Removal of Dyes from Water Using Aluminum-Based Water Treatment Sludge as a Low-Cost Coagulant: Use of Response Surface Methodology
Abstract
:1. Introduction
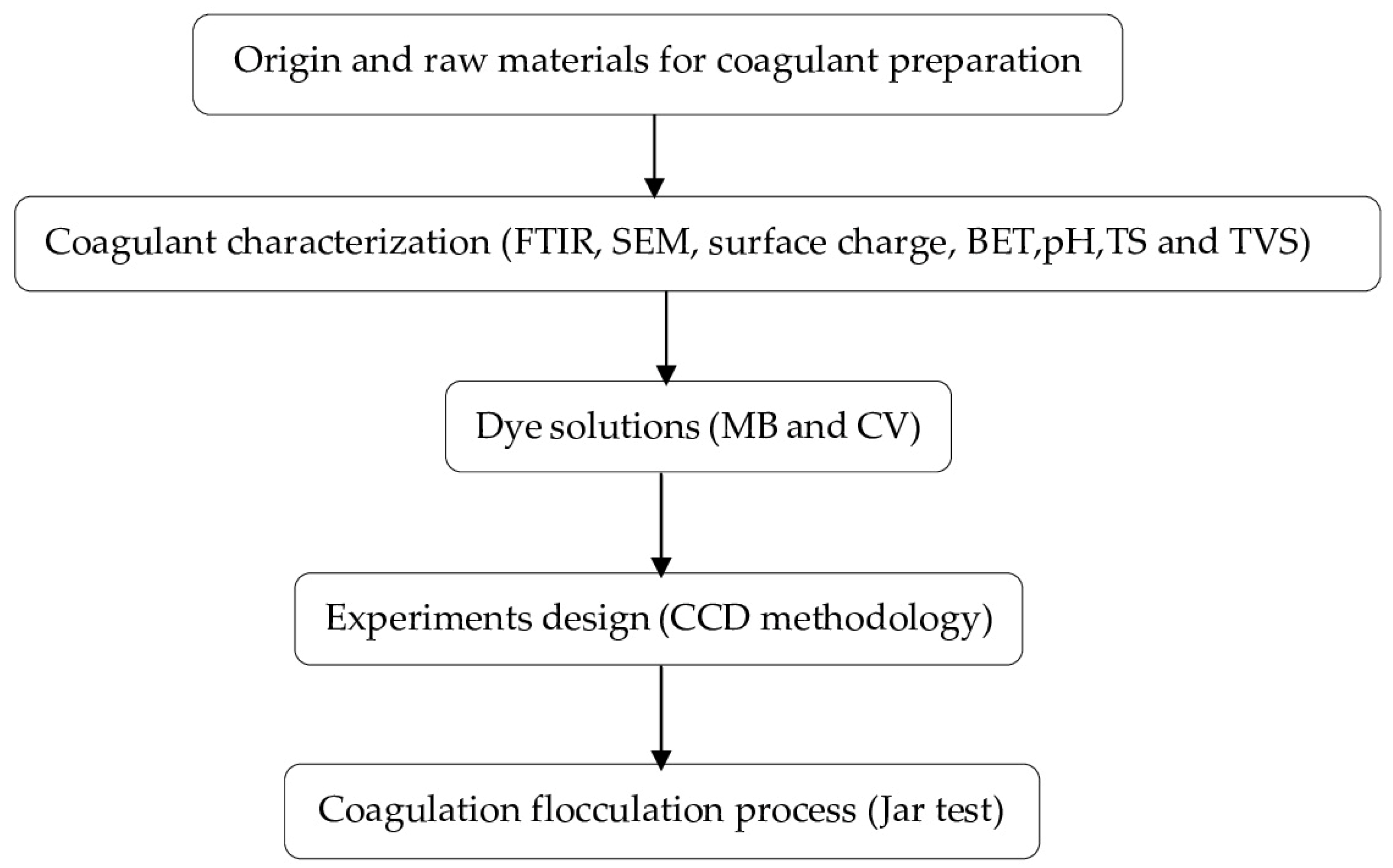
2. Materials and Methods
2.1. Origin and Raw Materials for Coagulant Preparation
- -
- Drying by placing the dehydrated sludge in an oven at 105 °C for 24 h.
- -
- Grinding using a domestic grinder.
- -
- Sieving using a vibrating sieve shaker, and finally, the powder form of the coagulant with homogeneous granulometry (148 μm).
2.2. Coagulant Characterization
2.3. Dye Solutions
2.4. Experimental Design
- ➢
- They require an experiment number (N) according to the relationship N = 2k + 2k + nc, where k is the factor number and nc is the replica number of the central point.
- ➢
- α depends on the number of variables and can be calculated by the relation α = 2k/4; for three variables, they are 1.68.
- ➢
- All factors are studied in five levels (−α, −1, 0, +1, + α).
- ➢
- Obtain the mathematical model linking the response (dye removal) with the factors studied.
- ➢
- Test the significance of the factor effects.
- ➢
- Identify the optimal conditions.
2.5. Coagulation–Flocculation Process (Jar Test)
- Fill beakers to a constant volume with contaminated water.
- Add a mass of dye (see Table 2).
- Adjust the pH according to Table 2 using solutions of NaOH (1 M) and H2SO4 (1 M).
- Add the coagulant doses according to the test matrix.
- Coagulation for 3 min at 160 rpm.
- Flocculation for 20 min at 30 rpm.
