google Scholar] [CrossRef]
- Wang, M.; Yang, H.; Ergas, S.J.; van der Steen, P. A novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo-sequencing batch reactor (PSBR). Water Res. 2015, 87, 38–48. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578. [Google Scholar] [CrossRef]
- Dan, N.H.; Rene, E.R.; Le Luu, T. Removal of nutrients from anaerobically digested swine wastewater using an intermittent cycle extended aeration system. Front. Microbiol. 2020, 11, 576438. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Deng, L.; Zheng, D.; Wang, L.; Liu, Y. Separation of swine wastewater into different concentration fractions and its contribution to combined anaerobic–aerobic process. J. Environ. Manag. 2016, 168, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Xue, T.; Li, B.; Dai, X.; Peng, Y. Treating low carbon/nitrogen (C/N) wastewater in simultaneous nitrification-endogenous denitrification and phosphorous removal (SNDPR) systems by strengthening anaerobic intracellular carbon storage. Water Res. 2015, 77, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, J.; He, X.; He, L.; Lin, Z.; Shi, S.; Zhou, J. Simultaneous nitrogen and phosphorus removal from simulated digested piggery wastewater in a single-stage biofilm process coupling anammox and intracellular carbon metabolism. Bioresour. Technol. 2021, 333, 125152. [Google Scholar] [CrossRef] [PubMed]
- Izadi, P.; Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Wang, J.; Xia, L.; Chen, J.; Wang, X.; Wu, H.; Li, D.; Wells, G.F.; Yang, J.; Hou, J.; He, X. Synergistic simultaneous nitrification-endogenous denitrification and EBPR for advanced nitrogen and phosphorus removal in constructed wetlands. Chem. Eng. J. 2021, 420, 127605. [Google Scholar] [CrossRef]
- Srinivasan, V.N.; Li, G.; Wang, D.; Tooker, N.B.; Dai, Z.; Onnis-Hayden, A.; Bott, C.; Dombrowski, P.; Schauer, P.; Pinto, A. Oligotyping and metagenomics reveal distinct Candidatus Accumulibacter communities in side-stream versus conventional full-scale enhanced biological phosphorus removal (EBPR) systems. Water Res. 2021, 206, 117725. [Google Scholar] [CrossRef]
- Mino, T.; Van Loosdrecht, M.; Heijnen, J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, W.; Meng, Q.; Fan, Z.; Peng, Y. Phosphorus removal performance, intracellular metabolites and clade-level community structure of Tetrasphaera-dominated polyphosphate accumulating organisms at different temperatures. Sci. Total Environ. 2022, 842, 156913. [Google Scholar] [CrossRef]