- The settling phase involves leaving the beakers to settle for 30 min.
| Standard Run | pH (X1) | Coagulant Dosage (mg/L) (X2) | Dye Concentration (mg/L) (X3) | RMB(%) | RCV(%) |
|---|---|---|---|---|---|
| 01 | 4.000 | 20.000 | 20.000 | 24.634 | 33.210 |
| 02 | 10.000 | 20.000 | 20.000 | 42.783 | 76.030 |
| 03 | 4.000 | 70.000 | 20.000 | 43.553 | 58.890 |
| 04 | 10.000 | 70.000 | 20.000 | 59.981 | 61.310 |
| 05 | 4.000 | 20.000 | 70.000 | 46.703 | 29.120 |
| 06 | 10.000 | 20.000 | 70.000 | 60.914 | 60.160 |
| 07 | 4.000 | 70.000 | 70.000 | 61.096 | 60.100 |
| 08 | 10.000 | 70.000 | 70.000 | 61.074 | 60.610 |
| 09 | 1.954 | 45.000 | 45.000 | 42.595 | 60.180 |
| 10 | 12.045 | 45.000 | 45.000 | 75.349 | 99.540 |
| 11 | 7.000 | 2.955 | 45.000 | 33.313 | 27.360 |
| 12 | 7.000 | 87.044 | 45.000 | 66.117 | 58.190 |
| 13 | 7.000 | 45.000 | 2.955 | 32.734 | 38.730 |
| 14 | 7.000 | 45.000 | 87.044 | 47.045 | 38.550 |
| 15 | 7.000 | 45.000 | 45.000 | 40.116 | 38.550 |
| 16 | 7.000 | 45.000 | 45.000 | 39.577 | 38.500 |