- Stokholm-Bjerregaard, M.; McIlroy, S.J.; Nierychlo, M.; Karst, S.M.; Albertsen, M.; Nielsen, P.H. A critical assessment of the microorganisms proposed to be important to enhanced biological phosphorus removal in full-scale wastewater treatment systems. Front. Microbiol. 2017, 8, 718. [Google Scholar] [CrossRef]
- Nielsen, P.H.; McIlroy, S.J.; Albertsen, M.; Nierychlo, M. Re-evaluating the microbiology of the enhanced biological phosphorus removal process. Curr. Opin. Biotechnol. 2019, 57, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, R.; Nguyen, H.T.T.; Saunders, A.M.; Nielsen, J.L.; Wimmer, R.; Le, V.Q.; McIlroy, S.J.; Petrovski, S.; Seviour, R.J.; Calteau, A. A metabolic model for members of the genus Tetrasphaera involved in enhanced biological phosphorus removal. ISME J. 2013, 7, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Zhang, F.; Yang, H.; Lu, J.; Ge, S.; Li, X.; Zhang, W. Tetrasphaera, rather than Candidatus Accumulibacter as performance indicator of free ammonia inhibition during the enhanced biological phosphorus removal processes. J. Environ. Chem. Eng. 2021, 9, 106219. [Google Scholar] [CrossRef]
- Oehmen, A.; Lemos, P.C.; Carvalho, G.; Yuan, Z.; Keller, J.; Blackall, L.L.; Reis, M.A. Advances in enhanced biological phosphorus removal: From micro to macro scale. Water Res. 2007, 41, 2271–2300. [Google Scholar] [CrossRef] [PubMed]
- He, S.; McMahon, K.D. Microbiology of ‘Candidatus Accumulibacter’ in activated sludge. Microb. Biotechnol. 2011, 4, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Skennerton, C.T.; Barr, J.J.; Slater, F.R.; Bond, P.L.; Tyson, G.W. Expanding our view of genomic diversity in Candidatus Accumulibacter clades. Environ. Microbiol. 2015, 17, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Nielsen, J.L.; Nielsen, P.H. Identity and ecophysiology of uncultured actinobacterial polyphosphate-accumulating organisms in full-scale enhanced biological phosphorus removal plants. Appl. Environ. Microbiol. 2005, 71, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.T.; Kristiansen, R.; Vestergaard, M.; Wimmer, R.; Nielsen, P.H. Intracellular accumulation of glycine in polyphosphate-accumulating organisms in activated sludge, a novel storage mechanism under dynamic anaerobic-aerobic conditions. Appl. Environ. Microbiol. 2015, 81, 4809–4818. [Google Scholar] [CrossRef]
- Marques, R.; Santos, J.; Nguyen, H.; Carvalho, G.; Noronha, J.P.; Nielsen, P.H.; Reis, M.A.; Oehmen, A. Metabolism and ecological niche of Tetrasphaera and Ca. Accumulibacter in enhanced biological phosphorus removal. Water Res. 2017, 122, 159–171. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research advances of the phosphorus-accumulating organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef]
- Qiu, G.; Zuniga-Montanez, R.; Law, Y.; Thi, S.S.; Nguyen, T.Q.N.; Eganathan, K.; Liu, X.; Nielsen, P.H.; Williams, R.B.; Wuertz, S. Polyphosphate-accumulating organisms in full-scale tropical wastewater treatment plants use diverse carbon sources. Water Res. 2019, 149, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zeng, W.; Fan, Z.; Wang, C.; Meng, Q.; Peng, Y. Effects of polyaluminium chloride addition on community structures of polyphosphate and glycogen accumulating organisms in biological phosphorus removal (BPR) systems. Bioresour. Technol. 2020, 297, 122431. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.L.; Nguyen, H.; Meyer, R.L.; Nielsen, P.H. Identification of glucose-fermenting bacteria in a full-scale enhanced biological phosphorus removal plant by stable isotope probing. Microbiology 2012, 158, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zeng, W.; Meng, Q.; Liu, H.; Liu, H.; Peng, Y. Achieving enhanced biological phosphorus removal utilizing waste activated sludge as sole carbon source and simultaneous sludge reduction in sequencing batch reactor. Sci. Total Environ. 2021, 799, 149291. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zeng, W.; Meng, Q.; Liu, H.; Ma, C.; Peng, Y. Achieving partial nitrification, enhanced biological phosphorus removal and in-situ fermentation (PNPRF) in continuous-flow system and mechanism analysis at transcriptional level. Chem. Eng. J. 2022, 428, 131098. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y. Simultaneous nitrogen and phosphorus recovery from sludge-fermentation liquid mixture and application of the fermentation liquid to enhance municipal wastewater biological nutrient removal. Environ. Sci. Technol. 2009, 43, 6164–6170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Y.; Li, B.; Zhang, Q.; Wu, L.; Li, X.; Peng, Y. Enhanced nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor with sludge fermentation products as carbon source. Bioresour. Technol. 2017, 244, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Coats, E.R.; Eyre, K.; Bryant, C.; Woodland, T.; Brinkman, C.K. Assessing the effects of RAS fermentation on EBPR performance and associated microbial ecology. Water Environ. Res. 2018, 90, 659–671. [Google Scholar] [CrossRef]