| 17 | 7.000 | 45.000 | 45.000 | 39.252 | 38.620 |
| 18 | 7.000 | 45.000 | 45.000 | 39.047 | 38.620 |
| 19 | 7.000 | 45.000 | 45.000 | 39.27 | 38.550 |
| 20 | 7.000 | 45.000 | 45.000 | 39.304 | 38.550 |
3. Results and Discussion
3.1. Coagulant Characterization (Sludge Powder)
3.1.1. Surface Charge and FTIR Characterization
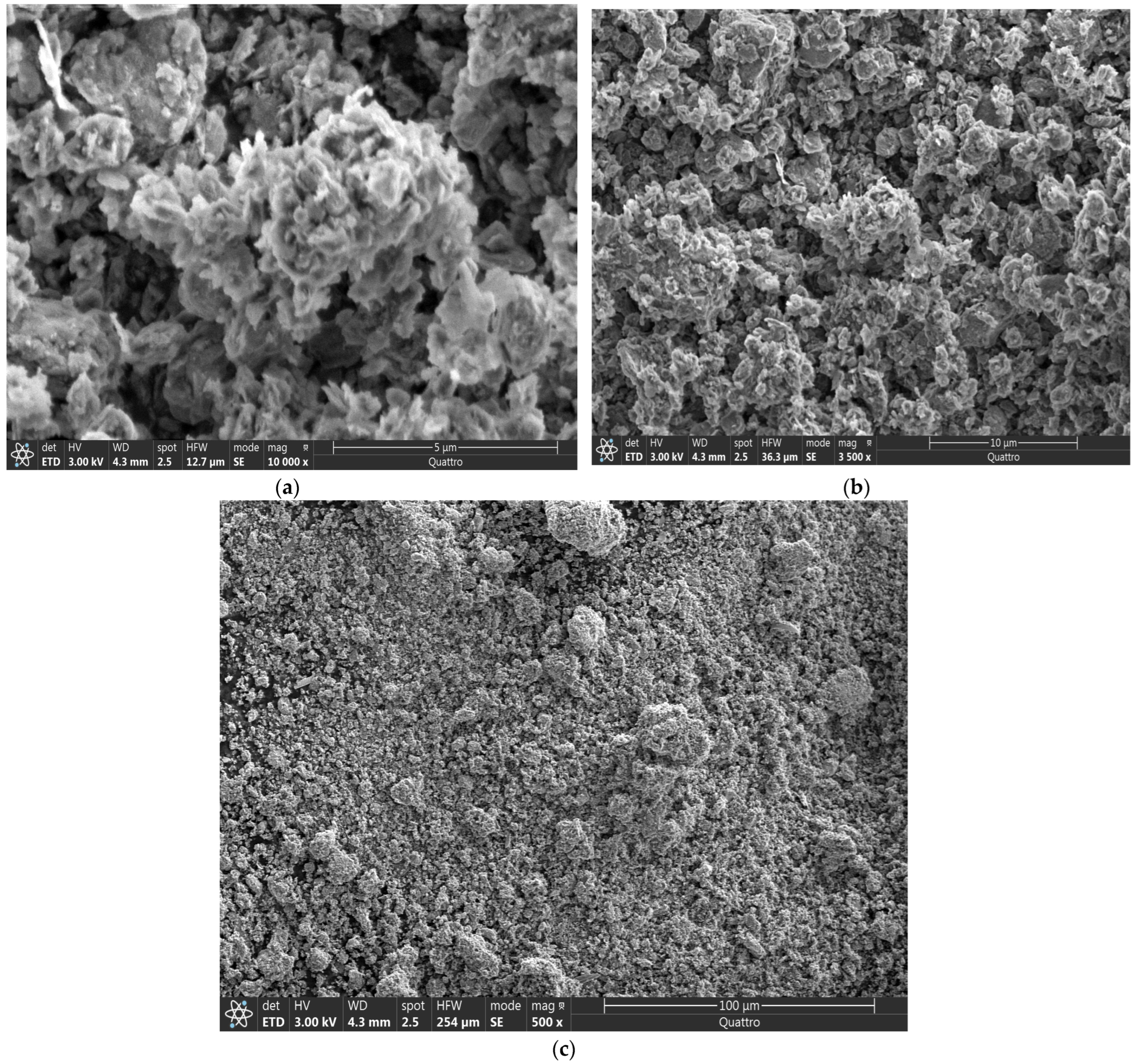
3.1.2. SEM Characterization
3.1.3. BET Surface Area Analysis
3.2. Study of Dye Removal Using Sludge as a Coagulant
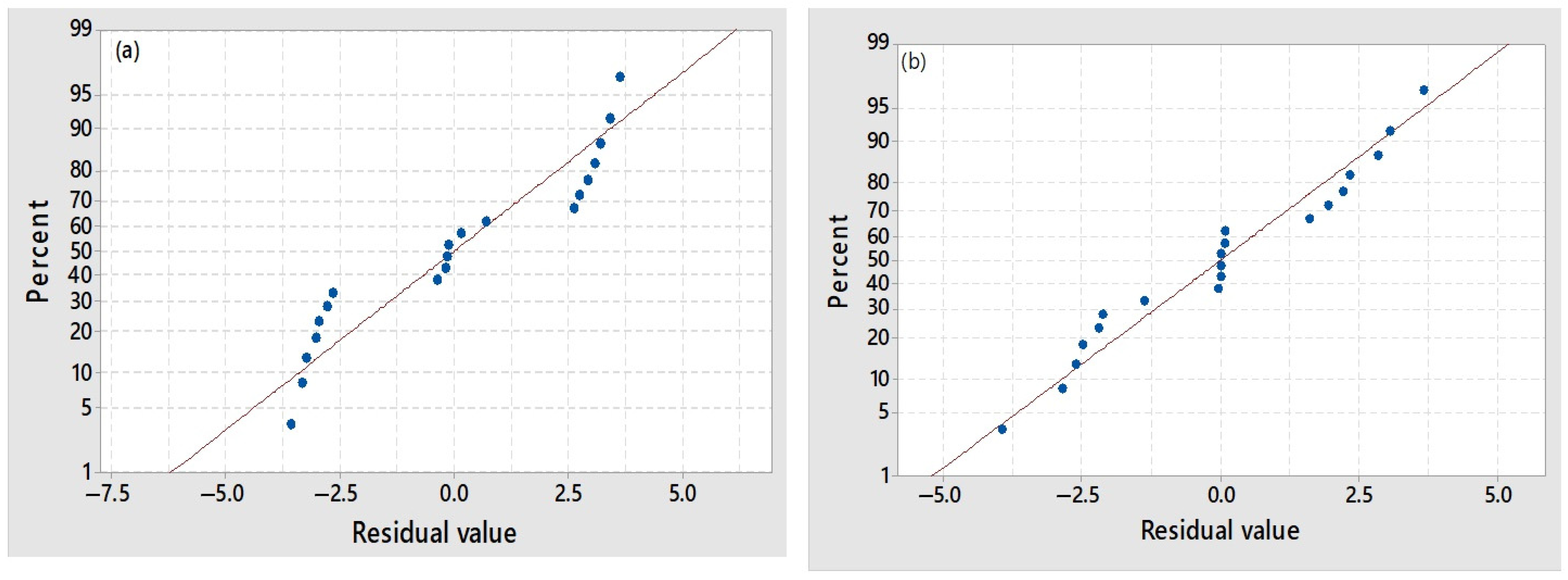
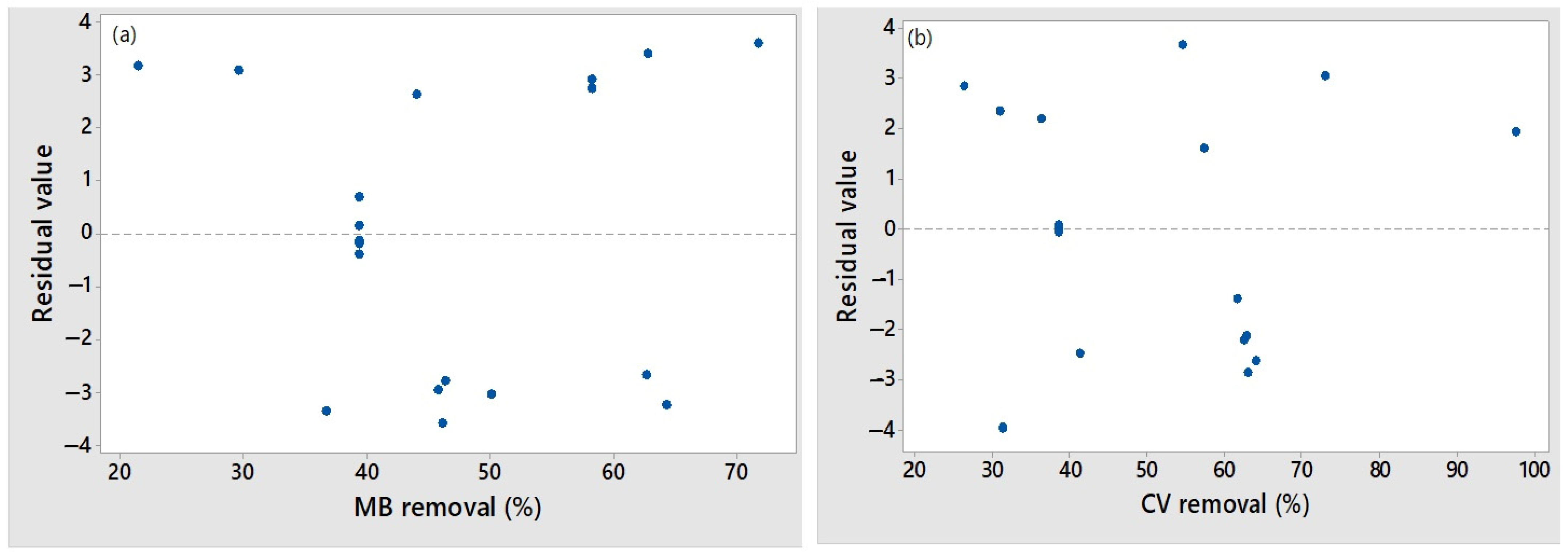
3.2.1. Statistical Analysis
3.2.2. Analysis of Variance (ANOVA)
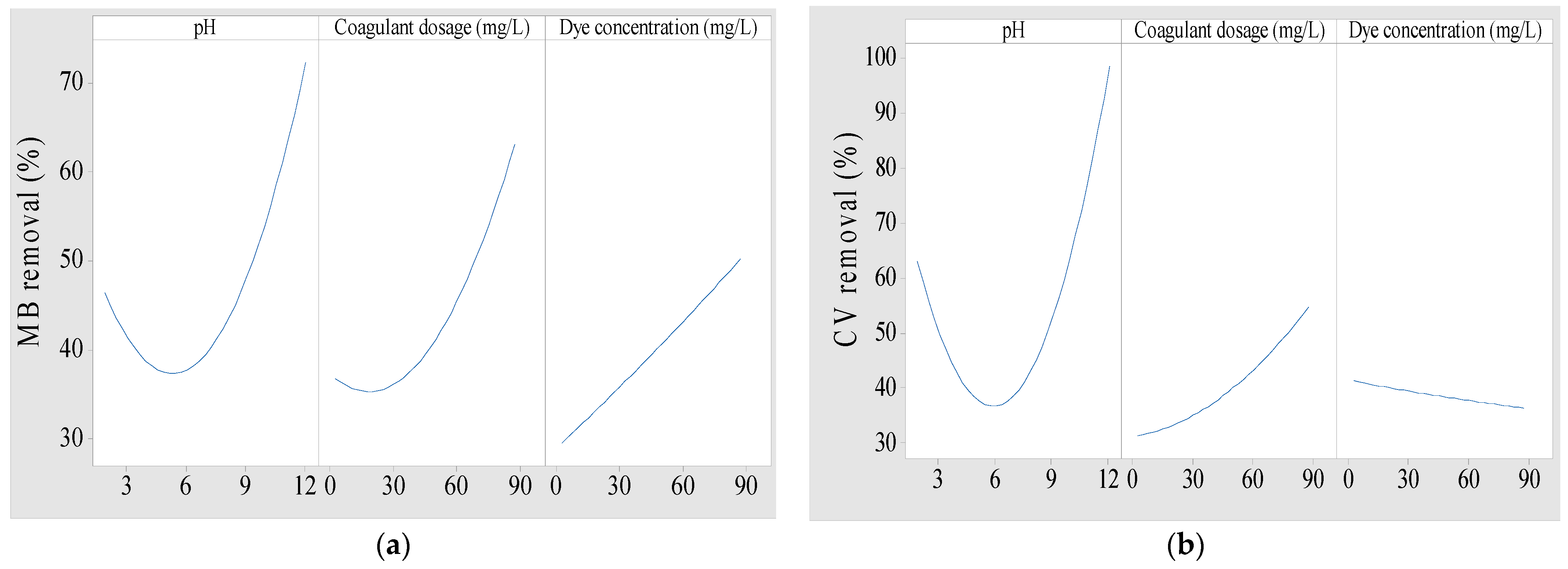
3.2.3. Effect of Main Factors on Dye Removal
3.2.4. Response Surface Plots (3D)
- ➢
- Interaction between the pH and coagulant dose (Figure 9a): CV reduction reaches its maximum level when a pH of 12 is combined with a coagulant dose of around 50 mg/L. This suggests that, at these specific pH and coagulant dose values, the best CV reduction is achieved (over 95%).
- ➢
- Interaction between the pH and dye concentration (Figure 9b): This figure shows that the CV removal value is highest when the pH is set at 12 and the dye concentration is around 50 mg/L. This indicates that, for these particular pH and dye concentration values, the maximum CV dye reduction is around 97%.
- ➢
- Interaction between the dye concentration and coagulant dosage (Figure 9c): The interaction graph reveals that the maximum CV removal (between 50 and 60%) is achieved for a coagulant dose of 75 mg/L and a dye concentration of around 25 mg/L or 75 mg/L.

3.2.5. Optimization and Validation
3.3. Economic Assessment
- The cost of the raw material. This study has shown that sludge is a reliable and readily available source in drinking water treatment plants. Thus, the cost of the raw material is very low or negligible because we have solved the problem of sludge stored in the plant; in addition, such a material is then economical compared to other dye treatment processes, such as microfiltration and advanced oxidation processes.
- The cost of the AS preparation. In this study, a simple and straightforward method was used to prepare this coagulant using a mill to obtain the powder form of the coagulant.
- The cost of the NaOH solution used to adjust the coagulation pH of the two dyes considered. A total volume of 2.1 mL (1 mol/L) and 2.4 mL (1 mol/L) was used to set the coagulation pH at 12.045 and 11.23, i.e., 96 and 84 mg NaOH were used to reduce MB and CV, respectively. According to the art-chemistry website, the cost of 1 kg NaoH was EUR 7.19 [60]; the cost of NaOH used to treat 1L of colored water was EUR 0.690 and 0.603 for MB and CV, respectively.
4. Conclusions
- The infrared spectrum showed the presence of several functional groups responsible for the dye removal process, namely, the OH group. The pH of zero charge (pHpzc) was used to assess the surface charge of this material (AS). The pHpzc value was 6.9. In this study, when the pH of the water was >pHpzc, the coagulation of MB and CV was favorable. The coagulant (AS) has an amorphous structural morphology, which favors the coagulation–flocculation process.
- Powder sludge treatment of colored effluents is effective according to the results obtained for the two dyes used. The experimental results highlighted the optimization and modeling of the coagulation–flocculation process, and the pH, initial dye concentration, and coagulant dosage were used as factors influencing the reduction in MB and CV in water using the centered composite design (CCD) as the experimental method. The results show that the correlation coefficients, R2 and R2 adjusted, for MB and CV were 95.80%, 92.02%, 98.44%, and 97.03%, respectively. In this case, the maximum reductions were 89.06% and 100% % at the following optimal conditions: pH (12.045 and 11.23), initial dye concentration (2.955 and 31.251 mg/L), and coagulant dosage (87.049 and 4.484 mg/L) for MB and CV, respectively. It can be concluded that the three factors considered influence the dye removal efficiency. This study also shows that the two models obtained are reliable. Consequently, these uses can be applied to waters with properties similar to this study, such as industrial textile waters.
- The present work shows that the sludge can be used effectively as a low-cost coagulant for dye removal.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Alum sludge |
| BET | Brunauer–Emmett–Telle |
| CCD | Central composite design |
| RSM | Response surface method |
| MB | Methylene blue |
| CV | Crystal violet |
| FAIR | Fourier transform infrared spectrophotometry |
| pHpzc | Point zero charge |
| SEM | Scanning electron microscopy |
| R | Dye removal efficiency |
| RCV | Removal of crystal violet |
| RMB | Removal of methylene blue |
| R2 | Coefficient of determination |
| R2adj | Adjusted coefficient of determination |
| TOC | Total organic carbon |
| TS | Total solids |
| TSS | Total suspended solids |
| TVS | Total volatile solids |
| WTS | Water treatment sludge |
References
- Djelal, H.; Rigail, M.; Boyer, L. Les Effluents Industriels et Leur Traitement. Manag. Avenir 2008, 20, 275–288. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Hassen, M.A.; Kerroum, D. Isotherm and Kinetics Study of Biosorption of Cationic Dye onto Banana Peel. Energy Procedia 2012, 19, 286–295. [Google Scholar] [CrossRef]
- Lemessa, F.; Simane, B.; Seyoum, A.; Gebresenbet, G. Assessment of the Impact of Industrial Wastewater on the Water Quality of Rivers around the Bole Lemi Industrial Park (BLIP), Ethiopia. Sustainability 2023, 15, 4290. [Google Scholar] [CrossRef]
- Owhonka, A.; Fubara, E.F.; Justice, O.B. Wastewater Quality-It’s Impact on the Environment and Human Physiology: A Review. Int. J. Adv. Res. Innov. 2021, 9, 43–58. [Google Scholar] [CrossRef]
- Li, X.; Bao, L.; Wei, Y.; Zhao, W.; Wang, F.; Liu, X.; Su, H.; Zhang, R. Occurrence, Bioaccumulation, and Risk Assessment of Microplastics in the Aquatic Environment: A Review. Water 2023, 15, 1768. [Google Scholar] [CrossRef]
- Hammami, S. Étude de Dégradation Des Colorants de Textile Par Les Procédés d’Oxydation Avancée. Application à La Dépollution Des Rejets Industriels. Ph.D. Thesis, Université de Marne la Vallée, Paris, France, 2008. [Google Scholar]
- Mansour, H.B.; Boughzala, O.; Barillier, D.; Mosrati, R. Les Colorants Textiles Sources de Contamination de l’eau: CRIBLAGE de La Toxicité et Des Méthodes de Traitement. Rev. Des Sci. L’Eau 2011, 24, 209–238. [Google Scholar] [CrossRef]
- Kasperchik, V.P.; Yaskevich, A.L.; Bil’Dyukevich, A.V. Wastewater Treatment for Removal of Dyes by Coagulation and Membrane Processes. Pet. Chem. 2012, 52, 545–556. [Google Scholar] [CrossRef]