- Li, X.; Chen, H.; Hu, L.; Yu, L.; Chen, Y.; Gu, G. Pilot-scale waste activated sludge alkaline fermentation, fermentation liquid separation, and application of fermentation liquid to improve biological nutrient removal. Environ. Sci. Technol. 2011, 45, 1834–1839. [Google Scholar] [CrossRef]
- Lu, H.; Oehmen, A.; Virdis, B.; Keller, J.; Yuan, Z. Obtaining highly enriched cultures of Candidatus Accumulibacter phosphates through alternating carbon sources. Water Res. 2006, 40, 3838–3848. [Google Scholar] [CrossRef]
- Sabba, F.; Farmer, M.; Jia, Z.; Di Capua, F.; Dunlap, P.; Barnard, J.; Qin, C.D.; Kozak, J.A.; Wells, G.; Downing, L. Impact of operational strategies on a sidestream enhanced biological phosphorus removal (S2EBPR) reactor in a carbon limited wastewater plant. Sci. Total Environ. 2023, 857, 159280. [Google Scholar] [CrossRef] [PubMed]
- Gu, A.Z.; Saunders, A.; Neethling, J.; Stensel, H.; Blackall, L. Functionally relevant microorganisms to enhanced biological phosphorus removal performance at full-scale wastewater treatment plants in the United States. Water Environ. Res. 2008, 80, 688–698. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 24th ed.; American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WEF); APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Zhao, J.; Xin, M.; Zhang, J.; Sun, Y.; Luo, S.; Wang, H.; Wang, Y.; Bi, X. Diclofenac inhibited the biological phosphorus removal: Performance and mechanism. Chemosphere 2020, 243, 125380. [Google Scholar] [CrossRef]
- Oehmen, A.; Zeng, R.J.; Yuan, Z.; Keller, J. Anaerobic metabolism of propionate by polyphosphate-accumulating organisms in enhanced biological phosphorus removal systems. Biotechnol. Bioeng. 2005, 91, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Amann, R.I. In situ identification of micro-organisms by whole cell hybridization with rRNA-targeted nucleic acid probes. In Molecular Microbial Ecology Manual; Springer: Berlin/Heidelberg, Germany, 1995; pp. 331–345. [Google Scholar]
- Miao, Y.; Zhang, L.; Yu, D.; Zhang, J.; Zhang, W.; Ma, G.; Zhao, X.; Peng, Y. Application of intermittent aeration in nitrogen removal process: Development, advantages and mechanisms. Chem. Eng. J. 2022, 430, 133184. [Google Scholar] [CrossRef]
- Carvalheira, M.; Oehmen, A.; Carvalho, G.; Reis, M.A. Survival strategies of polyphosphate accumulating organisms and glycogen accumulating organisms under conditions of low organic loading. Bioresour. Technol. 2014, 172, 290–296. [Google Scholar] [CrossRef]
- Kong, Y.; Xia, Y.; Nielsen, P.H. Activity and identity of fermenting microorganisms in full-scale biological nutrient removing wastewater treatment plants. Environ. Microbiol. 2008, 10, 2008–2019. [Google Scholar] [CrossRef]
- Izadi, P.; Izadi, P.; Eldyasti, A. Evaluation of PAO adaptability to oxygen concentration change: Development of stable EBPR under stepwise low-aeration adaptation. Chemosphere 2022, 286, 131778. [Google Scholar] [CrossRef]
- Smolders, G.; Van der Meij, J.; Van Loosdrecht, M.; Heijnen, J. Model of the anaerobic metabolism of the biological phosphorus removal process: Stoichiometry and pH influence. Biotechnol. Bioeng. 1994, 43, 461–470. [Google Scholar] [CrossRef]
- Acevedo, B.; Oehmen, A.; Carvalho, G.; Seco, A.; Borrás, L.; Barat, R. Metabolic shift of polyphosphate-accumulating organisms with different levels of polyphosphate storage. Water Res. 2012, 46, 1889–1900. [Google Scholar] [CrossRef]
- Lanham, A.B.; Oehmen, A.; Saunders, A.M.; Carvalho, G.; Nielsen, P.H.; Reis, M.A. Metabolic versatility in full-scale wastewater treatment plants performing enhanced biological phosphorus removal. Water Res. 2013, 47, 7032–7041. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tooker, N.B.; Srinivasan, V.; Li, G.; Fernandez, L.A.; Schauer, P.; Menniti, A.; Maher, C.; Bott, C.B.; Dombrowski, P. Side-stream enhanced biological phosphorus removal (S2EBPR) process improves system performance-A full-scale comparative study. Water Res. 2019, 167, 115109. [Google Scholar] [CrossRef] [PubMed]
- Comeau, Y.; Hall, K.; Hancock, R.; Oldham, W. Biochemical model for enhanced biological phosphorus removal. Water Res. 1986, 20, 1511–1521. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, H.; Liu, J.; Zuo, D.; Deng, L. Sequencing batch reactor (SBR) and anoxic and oxic process (A/O) display opposite performance for pollutant removal in treating digested effluent of swine wastewater with low and high COD/N ratios. J. Clean. Prod. 2022, 372, 133643. [Google Scholar] [CrossRef]
- Qi, B.; Jiang, X.; Wang, H.; Li, J.; Zhao, Q.; Li, R.; Wang, W. Resource recovery from liquid digestate of swine wastewater by an ultrafiltration membrane bioreactor (UF-MBR) and reverse osmosis (RO) process. Environ. Technol. Innov. 2021, 24, 101830. [Google Scholar] [CrossRef]