- Yao, S.; Fabbricino, M.; Race, M.; Ferraro, A.; Pontoni, L.; Aimone, O.; Chen, Y. Study of the Digestate as an Innovative and Low-Cost Adsorbent for the Removal of Dyes in Wastewater. Processes 2020, 8, 852. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Albayati, T.M.; Alrubaye, J.M. A Hybrid Adsorption Membrane Process for Removal of Dye from Synthetic and Actual Wastewater. Chem. Eng. Process.-Process Intensif. 2020, 157, 108113. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Khelifi, M.N.; Khelfaoui, A.; Benalia, A.; Derbal, K. The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design. Water 2022, 14, 2251. [Google Scholar] [CrossRef]
- Zou, H.; Ma, W.; Wang, Y. A Novel Process of Dye Wastewater Treatment by Linking Advanced Chemical Oxidation with Biological Oxidation. Arch. Environ. Prot. 2015, 41, 33–39. [Google Scholar] [CrossRef]
- Szpyrkowicz, L.; Juzzolino, C.; Kaul, S.N. A Comparative Study on Oxidation of Disperse Dyes by Electrochemical Process, Ozone, Hypochlorite and Fenton Reagent. Water Res. 2001, 35, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, C.; Suteu, D.; Muresan, A.; Muresan, R.; Popescu, A. Textile Wastewater Treatment by Homogeneous Oxidation with Hydrogen Peroxide. Environ. Eng. Manag. J. 2009, 8, 1359–1369. [Google Scholar] [CrossRef]
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Song, X.; Sand, W. Recent Advances in Anaerobic Biological Processes for Textile Printing and Dyeing Wastewater Treatment: A Mini-Review. World J. Microbiol. Biotechnol. 2018, 34, 165. [Google Scholar] [CrossRef] [PubMed]
- Ledakowicz, S.; Pa, K. Recent Achievements in Dyes Removal Focused on Advanced. Molecules 2021, 26, 870. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological Treatment of Model Dyes and Textile Wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Sivarajasekar, N. Agriculture Waste Biomass Valorisation for Cationic Dyes Sequestration: A Concise Review Liquid-Liquid Extraction for Wastewater Treatment View Project. Artic. J. Chem. Pharm. Res. 2015, 7, 737–748. [Google Scholar]
- Alsukaibi, A.K.D. Various Approaches for the Detoxification of Toxic Dyes in Wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Abba, A.B.; Saggai, S.; Touil, Y.; Al-Ansari, N.; Kouadri, S.; Nouasria, F.Z.; Najm, H.M.; Mashaan, N.S.; Eldirderi, M.M.A.; Khedher, K.M. Copper and Zinc Removal from Wastewater Using Alum Sludge Recovered from Water Treatment Plant. Sustainability 2022, 14, 9806. [Google Scholar] [CrossRef]
- Pierzynski, G.M. Plant Nutrient Aspects of Sewage Sludge. In Sewage Sludge: Land Utilization and the Environment; Clapp, C.E., Larson, W.E., Dowdy, R.H., Eds.; Wiley: Hoboken, NJ, USA, 1994; pp. 21–25. [Google Scholar]
- Pay, D.; Christopher, A.S.; Lica, R.-S.; Mark, R.; Akiça, B. L’irrigation Avec Des Eaux Usées et La Santé; CRDI, Ed.; Presses de l’Universite du Quebec: Québec, QC, Canada, 2012; ISBN 9782760531604. [Google Scholar]
- The Canadian Fertilizer. 2024. Available online: https://fertilizercanada.ca/news-events/events/canadian-fertilizer-products-forum-5/ (accessed on 18 April 2024).
- Sadri Moghaddam, S.; Alavi Moghaddam, M.R.; Arami, M. Coagulation/Flocculation Process for Dye Removal Using Sludge from Water Treatment Plant: Optimization through Response Surface Methodology. J. Hazard. Mater. 2010, 175, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Chu, W. Dye Removal from Textile Dye Wastewater Using Recycled Alum Sludge. Water Res. 2001, 35, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- Sadri Moghaddam, S.; Alavi Moghaddam, M.R.; Arami, M. Response Surface Optimization of Acid Red 119 Dye from Simulated Wastewater Using Al Based Waterworks Sludge and Polyaluminium Chloride as Coagulant. J. Environ. Manag. 2011, 92, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Jangkorn, S.; Kuhakaew, S.; Theantanoo, S.; Klinla-or, H.; Sriwiriyarat, T. Evaluation of Reusing Alum Sludge for the Coagulation of Industrial Wastewater Containing Mixed Anionic Surfactants. J. Environ. Sci. 2011, 23, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhao, Y.; Tang, C.; Addo-Bankas, O. Use of Aluminum-Based Water Treatment Sludge as Coagulant for Animal Farm Wastewater Treatment. J. Water Process Eng. 2022, 46, 102645. [Google Scholar] [CrossRef]
- Anesthesia, N.; Be, S.; In, M.O.; In, L.; Appropriate, W.; Equipment, R.; Are, D.; Available, I.; Manage, T.O.; Ii, G.; et al. Guidelines for Drinking-Water Quality, 4th ed: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; ISBN 9789240045064. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 9789241548151. [Google Scholar]
- Padhiyar, H.; Thanki, A.; Kumar Singh, N.; Pandey, S.; Yadav, M.; Chand Yadav, T. Parametric and Kinetic Investigations on Segregated and Mixed Textile Effluent Streams Using Moringa Oleifera Seed Powders of Different Sizes. J. Water Process Eng. 2020, 34, 101159. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the Characterization of Acidic and Basic Surface Sites on Carbons by Various Techniques. Carbon N. Y. 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Benalia, A.; Atime, L.; Baatache, O.; Khalfaoui, A.; Fadia, A. Removal of Lead in Water by Coagulation Flocculation Process Using Cactus—Based Natural Coagulant: Optimization and Modeling by Response Surface Methodology (RSM). Environ. Monit. Assess. 2024, 196, 244. [Google Scholar] [CrossRef] [PubMed]
- Federation, W.E. Standard Methods for the Examination of Water and Wastewater Standard Methods for the Examination of Water and Wastewater. Public Health 1999, 51, 940. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Webster, A.; Ntwampe, I.O.; Waanders, F.B. Coagulation/Flocculation Potential of Polyaluminium Chloride and Bentonite Clay Tested in the Removal of Methyl Red and Crystal Violet. Arab. J. Sci. Eng. 2017, 42, 1389–1397. [Google Scholar] [CrossRef]
- Gupta, K.N.; Kumar, R.; Thakur, A.K.; Khan, N.A. Treatment of Dyeing Wastewater Using Foam Separation: Optimization Studies. Water 2023, 15, 2236. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Boujraf, S.; Taouda, H.; Zerrouq, F. Assessment of Adsorption Kinetics for Removal Potential of Crystal Violet Dye from Aqueous Solutions Using Moroccan Pyrophyllite. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 23, 20–28. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Aberkane, I.; Guizah, Q.E.; Khalfaoui, A.; Pizzi, A. Valorization of Pine Cones (Pinus Nigras) for Industrial Wastewater Treatment and Crystal Violet Removal: A Sustainable Approach Based on Bio-Coagulants and a Bio-Adsorbent. Water 2024, 16, 260. [Google Scholar] [CrossRef]
- De Paula, H.M.; Sangoi, M.; Ilha, D.O.; Sarmento, A.P.; Andrade, L.S. Dosage Optimization of Moringa Oleifera Seed and Traditional Chemical Coagulants Solutions for Concrete Plant Wastewater Treatment. J. Clean. Prod. 2018, 174, 123–132. [Google Scholar] [CrossRef]
- Benalia, A. Extraction et Valorisation Des Produits Actifs Des Plantes Naturelles En Tant Que Bio Coagulants Utiles Dans l’amelioration de Qualite Des Eaux. Ph.D. Thesis, University of Constantine 3, El Khroub, Algeria, 2023. [Google Scholar]
- Benalia, A.; Chaibraa, W.; Djeghar, S.; Derbal, K.; Khalfaoui, A.; Mahfouf, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design. Water 2023, 15, 1984. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Pine Cone as Bio-Coagulant for Heavy Metal Removal from Industrial Wastewater: Use of Box—Behnken Design. Ind. Crop. Prod. 2024, 210, 118185. [Google Scholar] [CrossRef]
- Benalia, A.; Baatache, O.; Derbal, K.; Khalfaoui, A. The Use of Central Composite Design (CCD) to Optimize and Model the Coagulation-Flocculation Process Using a Natural Coagulant: Application in Jar Test and Semi-Industrial Scale. J. Water Process Eng. 2024, 57, 104704. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Benalia, A.; Selama, Z.; Hammoud, A.; Derbal, K.; Panico, A.; Pizzi, A. Removal of Chromium (VI) from Water Using Orange Peel as the Biosorbent: Experimental, Modeling, and Kinetic Studies on Adsorption Isotherms and Chemical Structure. Water 2024, 16, 742. [Google Scholar] [CrossRef]
- Chua, S.; Malek, M.A.; Chong, F.; Sujarwo, W.; Ho, Y. Red Lentil (Lens Culinaris) Extract as a Novel Natural Characterization and Performance Optimization Study. Water 2019, 11, 1686–1704. [Google Scholar] [CrossRef]
- Oliveira, E.; Val, A.; Araujo, E.B. De Application of Lolium Multiflorum as an Efficient Raw Material in the Production of Adsorbent for Removal of Methylene Blue. J. Carbon Res. 2023, 9, 44. [Google Scholar]
- Zainol, N.A.; Al Balqis Khalilullah, P.; Ghani, A.A.; Rashid, N.A.; Makhtar, S.M.Z. Turbidity Removal from Kaolin Synthetic Wastewater via Coagulation Process Using Sludge from Water Treatment Plant. Int. J. Integr. Eng. 2022, 14, 222–231. [Google Scholar] [CrossRef]
- Okolo, B.I.; Adeyi, O.; Oke, E.O.; Agu, C.M.; Nnaji, P.C.; Akatobi, K.N.; Onukwuli, D.O. Coagulation Kinetic Study and Optimization Using Response Surface Methodology for Effective Removal of Turbidity from Paint Wastewater Using Natural Coagulants. Sci. Afr. 2021, 14, e00959. [Google Scholar] [CrossRef]
- Elbariji, S.; Elamine, M.; Eljazouli, H.; Kabli, H. Traitement et valorisation des sous-produits du bois. Application à l’élimination des colorants industriels. Comptes Rendus Chim. 2006, 9, 1314–1321. [Google Scholar] [CrossRef]