- Provenzano, M.R.; Malerba, A.D.; Pezzolla, D.; Gigliotti, G. Chemical and spectroscopic characterization of organic matter during the anaerobic digestion and successive composting of pig slurry. Waste Manag. 2014, 34, 653–660. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Hao, J.; Li, D.; Wei, Z.; Teng, R.; Sun, B. Protein and carbohydrate drive microbial responses in diverse ways during different animal manures composting. Bioresour. Technol. 2019, 271, 482–486. [Google Scholar] [CrossRef]

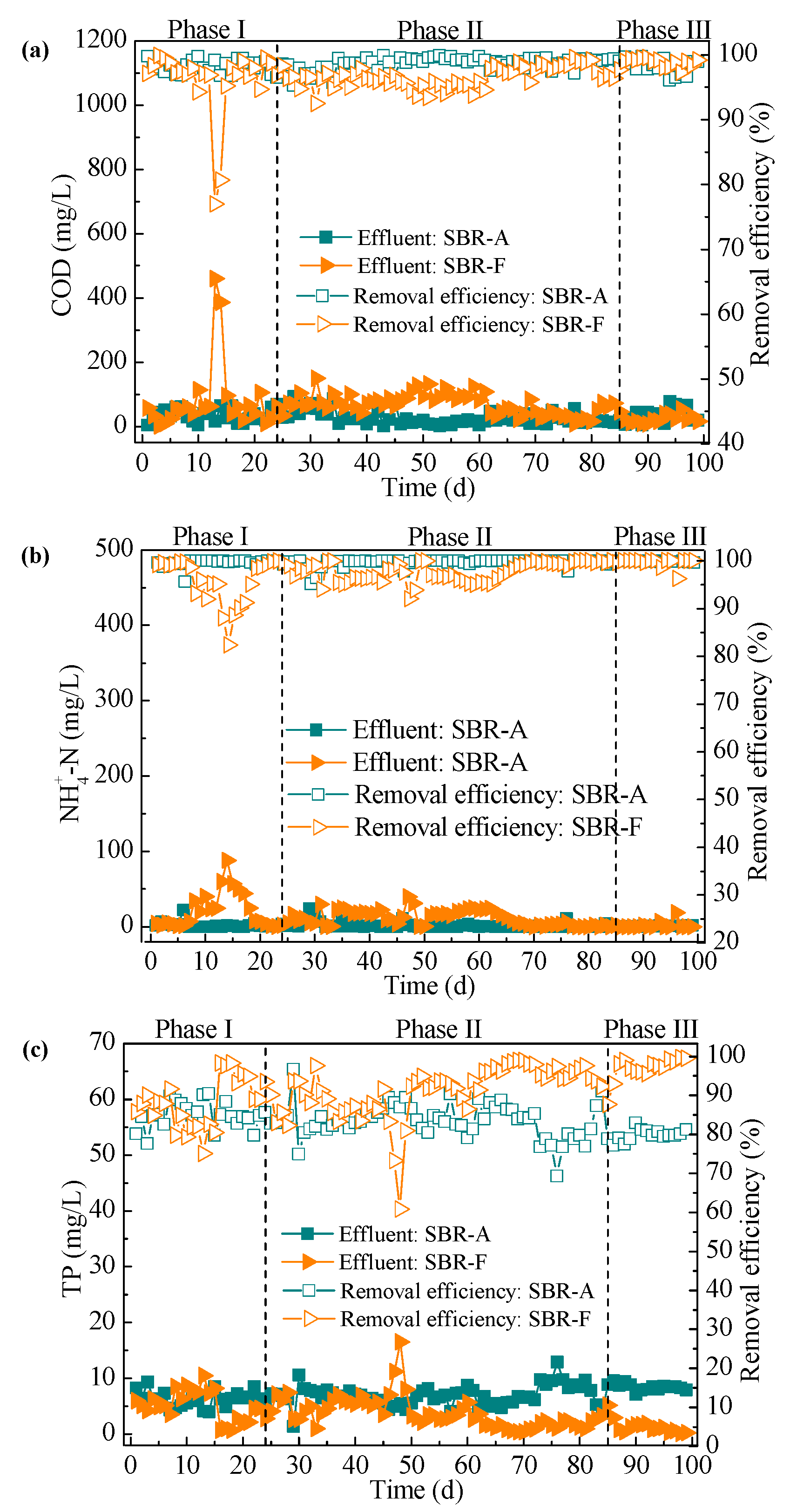

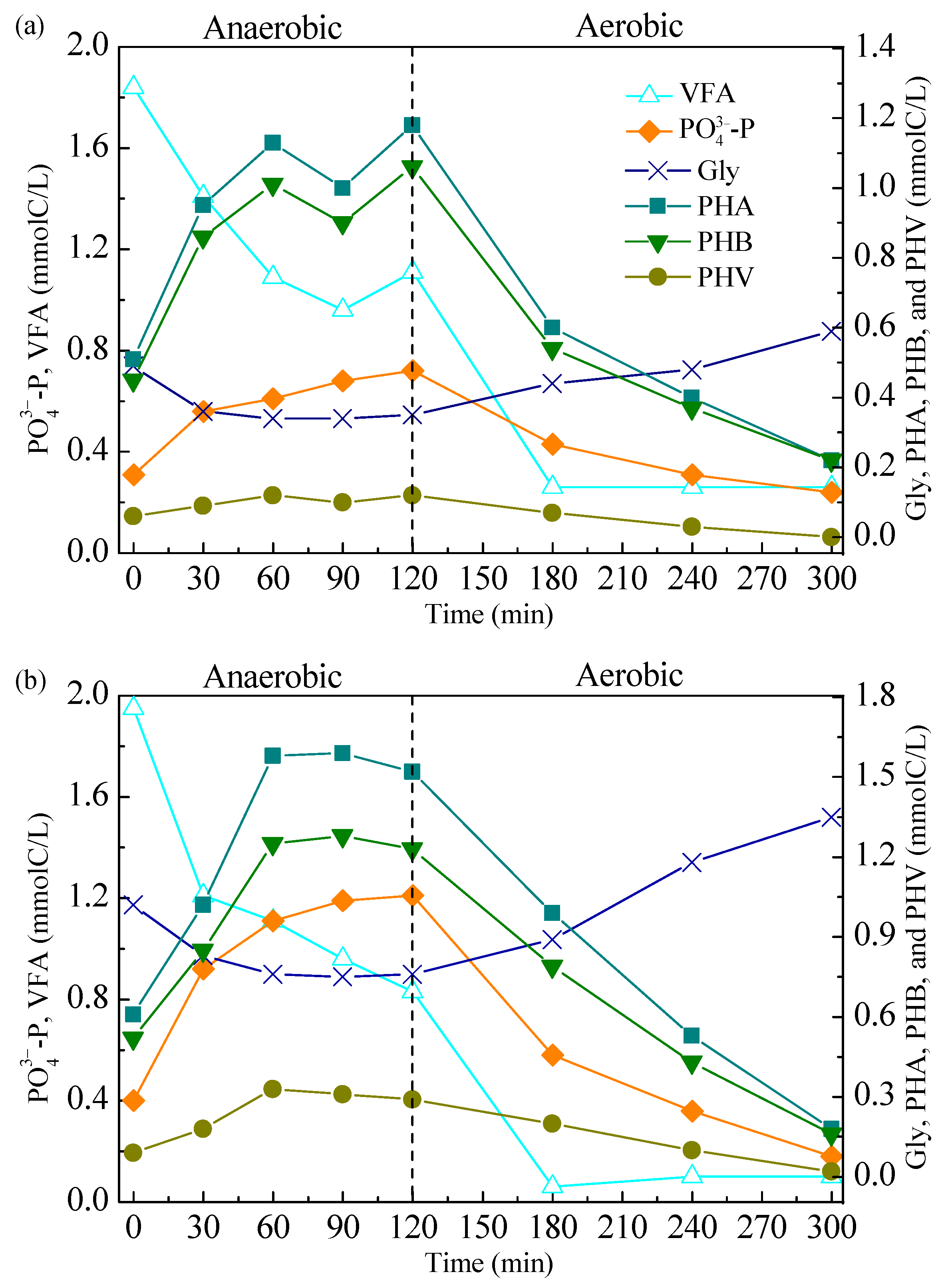
| Phase | Day (d) | Reactor | Carbon Source | Influent C/N a | TP | NH4+-N | COD | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Effluent (mg/L) b | Removal (%) b | Effluent (mg/L) b | Removal (%) b | Effluent (mg/L) b | Removal (%) b | |||||
| I to II | 1 to 84 | SBR-A | Wastewater | 4.0 | 6.7 ± 1.8 | 84.1 ± 4.2 | 2.0 ± 4.6 | 99.6 ± 0.9 | 29 ± 21 | 98.6 ± 1.0 |
| I | 1 to 6 | SBR-F | Wastewater | 4.0 | 5.2 ± 0.7 | 87.5 ± 1.8 | 3.0 ± 1.5 | 99.4 ± 0.3 | 31 ± 23 | 98.5 ± 1.2 |
| 7 to 24 | SBR-F | Wastewater + fermentation liquid | N/A | 5.0 ± 3.2 | 88.2 ± 7.5 | 28.8 ± 29.8 | 96.2 ± 6.0 | 98 ± 122 | 95.1 ± 6.1 | |
| II-A | 25 to 30 | SBR-F | Wastewater + casein hydrolysate | 6.5 | 5.0 ± 2.2 | 88.1 ± 5.2 | 8.5 ± 4.5 | 98.3 ± 0.9 | 69 ± 22 | 96.6 ± 1.1 |
| 31 to 42 | SBR-F | 7.5 | 5.1 ± 1.6 | 87.9 ± 3.9 | 17.8 ± 8.9 | 96.4 ± 1.8 | 79 ± 28 | 96.0 ± 1.4 | ||
| 43 to 48 | SBR-F | 8.0 | 8.2 ± 4.8 | 80.6 ± 11.5 | 17.6 ± 14.3 | 96.5 ± 2.9 | 84 ± 17 | 95.8 ± 0.9 | ||
| II-B | 49 to 60 | SBR-F | Wastewater + glucose | 8.0 | 3.7 ± 1.6 | 91.1 ± 3.9 | 16.1 ± 8.1 | 96.8 ± 1.6 | 102 ± 19 | 94.9 ± 0.9 |
| 61 to 74 | SBR-F | 6.0 | 1.5 ± 0.9 | 96.5 ± 2.2 | 8.8 ± 8.6 | 98.2 ± 1.7 | 48 ± 23 | 97.6 ± 1.2 | ||
| 75 to 84 | SBR-F | 4.2 | 2.0 ± 0.7 | 95.3 ± 1.8 | 1.5 ± 1.9 | 99.7 ± 0.4 | 39 ± 24 | 98.0 ± 1.2 | ||
| III | 85 to 99 | SBR-F | Wastewater + glucose | 6.0 | 8.5 ± 0.6 | 79.8 ± 1.5 | 0.7 ± 0.6 | 99.9 ± 0.1 | 36 ± 24 | 98.2 ± 1.2 |
| SBR-F | 1.3 ± 1.3 | 96.8 ± 3.1 | 2.0 ± 5.0 | 99.6 ± 1.0 | 25 ± 14 | 98.8 ± 0.7 | ||||
| Parameters | Unit | SBR-A | SBR-F | |
|---|---|---|---|---|
| Kinetic rates | VFAup | mg C/(g VSS·h) | 35.70 | 67.40 |
| Prel | mg P/(g VSS·h) | 12.60 | 24.50 | |
| Pup | mg P/(g VSS·h) | 7.30 | 15.00 | |
| Pup/Prel | 0.78 | 0.85 | ||
| Stoichiometric parameters | P/VFA | mol P/mol C | 0.56 | 0.72 |
| Gly/VFA | mol C/mol C | 0.19 | 0.23 | |
| PHA/VFA | mol C/mol C | 0.91 | 0.81 | |
| P/PHA | mol P/mol C | 0.50 | 0.77 | |
| Gly/PHA | mol C/mol C | 0.26 | 0.43 |
| Relative Abundance | SBR-A (%) a | SBR-F (%) a |
|---|---|---|
| Candidatus Accumulibacter | 30.7 ± 4.3 | 44.7 ± 3.1 |
| Tetrasphaera | 1.8 ± 1.7 | 18.1 ± 6.6 |
| Reference | Dan et al. [11] | Yang et al. [12] | Huang et al. [14] | Cai et al. [56] | Qi et al. [57] | This Study | |
|---|---|---|---|---|---|---|---|
| Wastewater | Digested swine wastewater | Digested swine wastewater | Simulated digested swine wastewater | Mixture of raw swine wastewater and digested effluent | Digested swine wastewater | Digested swine wastewater | |
| Configuration | ICEAS | A/O | SBBR | SBR | A/O | UF-MBR | EBPR-F |
| Influent quality (mg/L) | |||||||
| C/N | 4.8 | 14.0 | 1.5 | 4.3 | 4.3 | 1.9 | 4.0 |
| COD | 2267.62 | 8375 ± 152 | 600 | 4328 ± 899 | 4328 ± 899 | 1009.50 ± 17.68 | 2000 ± 50 |
| NH4+-N | 476.35 | 603 ± 7.95 | 400 | 1010 ± 93.4 | 1010 ± 93.4 | 532.36 ± 5.24 | 400 ± 20 |
| TP | 415.34 | 216 ± 3.78 | 20 | 212 ± 58.1 | 212 ± 58.1 | 41.94 ± 0.41 | 42 ± 3 |
| Effluent quality (mg/L) | |||||||
| COD | 157.78 | 256 ± 6.23 | 50 | 414 ± 74.3 | 350 ± 48.5 | 509.16 ± 54.51 | 25 ± 14 |
| NH4+-N | 10.94 | 2.07 ± 0.79 | 1.79 ± 1.39 | 24.3 ± 36.5 | 57.1 ± 58.3 | 55.27 ± 5.72 | 2.0 ± 5.0 |
| TP | 52.46 | 29.27 ± 1.91 | 3.96 ± 0.82 | 111 ± 39.7 | 117 ± 56.8 | 10.87 ± 1.02 | 0.9 ± 0.6 |
| Removal (%) | |||||||
| COD | 93 | 97 | 91.7 | 89.9 | 91.5 | 49.6 | 98.8 |
| NH4+-N | 98 | 99 | 99.6 | 97.6 | 94.3 | 89.6 | 99.6 |
| TP | 87 | 86 | 83.7 | 47.5 | 44.7 | 74.1 | 97.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Zhang, C.; Li, P.; Feng, T.; Wu, J. Coupled In-Situ Fermentation for Enhanced Biological Phosphorus Removal from Digested Swine Wastewater. Water 2024, 16, 80. https://doi.org/10.3390/w16010080
Liao Y, Zhang C, Li P, Feng T, Wu J. Coupled In-Situ Fermentation for Enhanced Biological Phosphorus Removal from Digested Swine Wastewater. Water. 2024; 16(1):80. https://doi.org/10.3390/w16010080
Chicago/Turabian StyleLiao, Yifang, Chiqian Zhang, Ping Li, Tao Feng, and Jinhua Wu. 2024. "Coupled In-Situ Fermentation for Enhanced Biological Phosphorus Removal from Digested Swine Wastewater" Water 16, no. 1: 80. https://doi.org/10.3390/w16010080
APA StyleLiao, Y., Zhang, C., Li, P., Feng, T., & Wu, J. (2024). Coupled In-Situ Fermentation for Enhanced Biological Phosphorus Removal from Digested Swine Wastewater. Water, 16(1), 80. https://doi.org/10.3390/w16010080