- Verma, S.; Prasad, B.; Mishra, I.M. Pretreatment of Petrochemical Wastewater by Coagulation and Flocculation and the Sludge Characteristics. J. Hazard. Mater. 2010, 178, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Adaobi, C.; Ighalo, J.O.; Iwuozor, K.O.; Dominic, O.; Ugochukwu, P.; Al-rawajfeh, A.E. Prediction and Optimisation of Coagulation-Flocculation Process for Turbidity Removal from Aquaculture Effluent Using Garcinia Kola Extract: Response Surface and Artificial Neural Network Methods. Clean. Chem. Eng. 2022, 4, 100076. [Google Scholar] [CrossRef]
- Chahid, L.; Yaacoubi, A.; Bacaoui, A.; Lakhal, E. Valorization of Drinking Water Treatment Sludge (DWTS): Characterization and Applications as Coagulant and Sorbent for Olive Mill Wastewater (OMW). J. Mater. Environ. Sci. 2015, 6, 2520–2533. [Google Scholar]
- Li, C.W.; Lin, J.L.; Kang, S.F.; Liang, C.L. Acidification and Alkalization of Textile Chemical Sludge: Volume/Solid Reduction, Dewaterability, and Al(III) Recovery. Sep. Purif. Technol. 2005, 42, 31–37. [Google Scholar] [CrossRef]
- Laggoun, Z.; Khalfaoui, A.; Benalia, A.; Ghomrani, A.F.; Bouchareb, R.; Mahfouf, A.; Pizzi, A.; Panico, A.; Derbal, K. Applied Sciences Application of Response Surface Design for Optimization of Direct Red Dye Biosorption onto Cockleshells. Appl. Sci. 2023, 13, 12333. [Google Scholar] [CrossRef]
- Imtiazuddin, S.M.; Mumtaz, M.; Khalil, A. Mallick Pollutants of Wastewater Characteristics in Textile Industries. J. Basic Appl. Sci. 2012, 8, 554–556. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K. Etude Expérimentale et Modélisation Du Processus de La Coagulation Floculation: Application Aux Eaux Destinée a La Consommation. Master’s Thesis, University of Constantine 3, El Khroub, Algeria, 2015. [Google Scholar]
- Ezemagu, I.G.; Ejimofor, M.I.; Menkiti, M.C.; Nwobi-okoye, C.C. Modeling and Optimization of Turbidity Removal from Produced Water Using Response Surface Methodology and Artificial Neural Network. S. Afr. J. Chem. Eng. 2021, 35, 78–88. [Google Scholar] [CrossRef]
- Van Truong, T.; Tiwari, D.; Mok, Y.S.; Kim, D.J. Recovery of Aluminum from Water Treatment Sludge for Phosphorus Removal by Combined Calcination and Extraction. J. Ind. Eng. Chem. 2021, 103, 195–204. [Google Scholar] [CrossRef]
- Art-Chimie Hydroxyde de Sodium Technique. Available online: https://www.art-chimie-online.com/ (accessed on 17 April 2024).


| Parameters | |||
|---|---|---|---|
| Coded Values | pH | Coagulant Dosage (mg/L) | Dye Concentration (mg/L) |
| −1.68 | 1.954 | 2.955 | 2.955 |
| −1 | 4.000 | 20.000 | 20.000 |
| 0 | 7.000 | 45.000 | 45.000 |
| +1 | 10.000 | 70.000 | 70.000 |
| +1.68 | 12.045 | 87.044 | 87.044 |
| Source of Variance | Degree of Freedom | Sum of Square (SS) | Mean Square (MS) | p-Value | R2 | R2adj | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MB | CV | MB | CV | MB | CV | MB | CV | MB | CV | MB | CV | |
| Regression | 9 | 9 | 3076.70 | 6001.91 | 341.855 | 666.38 | 0.000 | 0.000 | 95.80 | 98.44 | 92.02 | 97.03 |
| Residual | 10 | 10 | 134.83 | 95.36 | 13.483 | 9.54 | ||||||
| Total | 19 | 19 | 3211.54 | 6027.27 | ||||||||
| Dye | Factors | Validation | |||
|---|---|---|---|---|---|
| pH | Coagulant Dosage (mg/L) | Dye Concentration (mg/L) | Y Predit * (%) | Y Experimental ** (%) | |
| MB | 12.045 | 87.044 | 2.955 | 94.444 | 89.06 |
| CV | 11.230 | 4.484 | 2.955 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benalia, A.; Derbal, K.; Baatache, O.; Lehchili, C.; Khalfaoui, A.; Pizzi, A. Removal of Dyes from Water Using Aluminum-Based Water Treatment Sludge as a Low-Cost Coagulant: Use of Response Surface Methodology. Water 2024, 16, 1400. https://doi.org/10.3390/w16101400
Benalia A, Derbal K, Baatache O, Lehchili C, Khalfaoui A, Pizzi A. Removal of Dyes from Water Using Aluminum-Based Water Treatment Sludge as a Low-Cost Coagulant: Use of Response Surface Methodology. Water. 2024; 16(10):1400. https://doi.org/10.3390/w16101400
Chicago/Turabian StyleBenalia, Abderrezzaq, Kerroum Derbal, Ouiem Baatache, Cheima Lehchili, Amel Khalfaoui, and Antonio Pizzi. 2024. "Removal of Dyes from Water Using Aluminum-Based Water Treatment Sludge as a Low-Cost Coagulant: Use of Response Surface Methodology" Water 16, no. 10: 1400. https://doi.org/10.3390/w16101400
APA StyleBenalia, A., Derbal, K., Baatache, O., Lehchili, C., Khalfaoui, A., & Pizzi, A. (2024). Removal of Dyes from Water Using Aluminum-Based Water Treatment Sludge as a Low-Cost Coagulant: Use of Response Surface Methodology. Water, 16(10), 1400. https://doi.org/10.3390/w16101400








